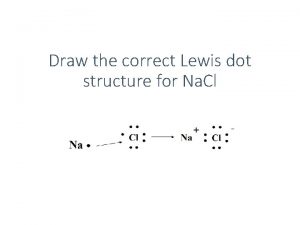
Soil Acidity Effect of p H and Aluminum














- Slides: 57

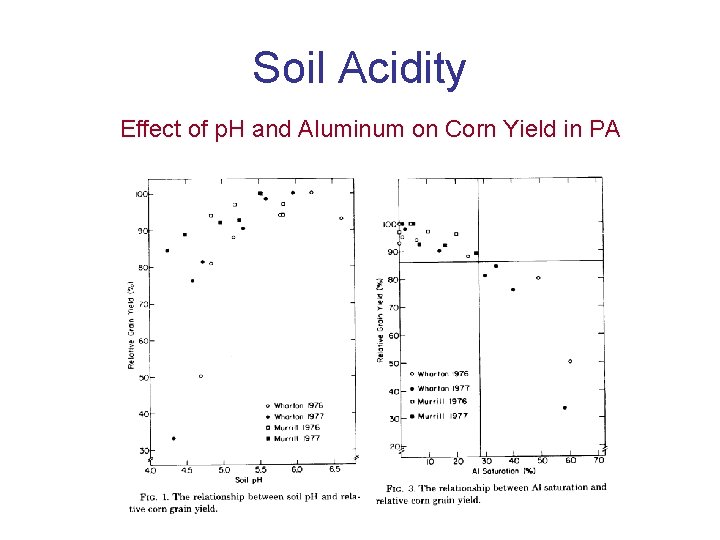
Soil Acidity Effect of p. H and Aluminum on Corn Yield in PA

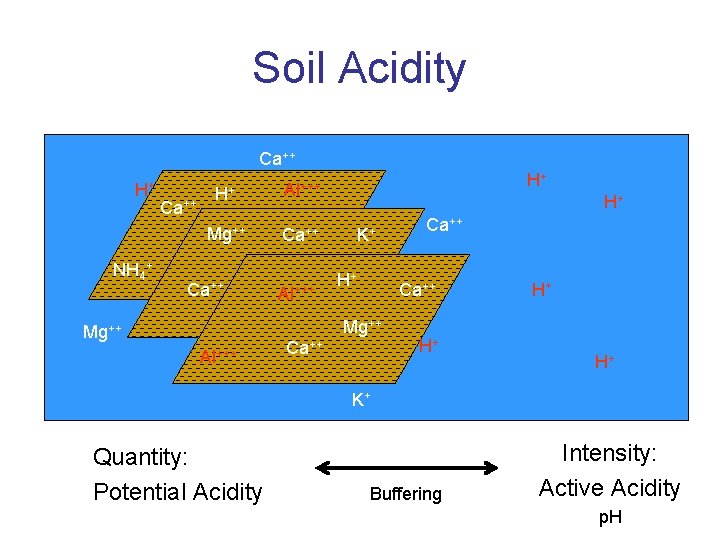
Soil Acidity • Active Acidity • Potential Acidity

Soil Acidity Ca++ H+ NH 4+ Ca++ H+ Al+++ Mg++ Ca++ Al+++ H+ H+ K+ H+ Ca++ Mg++ Al+++ Ca++ H+ H+ H+ K+ Quantity: Potential Acidity Buffering Intensity: Active Acidity p. H

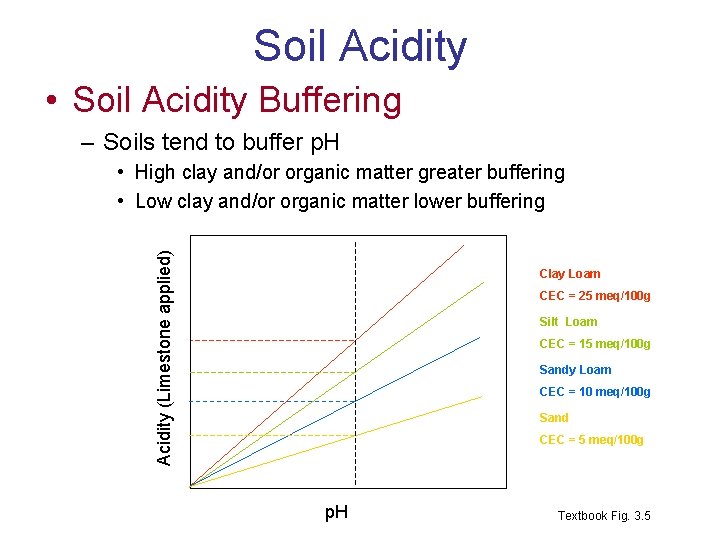
Soil Acidity • Soil Acidity Buffering – Soils tend to buffer p. H Acidity (Limestone applied) • High clay and/or organic matter greater buffering • Low clay and/or organic matter lower buffering Clay Loam CEC = 25 meq/100 g Silt Loam CEC = 15 meq/100 g Sandy Loam CEC = 10 meq/100 g Sand CEC = 5 meq/100 g p. H Textbook Fig. 3. 5

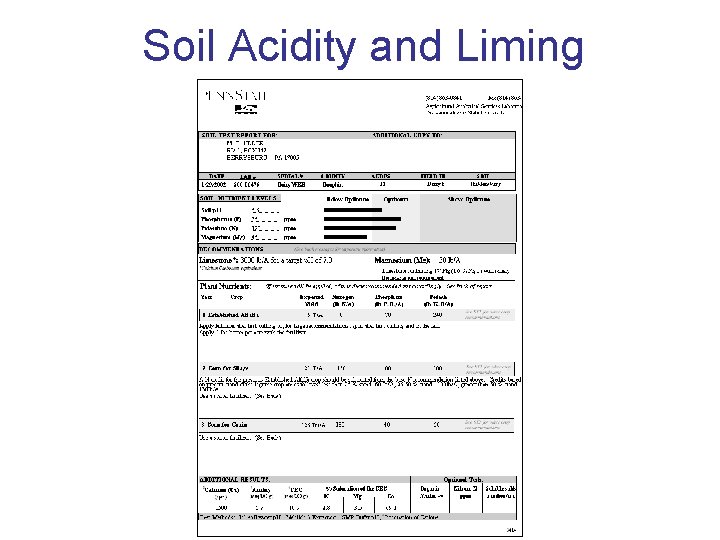
Soil Acidity and Liming

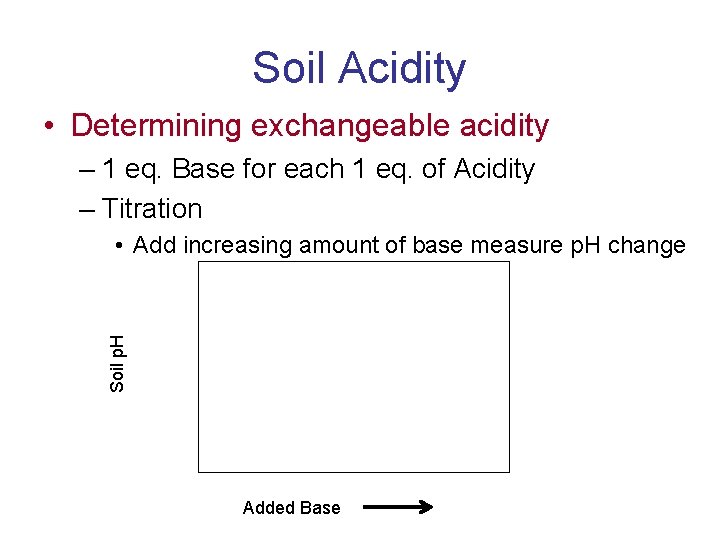
Soil Acidity • Determining exchangeable acidity – 1 eq. Base for each 1 eq. of Acidity – Titration Soil p. H • Add increasing amount of base measure p. H change Added Base

Soil Acidity • Determining exchangeable acidity – Buffer p. H • Add a p. H buffer solution and measure p. H change • Known relationship between p. H change and change in acidity • Common p. H buffers – SMP (used in PA) » Triethanolamine » Paranitrophenol » Potassium chromate » p. H 7. 5 – Adams-Evans – Mehlich – Woodruff

Soil Acidity NOTES • Determining exchangeable acidity – Buffer p. H • Add a p. H buffer solution and measure p. H change • Known relationship between p. H change and change in acidity • Common p. H buffers – SMP (used in PA) » Triethanolamine » Paranitrophenol » Potassium chromate » p. H 7. 5 – Adams-Evans – Mehlich - Many states including PA going to this – Woodruff

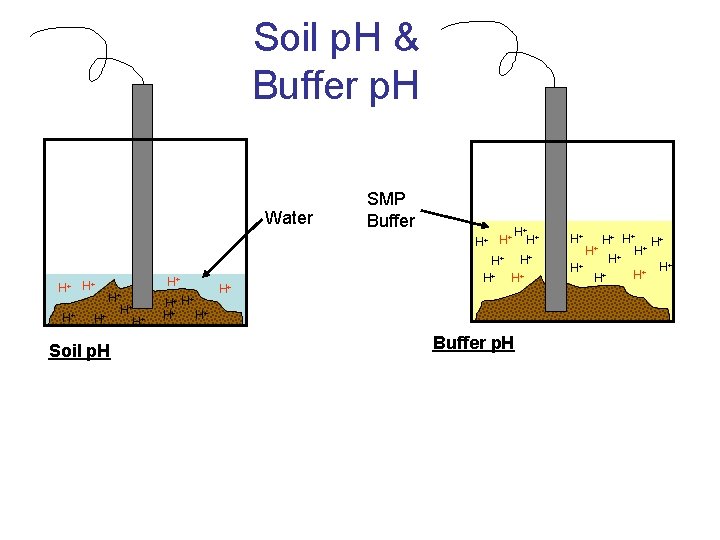
Soil p. H & Buffer p. H Water SMP Buffer H+ + H+ H+ + H H+ + H H H H+ H+ + + H H H+ H+ H+ Soil p. H H+ H+ H+ Buffer p. H

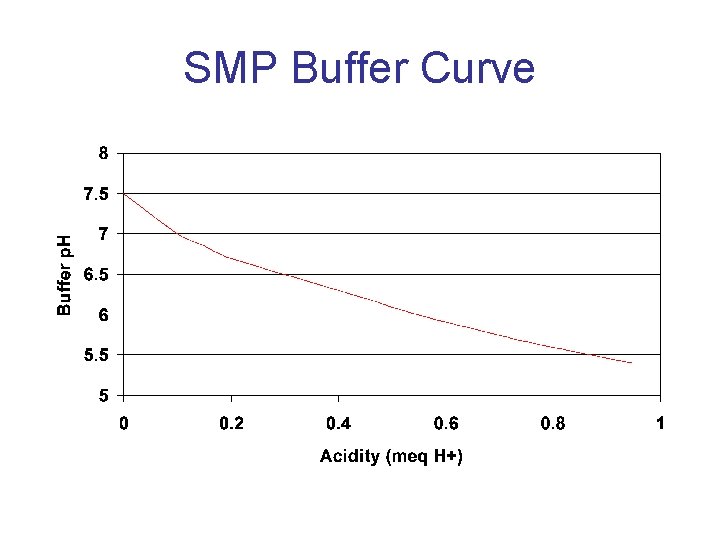
SMP Buffer Curve

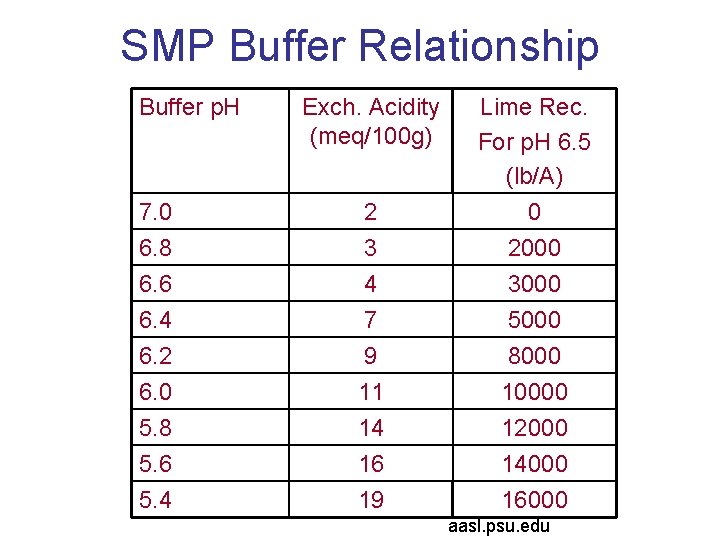
SMP Buffer Relationship Buffer p. H Exch. Acidity (meq/100 g) 7. 0 2 Lime Rec. For p. H 6. 5 (lb/A) 0 6. 8 6. 6 6. 4 6. 2 6. 0 5. 8 5. 6 5. 4 3 4 7 9 11 14 16 19 2000 3000 5000 8000 10000 12000 14000 16000 aasl. psu. edu

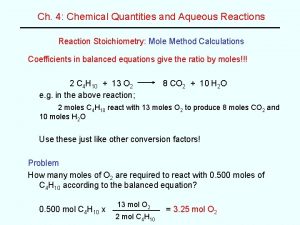
Soil Acidity and Liming • Determining Lime requirement – Exchangeable Acidity • 1 meq. Base/100 g for each 1 meq. of Acidity/100 g • How many pounds of lime do we need per acre?

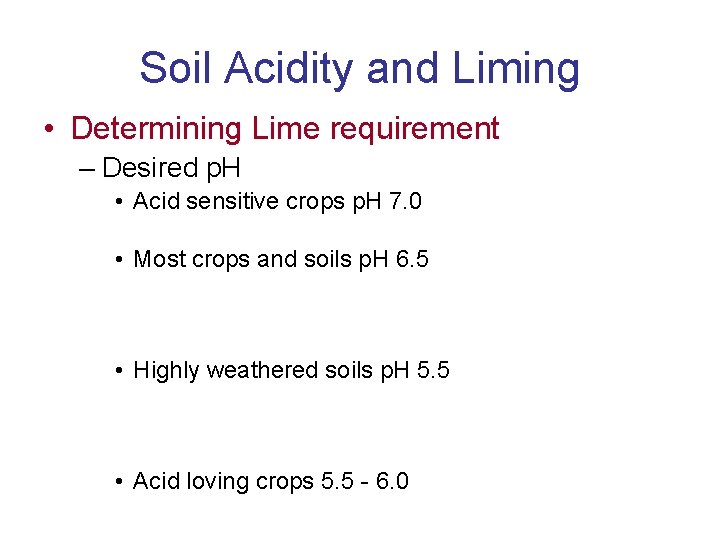
Soil Acidity and Liming • Determining Lime requirement – Desired p. H • Acid sensitive crops p. H 7. 0 • Most crops and soils p. H 6. 5 • Highly weathered soils p. H 5. 5 • Acid loving crops 5. 5 - 6. 0

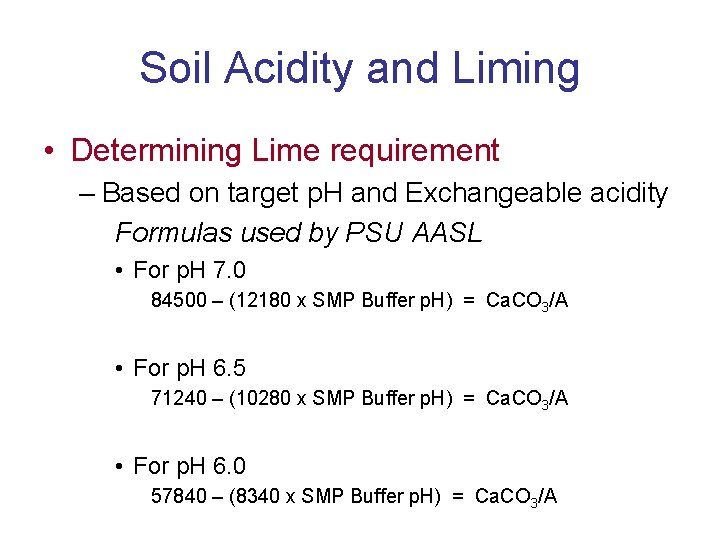
Soil Acidity and Liming • Determining Lime requirement – Based on target p. H and Exchangeable acidity Formulas used by PSU AASL • For p. H 7. 0 84500 – (12180 x SMP Buffer p. H) = Ca. CO 3/A • For p. H 6. 5 71240 – (10280 x SMP Buffer p. H) = Ca. CO 3/A • For p. H 6. 0 57840 – (8340 x SMP Buffer p. H) = Ca. CO 3/A

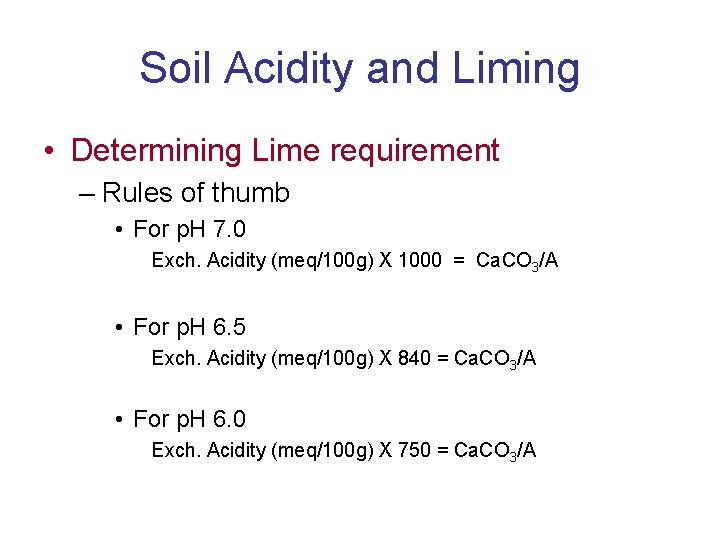
Soil Acidity and Liming • Determining Lime requirement – Rules of thumb • For p. H 7. 0 Exch. Acidity (meq/100 g) X 1000 = Ca. CO 3/A • For p. H 6. 5 Exch. Acidity (meq/100 g) X 840 = Ca. CO 3/A • For p. H 6. 0 Exch. Acidity (meq/100 g) X 750 = Ca. CO 3/A

Soil Acidity and Liming • Liming Materials – Material that will neutralize soil acidity • Calcium Oxide (Ca. O) – Lime, burnt lime, quick lime • Calcium Hydroxide (Ca(OH)2) – Hydrated lime, slaked lime • Calcium Carbonate (Ca. CO 3) – Calcitic limestone • Calcium/Magnesium Carbonate (Ca, Mg. CO 3) – Dolomitic limestone

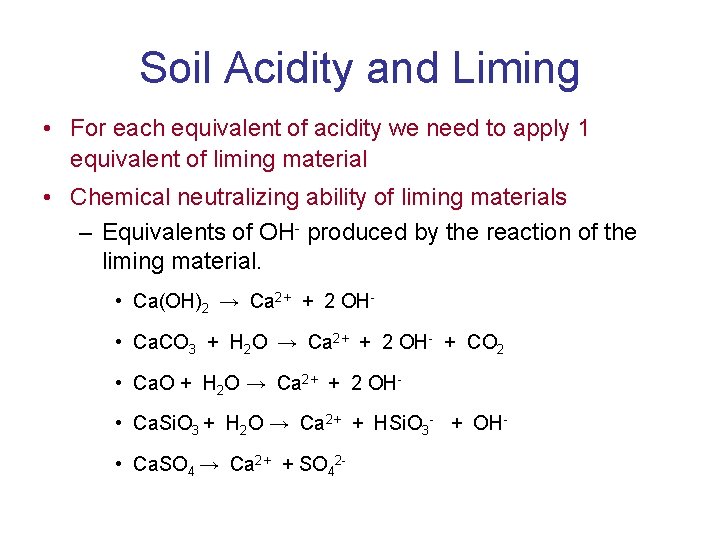
Soil Acidity and Liming • For each equivalent of acidity we need to apply 1 equivalent of liming material • Chemical neutralizing ability of liming materials – Equivalents of OH- produced by the reaction of the liming material. • Ca(OH)2 → Ca 2+ + 2 OH • Ca. CO 3 + H 2 O → Ca 2+ + 2 OH- + CO 2 • Ca. O + H 2 O → Ca 2+ + 2 OH • Ca. Si. O 3 + H 2 O → Ca 2+ + HSi. O 3 - + OH • Ca. SO 4 → Ca 2+ + SO 42 -

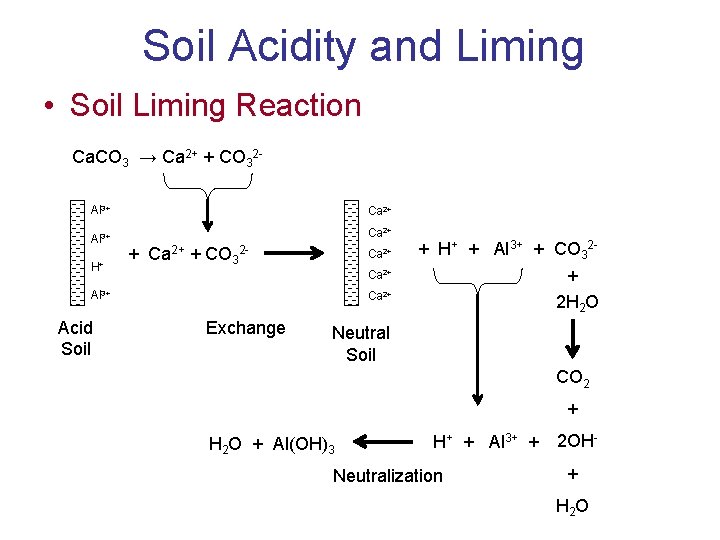
Soil Acidity and Liming • Soil Liming Reaction Ca. CO 3 → Ca 2+ + CO 32 Al 3+ Ca 2+ H+ + Ca 2+ + CO 32 - Ca 2+ + 2 H 2 O Ca 2+ Al 3+ Acid Soil + H+ + Al 3+ + CO 32 - Ca 2+ Exchange Neutral Soil CO 2 + H 2 O + Al(OH)3 H+ + Al 3+ + Neutralization 2 OH+ H 2 O

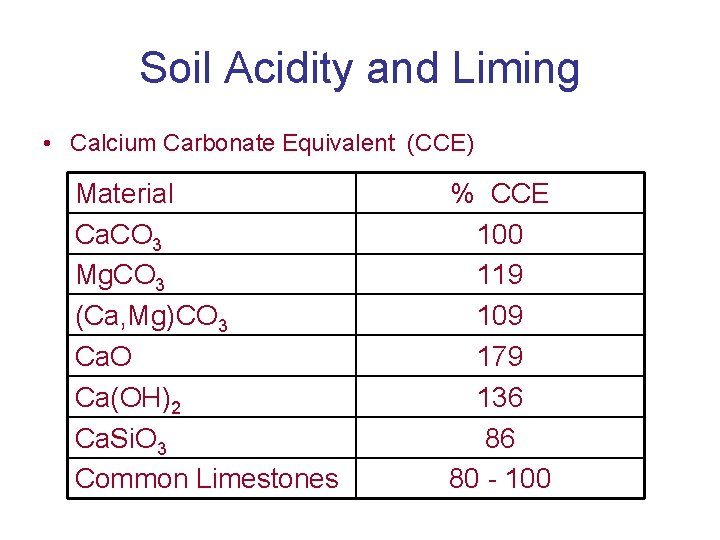
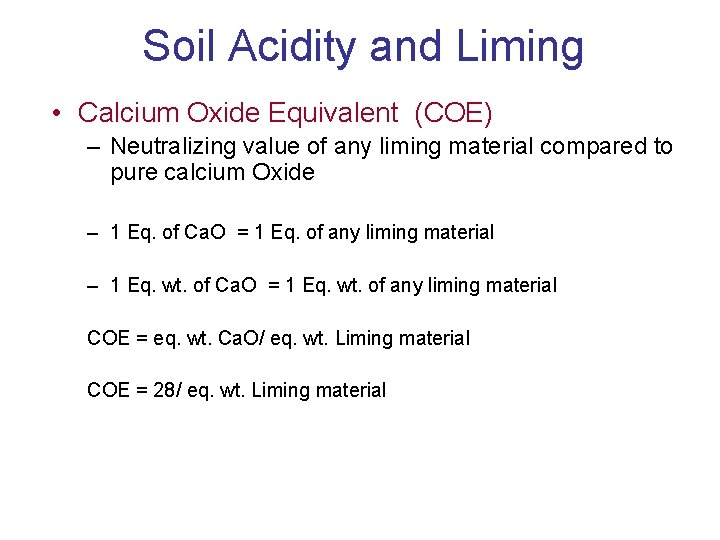
Soil Acidity and Liming • Liming material quality • Calcium Carbonate Equivalent (CCE) – Neutralizing value of any liming material compared to pure calcium carbonate

Soil Acidity and Liming • Calcium Carbonate Equivalent (CCE) – 1 Eq. of Ca. CO 3 = 1 Eq. of any liming material

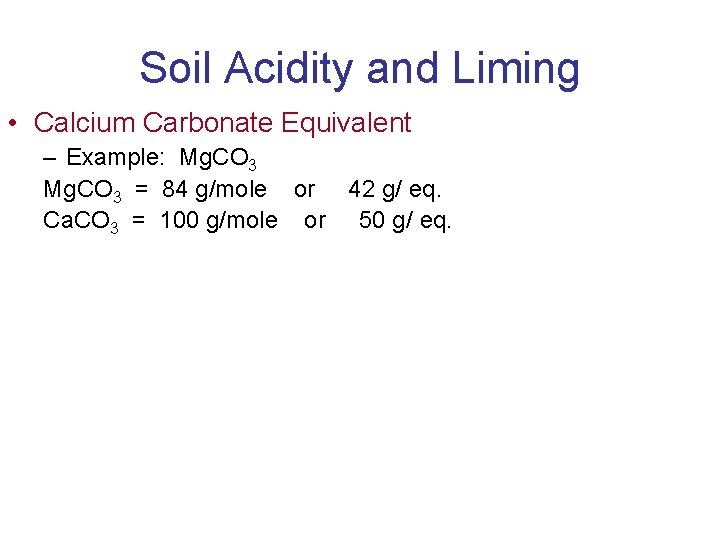
Soil Acidity and Liming • Calcium Carbonate Equivalent – Example: Mg. CO 3 = 84 g/mole or 42 g/ eq. Ca. CO 3 = 100 g/mole or 50 g/ eq.

Soil Acidity and Liming • Calcium Carbonate Equivalent (CCE) Material Ca. CO 3 Mg. CO 3 (Ca, Mg)CO 3 Ca. O Ca(OH)2 Ca. Si. O 3 Common Limestones % CCE 100 119 109 179 136 86 80 - 100

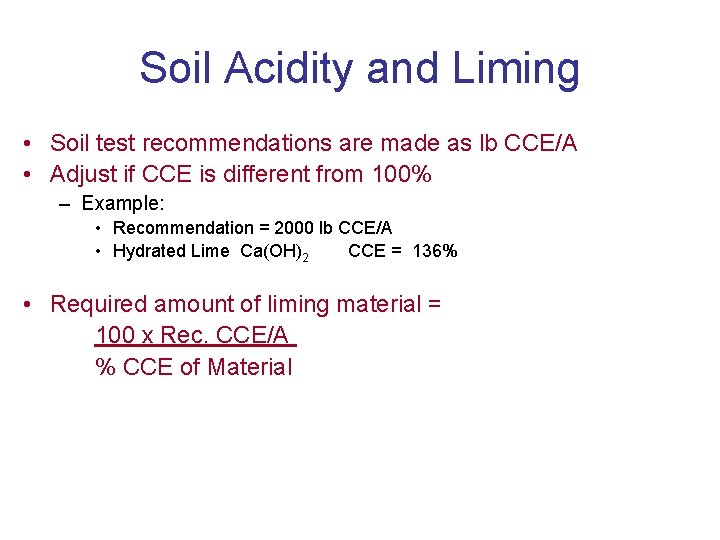
Soil Acidity and Liming • Soil test recommendations are made as lb CCE/A • Adjust if CCE is different from 100% – Example: • Recommendation = 2000 lb CCE/A • Hydrated Lime Ca(OH)2 CCE = 136% • Required amount of liming material = 100 x Rec. CCE/A % CCE of Material

Soil Acidity and Liming • Real World – Not usually dealing with pure materials, so we can't always calculate the CCE – Measure CCE in lab – Required in most states • Titration with standard acid – CCE is provide on the label for all liming materials sold in PA – Be careful calculating CCE on unknown materials

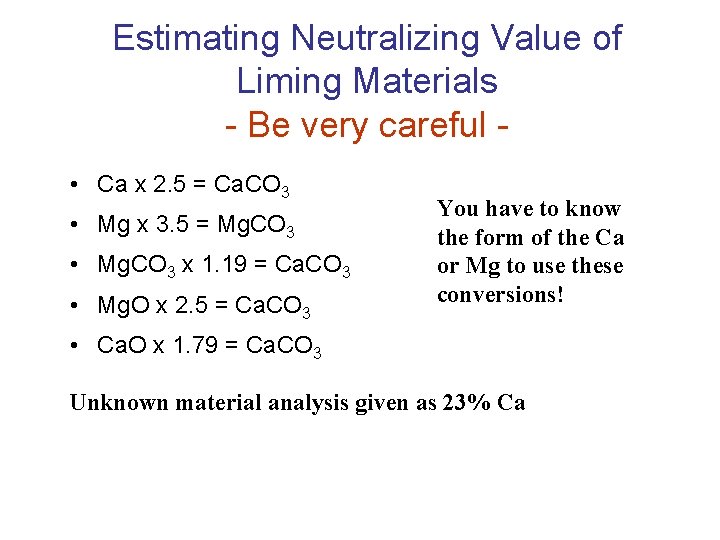
Estimating Neutralizing Value of Liming Materials - Be very careful • Ca x 2. 5 = Ca. CO 3 • Mg x 3. 5 = Mg. CO 3 • Mg. CO 3 x 1. 19 = Ca. CO 3 • Mg. O x 2. 5 = Ca. CO 3 You have to know the form of the Ca or Mg to use these conversions! • Ca. O x 1. 79 = Ca. CO 3 Unknown material analysis given as 23% Ca

Estimating Neutralizing Value of Liming Materials - Be very careful • If you don’t know what the material is made of then you need to measure the CCE directly! • Cost ~ $10 -15

Soil Acidity and Liming • Calcium Oxide Equivalent (COE) – Neutralizing value of any liming material compared to pure calcium Oxide – 1 Eq. of Ca. O = 1 Eq. of any liming material – 1 Eq. wt. of Ca. O = 1 Eq. wt. of any liming material COE = eq. wt. Ca. O/ eq. wt. Liming material COE = 28/ eq. wt. Liming material

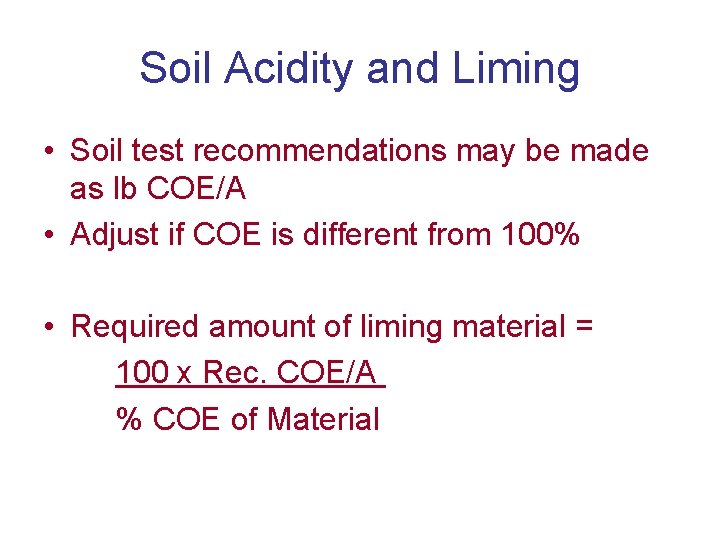
Soil Acidity and Liming • Soil test recommendations may be made as lb COE/A • Adjust if COE is different from 100% • Required amount of liming material = 100 x Rec. COE/A % COE of Material

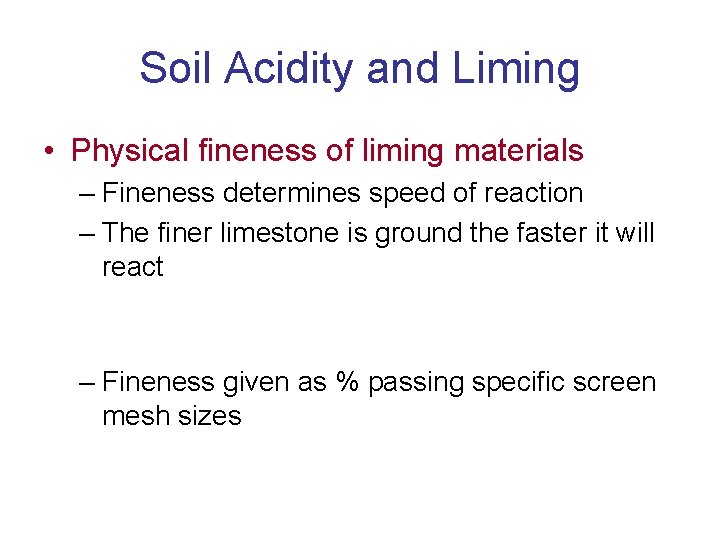
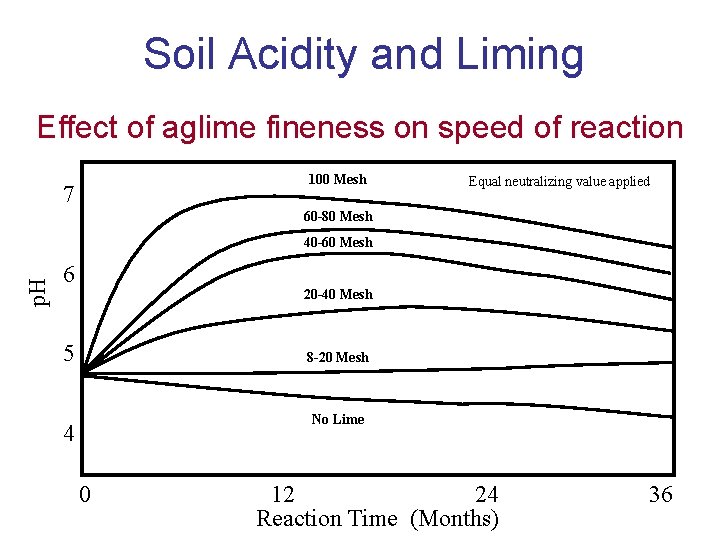
Soil Acidity and Liming • Physical fineness of liming materials – Fineness determines speed of reaction – The finer limestone is ground the faster it will react – Fineness given as % passing specific screen mesh sizes

Soil Acidity and Liming Effect of aglime fineness on speed of reaction 100 Mesh 7 Equal neutralizing value applied 60 -80 Mesh p. H 40 -60 Mesh 6 20 -40 Mesh 5 8 -20 Mesh No Lime 4 0 12 24 Reaction Time (Months) 36

Soil Acidity and Liming • Physical fineness of liming materials – Practical Limits to fineness – Larger than 20 mesh – not effective – Smaller than 100 mesh – little added benefit

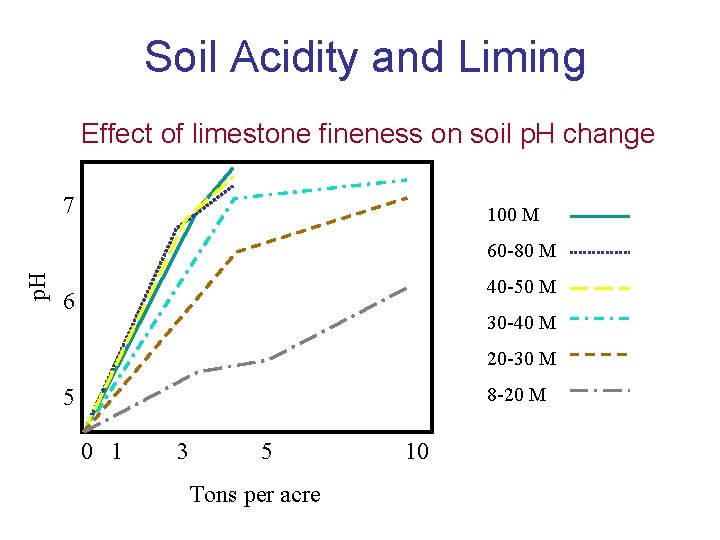
Soil Acidity and Liming Effect of limestone fineness on soil p. H change 7 100 M p. H 60 -80 M 40 -50 M 6 30 -40 M 20 -30 M 8 -20 M 5 0 1 3 5 Tons per acre 10

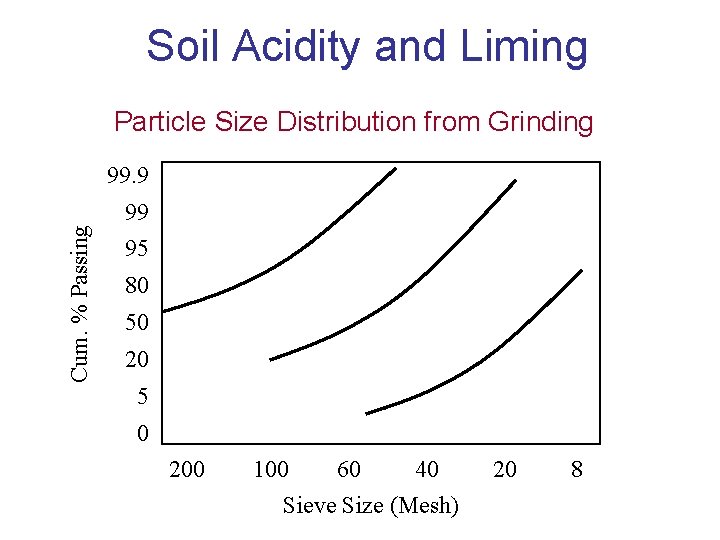
Soil Acidity and Liming Particle Size Distribution from Grinding 99. 9 Cum. % Passing 99 95 80 50 20 5 0 200 100 60 40 Sieve Size (Mesh) 20 8

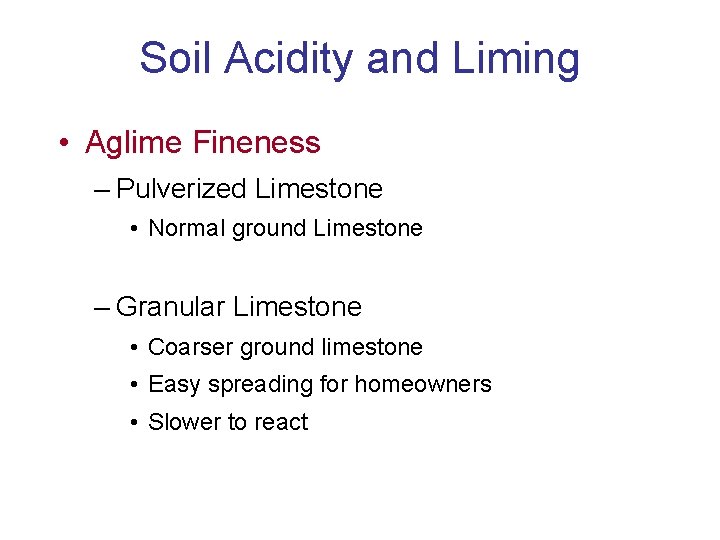
Soil Acidity and Liming • Aglime Fineness – Pulverized Limestone • Normal ground Limestone – Granular Limestone • Coarser ground limestone • Easy spreading for homeowners • Slower to react

Aglime Fineness definitions for PA Fine Sized Materials Medium Sized Materials 95% - 20 mesh sieve 90% - 20 mesh sieve 60% - 60 mesh sieve 50% - 100 mesh sieve 30% - 100 mesh sieve Coarse sized materials - all liming materials failing to meet the above fineness criteria

Aglime Quality • Acid-Base Chemistry and Fineness • Ca. CO 3 + H 2 O Ca 2+ + H 2 CO 3 + 2 OH- + 2 H+ 2 H 2 O For a given amount of acidity an equivalent amount of base (liming material) will be required regardless if it is ground very fine.

Aglime Quality • Acid-Base Chemistry in soils 1 meq/100 g acidity in the soil will require 1 meq/100 g of Ca. CO 3 to neutralize it. 1 meq Ca. CO 3/100 g soil = 1000 lb Ca. CO 3/A

Soil Acidity and Liming • Aglime Fineness – No matter how fine you grind limestone you cannot increase the chemical neutralizing ability – Finer the better, because it will react faster, but there are practical limitations – Distribution in “Fine Size” Limestone – usually adequate for practical liming for field crops – Little difference between calcite and dolomite

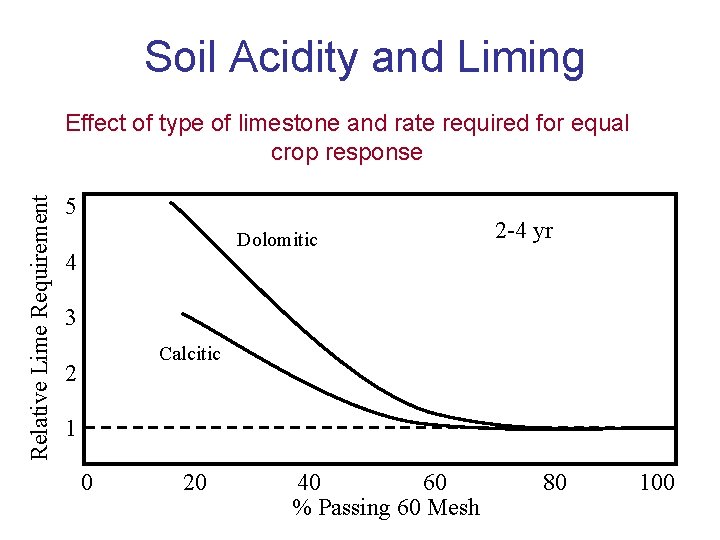
Soil Acidity and Liming Relative Lime Requirement Effect of type of limestone and rate required for equal crop response 5 Dolomitic 4 2 -4 yr 3 Calcitic 2 1 0 20 40 60 % Passing 60 Mesh 80 100

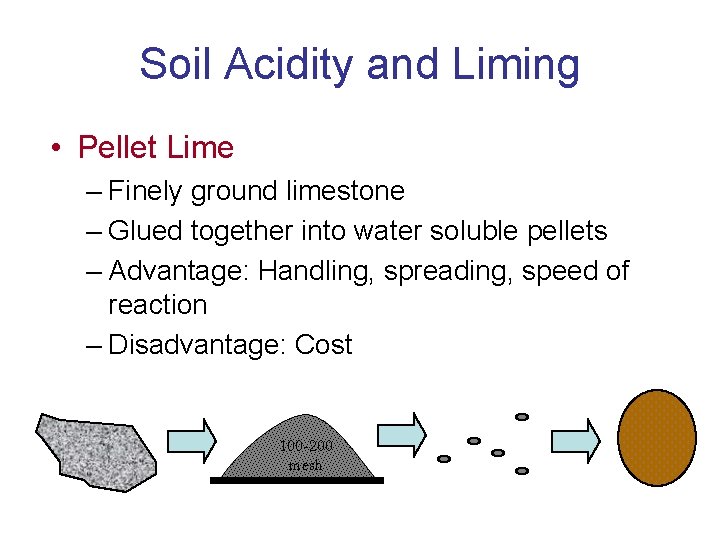
Soil Acidity and Liming • Pellet Lime – Finely ground limestone – Glued together into water soluble pellets – Advantage: Handling, spreading, speed of reaction – Disadvantage: Cost 100 -200 mesh

Soil Acidity and Liming • Fluid Lime – Finely ground limestone – Suspended in water with clay – Approx. 1000 lb CCE/ton material – Advantage: Spreading, speed of reaction – Disadvantage: Cost

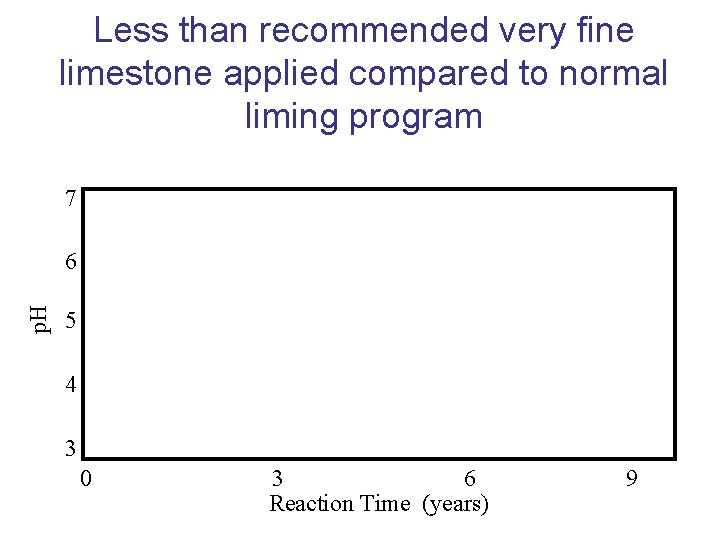
Less than recommended very fine limestone applied compared to normal liming program 7 p. H 6 5 4 3 0 3 6 Reaction Time (years) 9

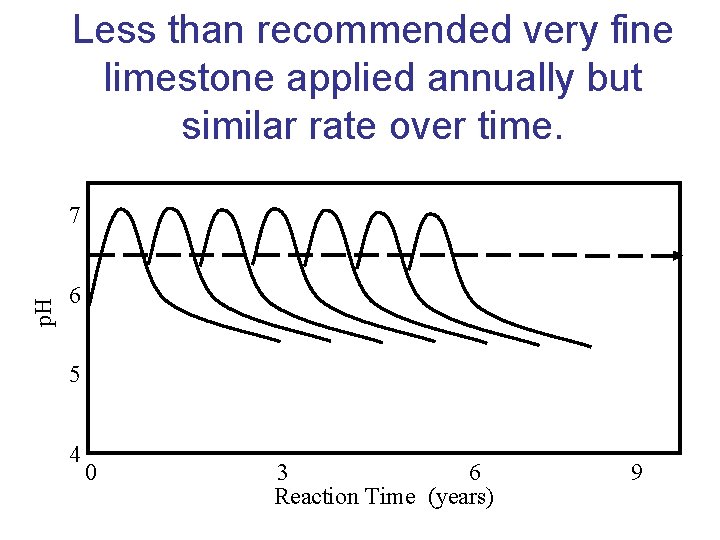
Less than recommended very fine limestone applied annually but similar rate over time. p. H 7 6 5 4 0 3 6 Reaction Time (years) 9

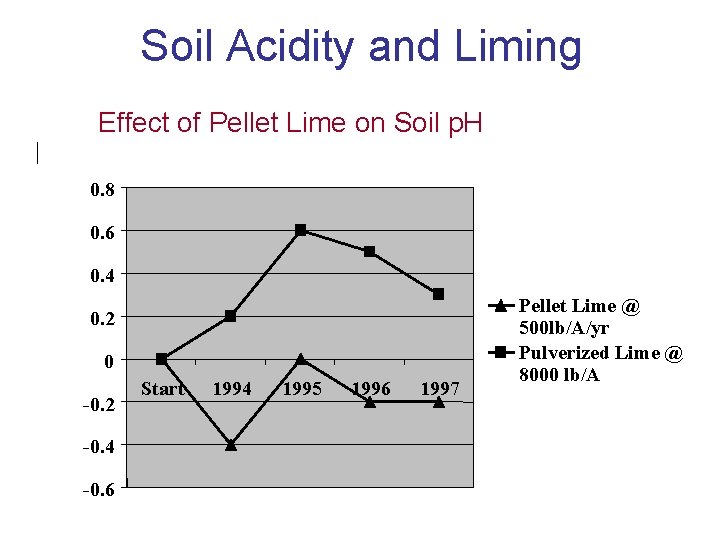
Soil Acidity and Liming Effect of Pellet Lime on Soil p. H 0. 8 Change in p. H 0. 6 0. 4 0. 2 0 -0. 2 -0. 4 -0. 6 Start 1994 1995 1996 1997 Pellet Lime @ 500 lb/A/yr Pulverized Lime @ 8000 lb/A

Soil Acidity and Liming • Calcium and Magesium – Normal liming practices will also supply required calcium and magnesium • At normal rates usually adequate Ca will be supplied for most crops • Magnesium will depend on the type of limestone used – If Mg is required use a Mg containing limestone (dolomitic) – Mg recommendations » pounds Mg/A » % Mg in recommended limestone

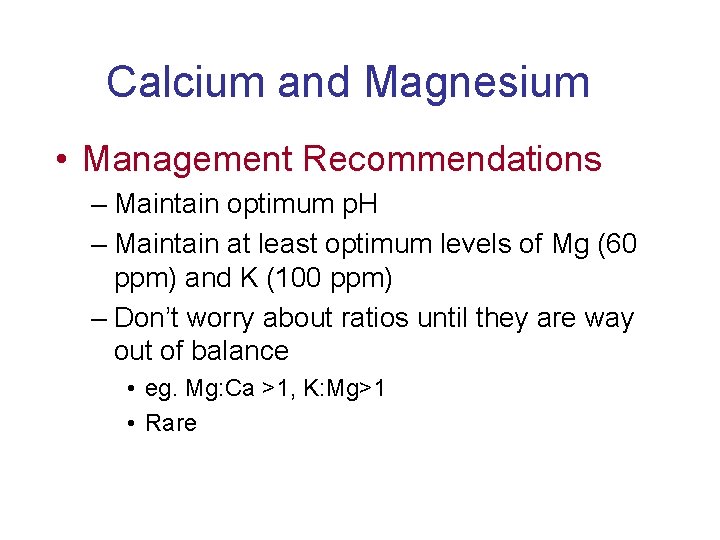
Calcium and Magnesium • Management Recommendations – Maintain optimum p. H – Maintain at least optimum levels of Mg (60 ppm) and K (100 ppm) – Don’t worry about ratios until they are way out of balance • eg. Mg: Ca >1, K: Mg>1 • Rare

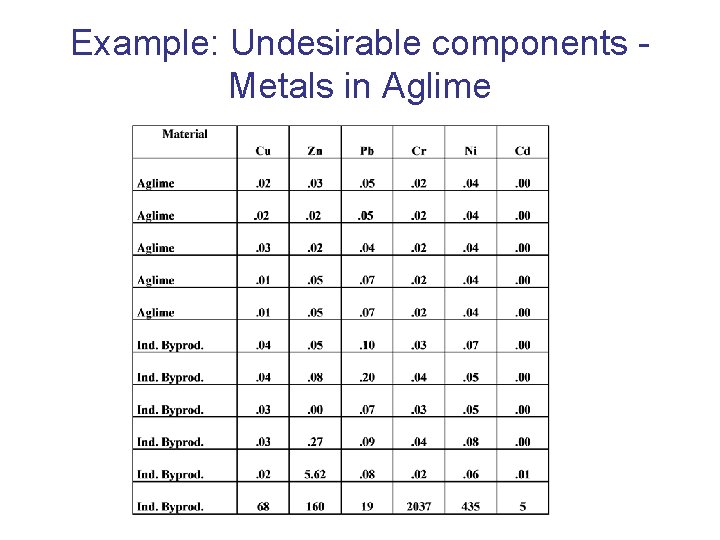
By-product Liming Materials • Quality –Liming value –Undesirable components

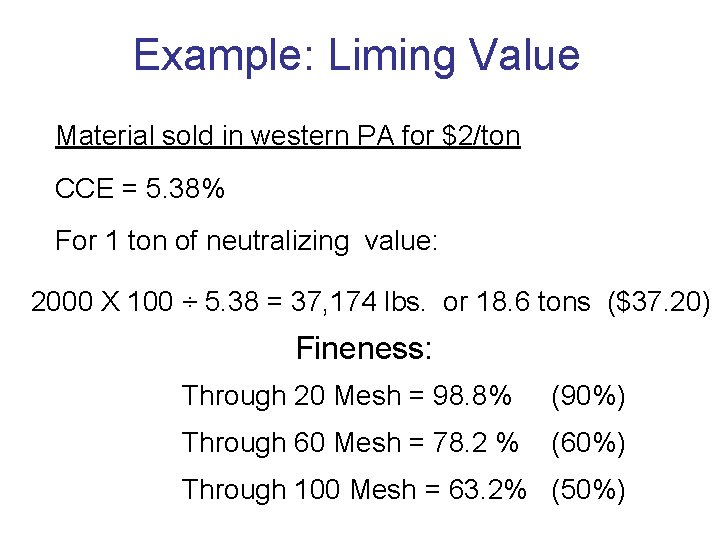
Example: Liming Value Material sold in western PA for $2/ton CCE = 5. 38% For 1 ton of neutralizing value: 2000 X 100 ÷ 5. 38 = 37, 174 lbs. or 18. 6 tons ($37. 20) Fineness: Through 20 Mesh = 98. 8% (90%) Through 60 Mesh = 78. 2 % (60%) Through 100 Mesh = 63. 2% (50%)

Gypsum • Ca. SO 4 • Excellent source of Ca and S – 33% Ca & 27% S • No neutralizing value • Not a liming material

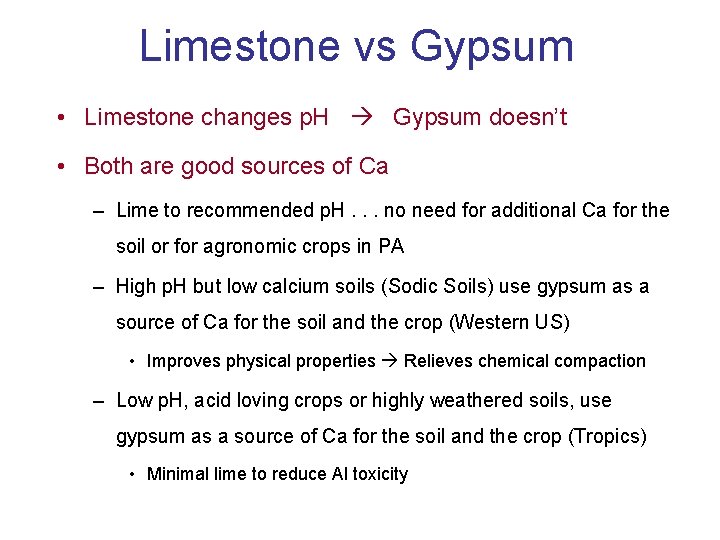
Limestone vs Gypsum • Limestone changes p. H Gypsum doesn’t • Both are good sources of Ca – Lime to recommended p. H. . . no need for additional Ca for the soil or for agronomic crops in PA – High p. H but low calcium soils (Sodic Soils) use gypsum as a source of Ca for the soil and the crop (Western US) • Improves physical properties Relieves chemical compaction – Low p. H, acid loving crops or highly weathered soils, use gypsum as a source of Ca for the soil and the crop (Tropics) • Minimal lime to reduce Al toxicity

Byproduct Materials Undesirable components • Should be registered (PDA) • Must be approved for land application by DEP – May limit lifetime application • Problem with unregistered materials and materials from out of state – May be little or no checking of quality • Determine the source – Dust, screenings – Stainless steel slag • Ask what contaminants might be present • If in doubt. . . get it analyzed OR don’t use it

Example: Undesirable components Metals in Aglime

Other Materials • Biosolids and Water Treatment Sludges – Often have significant neutralizing value – Must be clean – Must be registered if sold as liming materials

Other Materials Organic Calcium Compounds • Promesol 30, Liquid Lime – Trihydroxy glutaric acid 25% Ca – 1 gallon = 500 -750 lb CCE? • Liqui-Til – Trihydroxy glutaric acid – Neutralizes p. H in alkaline soil? • KK Organic Soil Builder – Neutralizes both acidic and alkaline soils? • Liqui-Cal – 8% Ca – 1 gallon = 500 lb Ca. CO 3? • Liquid Calcium – 1 gallon = Ca in 500 lb Ca. CO 3? – Suggest it is a lime substitute • Golden Cal – Glucoheptomic acid – 1 gallon = 500 lb CCE? • p. H Plus – 1 gallon = 500 -750 lb CCE?

Other Materials • Remember: 1 Eq. of base is required to neutralize 1 Eq. of acid • Watch out for materials that contain Ca with unwritten or suggestive claims for liming value

Soil Acidity and Liming • Limestone Application – Apply limestone far enough ahead of time to be effective – Spread limestone uniformly • • Spinner spreaders Boom spreaders Damp lime Dust – Spit high rates of limestone (>4 ton/A) – Time of year is not too critical • Consider soil quality issues – compaction – Mix limestone as much as practical • Adjust for depth of mixing - 6 2/3 in. standard depth • No till – Correct p. H before going to no-till

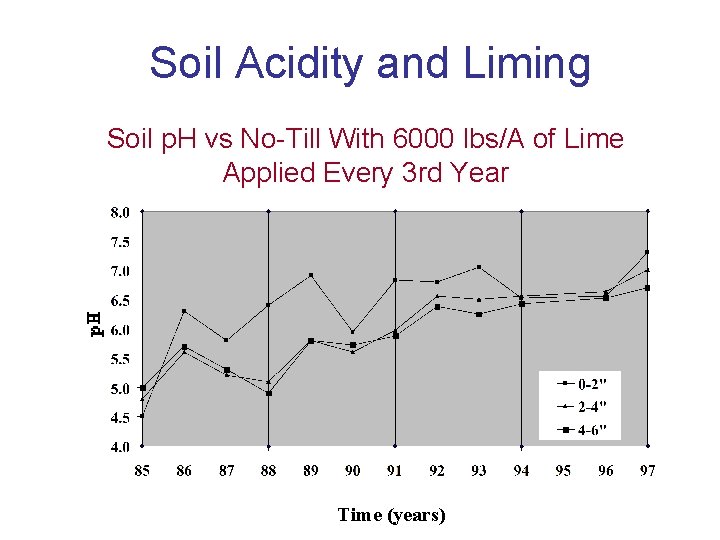
Soil Acidity and Liming Soil p. H vs No-Till With 6000 lbs/A of Lime Applied Every 3 rd Year Time (years)
 Soil compaction ppt
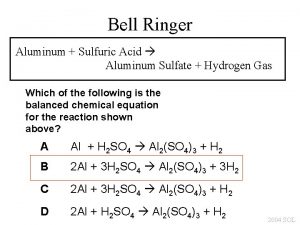
Soil compaction ppt Aluminium and sulfuric acid
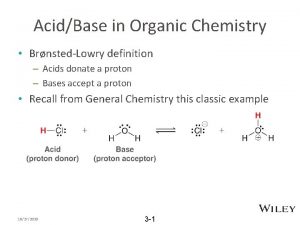
Aluminium and sulfuric acid Effect of hybridization on acidity
Effect of hybridization on acidity Thiol molecules
Thiol molecules Carboxylic acid to an ester
Carboxylic acid to an ester What does na2cr2o7 do
What does na2cr2o7 do Acidity of alcohols
Acidity of alcohols Nh4 base or acid
Nh4 base or acid Acidity of thiols
Acidity of thiols Carboxylic acid or base
Carboxylic acid or base Acidity trends periodic table
Acidity trends periodic table 3 methylbutanoic acid
3 methylbutanoic acid Acidity of phenol
Acidity of phenol Iontový součin vody
Iontový součin vody Qualitative organic analysis
Qualitative organic analysis Ario organic chemistry
Ario organic chemistry Living soil vs dead soil
Living soil vs dead soil Four major spheres of the earth
Four major spheres of the earth Bohr effect in respiration
Bohr effect in respiration Marshallian demand function
Marshallian demand function Aluminum and iron iii oxide balanced equation
Aluminum and iron iii oxide balanced equation Aluminum and oxygen ionic compound formula
Aluminum and oxygen ionic compound formula Replacement reaction example
Replacement reaction example Aluminum and its alloys
Aluminum and its alloys Aluminum and its alloys
Aluminum and its alloys Aluminum sulfate + calcium hydroxide
Aluminum sulfate + calcium hydroxide Bottleneck effect
Bottleneck effect Chemical properties of aluminum
Chemical properties of aluminum Rust chemical reaction
Rust chemical reaction Aluminum foil is cut in half physical or chemical change
Aluminum foil is cut in half physical or chemical change Aluminum ion symbol
Aluminum ion symbol Aluminum perbromate
Aluminum perbromate Ionic bonding worksheet answers
Ionic bonding worksheet answers How to draw the bohr diagram
How to draw the bohr diagram Hatchet chapter 1 questions
Hatchet chapter 1 questions Eb welding aluminum
Eb welding aluminum Aluminum winding dry type transformer
Aluminum winding dry type transformer What mass of aluminum hydroxide are needed to decompose
What mass of aluminum hydroxide are needed to decompose Ionic equation of caco3 and hcl
Ionic equation of caco3 and hcl Define bulk gaining industry
Define bulk gaining industry Custom tree grates
Custom tree grates Skc aluminum cyclone
Skc aluminum cyclone Sheet metal extrusion process
Sheet metal extrusion process Is aluminum brittle
Is aluminum brittle Computed radiography cassette
Computed radiography cassette Mach disk
Mach disk How to build a boat with aluminum foil
How to build a boat with aluminum foil Uhv equipment
Uhv equipment Aluminum bohr model
Aluminum bohr model Perfluorate
Perfluorate Word equation chemistry
Word equation chemistry Aluminum relative permittivity
Aluminum relative permittivity On average 113 204 aluminum cans
On average 113 204 aluminum cans An aluminum master cylinder ______.
An aluminum master cylinder ______. Magnesium pes spectrum
Magnesium pes spectrum What is the correct lewis dot structure for arsenic
What is the correct lewis dot structure for arsenic Transparent and translucent
Transparent and translucent Atoms tend to gain lose or share electrons
Atoms tend to gain lose or share electrons