Soil Acidity and Nutrients Section D Soil Fertility







![3+ Al(H 2 O)6 [Al(H 2 O)5 OH] 2+ + H+ Al 3+ H 3+ Al(H 2 O)6 [Al(H 2 O)5 OH] 2+ + H+ Al 3+ H](https://slidetodoc.com/presentation_image_h/333a6f792760a4508d070470a762ed78/image-12.jpg)
![[Al(H 2 O)5 OH] 2+ [Al(H 2 O)4(OH) 2] + + H+ Al 3+ [Al(H 2 O)5 OH] 2+ [Al(H 2 O)4(OH) 2] + + H+ Al 3+](https://slidetodoc.com/presentation_image_h/333a6f792760a4508d070470a762ed78/image-13.jpg)
![[Al(H 2 O)5 OH] + [Al(H 2 O)3(OH)3] + H+ Al 3+ H 2 [Al(H 2 O)5 OH] + [Al(H 2 O)3(OH)3] + H+ Al 3+ H 2](https://slidetodoc.com/presentation_image_h/333a6f792760a4508d070470a762ed78/image-14.jpg)







- Slides: 29

Soil Acidity and Nutrients Section D Soil Fertility and Plant Nutrition

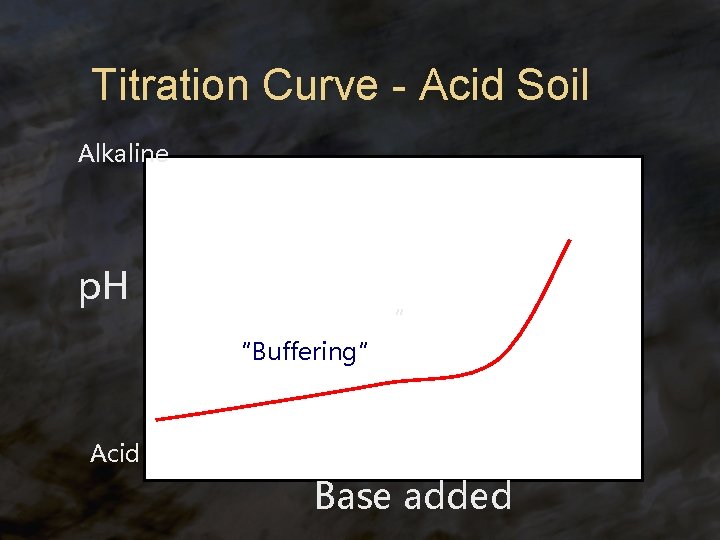
Soils ® Soils behave as weak acids or bases ® Some soils behave like a buffered weak acid: are acid, but resist increases in p. H ® have a “buffering capacity” ® ® Some soils behave like a buffered weak base are basic, but resist decreases in p. H ® have a “buffering capacity” ®

Titration Curve - Acid Soil Alkaline p. H “ “Buffering” Acid Base added

Soils ® Many soils behave like weak acids because: ® production of CO 2 ® organic acids in SOM, root exudates ® EXCHANGEABLE CATIONS ® Exchangeable cations are the most important influence on soil p. H. The relative proportion of acid or basic cations on the cation exchange sites determine the soil p. H in almost all cases.

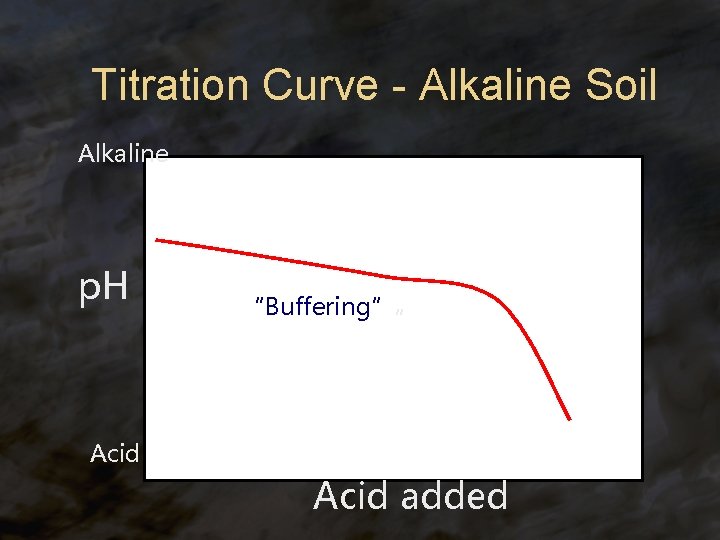
Titration Curve - Alkaline Soil Alkaline p. H “Buffering” “ Acid added

Soils ® Some soils behave like weak bases because: ® presence of Ca. CO 3 ® it is a weak base that will buffer soil solutions against decreases in p. H

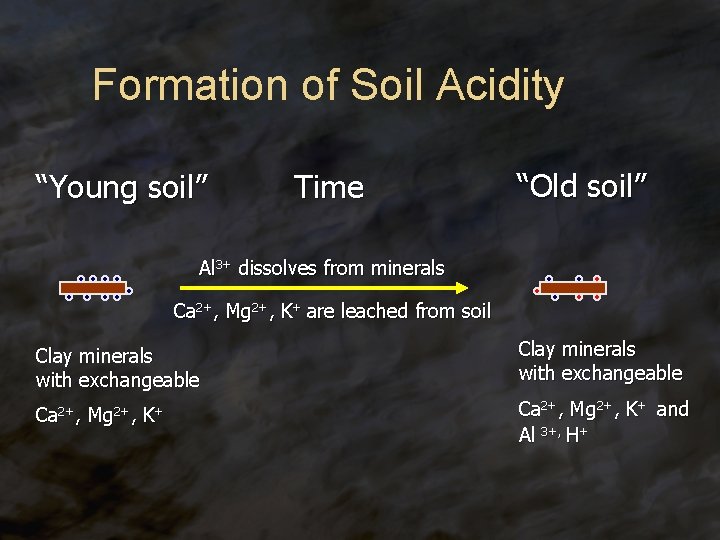
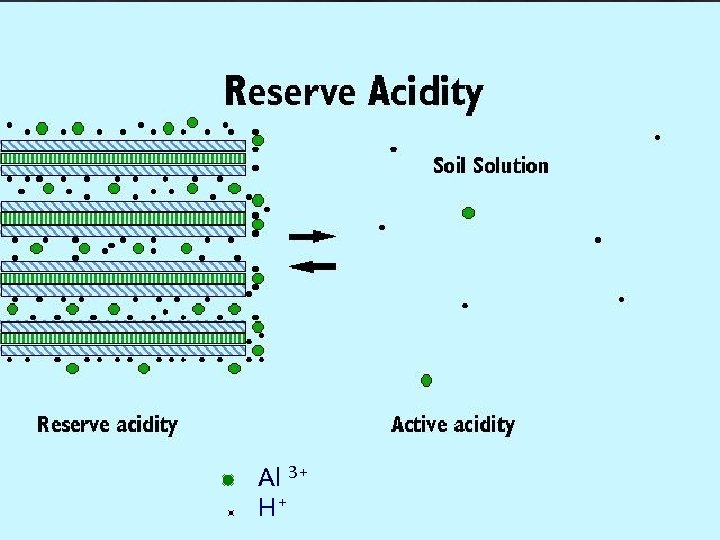
Formation of Soil Acidity “Young soil” Time “Old soil” Al 3+ dissolves from minerals Ca 2+, Mg 2+, K+ are leached from soil Clay minerals with exchangeable Ca 2+, Mg 2+, K+ and Al 3+, H+

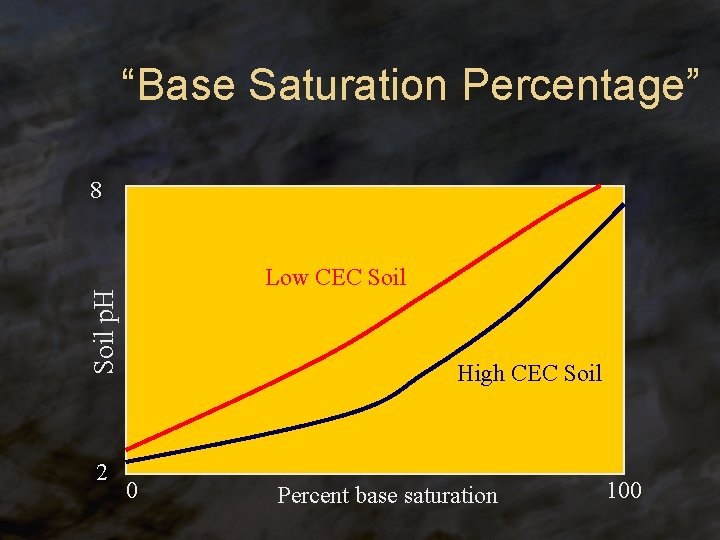
Exchangeable Cations ® Ca 2+, Mg 2+, Na+, and K+ do not produce H+ in soils, and so are called the “basic cations” ® Al 3+ and Fe 3+ react to produce H+ in soils, and so are called the “acid cations” ® The “base saturation” is the percentage of the CEC occupied by the basic cations. It is highly related to soil p. H.

“Base Saturation Percentage” 8 Soil p. H Low CEC Soil 2 High CEC Soil 0 Percent base saturation 100

Why is ® Al 3+ 3+ Al called “acidic”? ions in solution are surrounded by water molecules (octahedral coordination). ® The high positive charge of Al 3+ causes it to pull electrons from O on H 2 O. ® This makes H 2 O more acidic. ® This is called “Al hydrolysis” ® Fe 3+ behaves similarly.

![3 AlH 2 O6 AlH 2 O5 OH 2 H Al 3 H 3+ Al(H 2 O)6 [Al(H 2 O)5 OH] 2+ + H+ Al 3+ H](https://slidetodoc.com/presentation_image_h/333a6f792760a4508d070470a762ed78/image-12.jpg)
3+ Al(H 2 O)6 [Al(H 2 O)5 OH] 2+ + H+ Al 3+ H 2 O
![AlH 2 O5 OH 2 AlH 2 O4OH 2 H Al 3 [Al(H 2 O)5 OH] 2+ [Al(H 2 O)4(OH) 2] + + H+ Al 3+](https://slidetodoc.com/presentation_image_h/333a6f792760a4508d070470a762ed78/image-13.jpg)
[Al(H 2 O)5 OH] 2+ [Al(H 2 O)4(OH) 2] + + H+ Al 3+ H 2 O
![AlH 2 O5 OH AlH 2 O3OH3 H Al 3 H 2 [Al(H 2 O)5 OH] + [Al(H 2 O)3(OH)3] + H+ Al 3+ H 2](https://slidetodoc.com/presentation_image_h/333a6f792760a4508d070470a762ed78/image-14.jpg)
[Al(H 2 O)5 OH] + [Al(H 2 O)3(OH)3] + H+ Al 3+ H 2 O

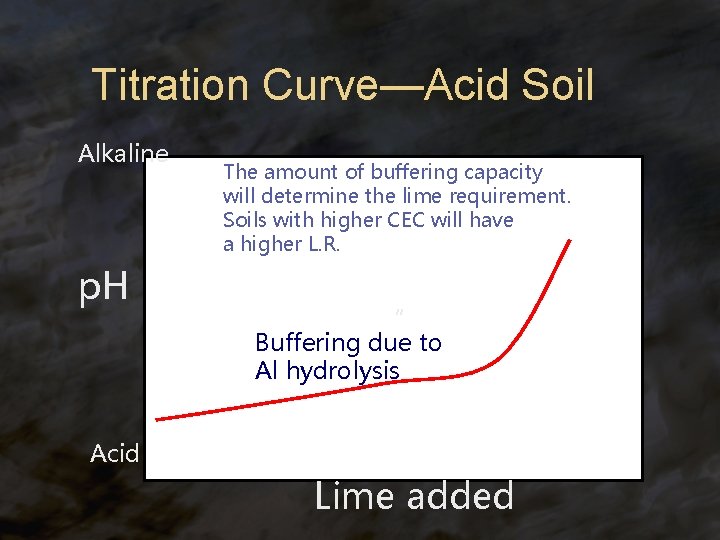
Titration Curve—Acid Soil Alkaline p. H The amount of buffering capacity will determine the lime requirement. Soils with higher CEC will have a higher L. R. “ Buffering due to Al hydrolysis Acid Lime added

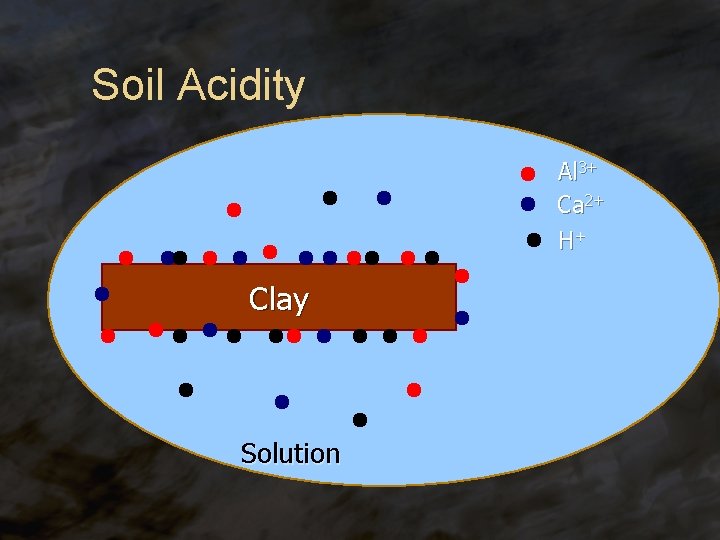
Soil Acidity Al 3+ Ca 2+ H+ Clay Solution

Al 3+ H+


Sulfide Oxidation Mine tailings (spoils) may become acidic because of the oxidation of pyrite (Fe. S 2): Fe. S 2 + H 2 O + 7/2 O 2 Fe. SO 4 + H 2 SO 4


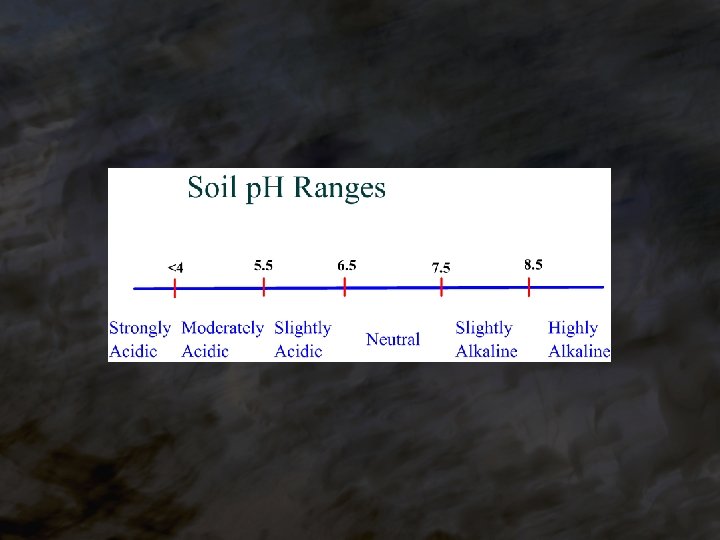
Al Toxicity ® Below a p. H of ______, 5. 5 Al 3+ may be present in concentrations that are toxic to plants. Al solubility is inversely proportional to p. H. ® Severely inhibits root growth, interferes with P uptake. ® Al toxicity is the #1 problem in acid soils. Mn and Fe may be toxic as well, because they soluble under acid become more _____ conditions.



Plants and p. H ® Differing plant tolerance to acidity is mostly due to: ® Different nutrient requirements ® Ability to immobilize Al 3+ in the rhizosphere or to detoxify Al 3+ within the plant.

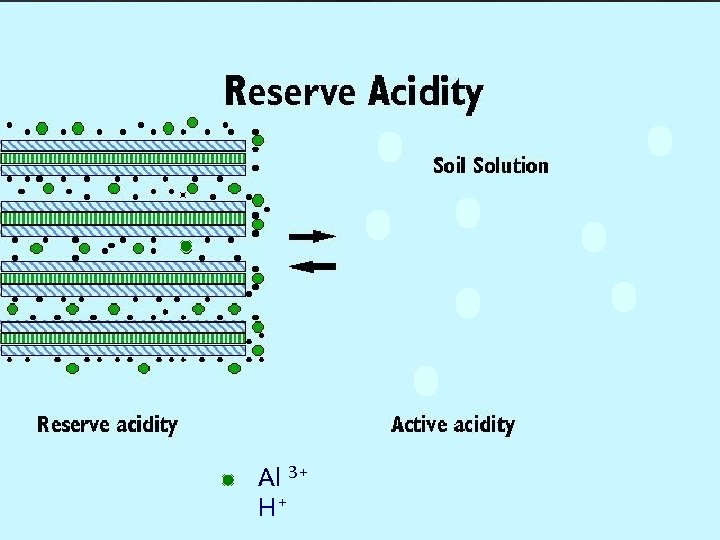
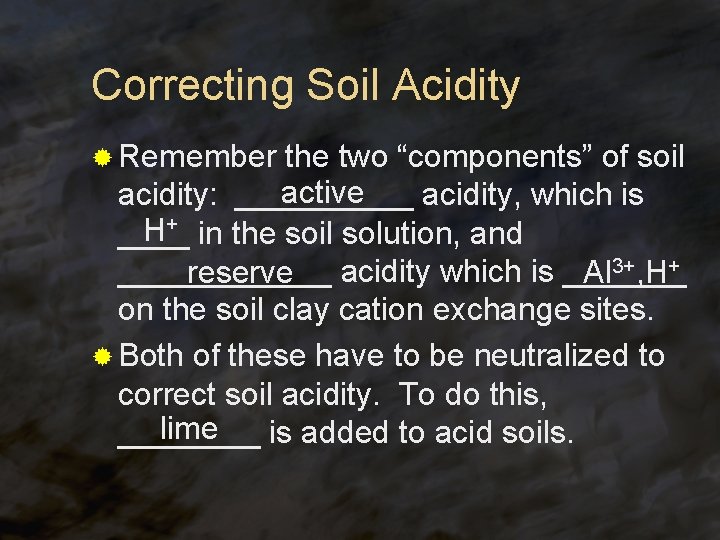
Correcting Soil Acidity ® Remember the two “components” of soil active acidity: _____ acidity, which is H+ in the soil solution, and ________ reserve acidity which is _______ Al 3+, H+ on the soil clay cation exchange sites. ® Both of these have to be neutralized to correct soil acidity. To do this, lime ____ is added to acid soils.

Al 3+ H+

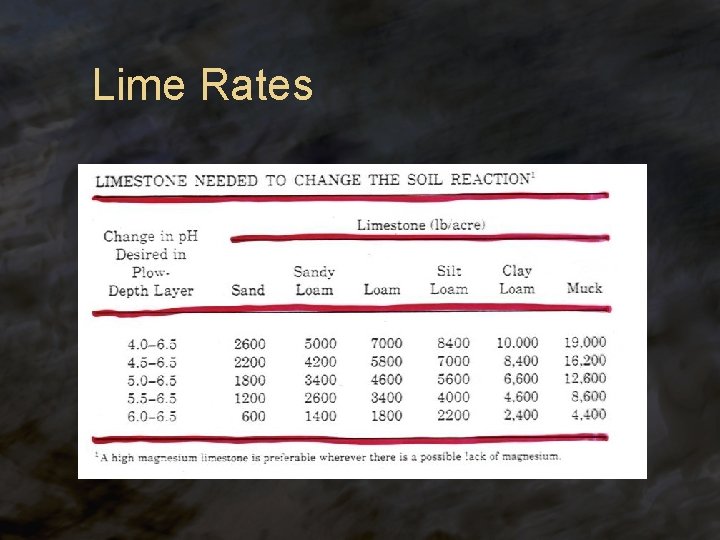
Liming Acid Soils ® Lime ® Sources: Ca. O (quicklime), Ca(OH)2 (hydrated lime), Ca. CO 3 (ag lime), Ca. Mg(CO 3)2 (dolomitic lime) ® The amount of lime to add to a soil (lime requirement) depends on: ® ® p. H Cation exchange capacity ® L. R. is usually determined by a soil test

Lime Rates

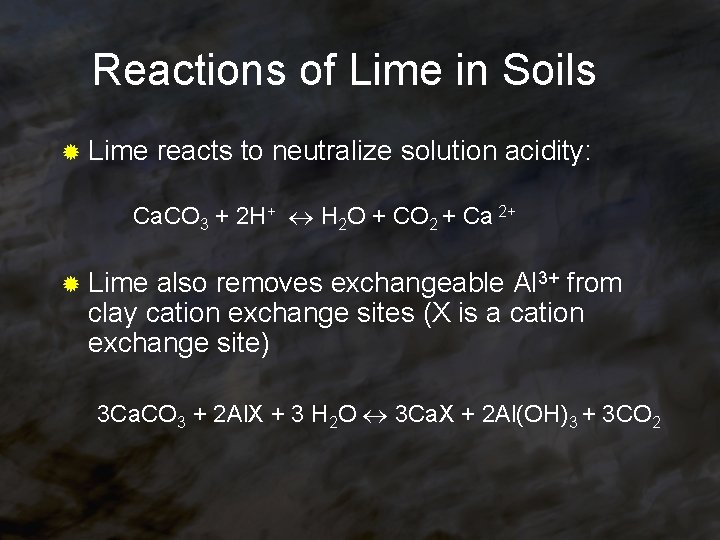
Reactions of Lime in Soils ® Lime reacts to neutralize solution acidity: Ca. CO 3 + 2 H+ H 2 O + CO 2 + Ca 2+ ® Lime also removes exchangeable Al 3+ from clay cation exchange sites (X is a cation exchange site) 3 Ca. CO 3 + 2 Al. X + 3 H 2 O 3 Ca. X + 2 Al(OH)3 + 3 CO 2