SOIL REACTIONS SOIL ACIDITY SOIL ALKALINITY CONDUCTIVITY REDOX















- Slides: 22

SOIL REACTIONS, SOIL ACIDITY SOIL ALKALINITY, CONDUCTIVITY, REDOX POTENTIAL

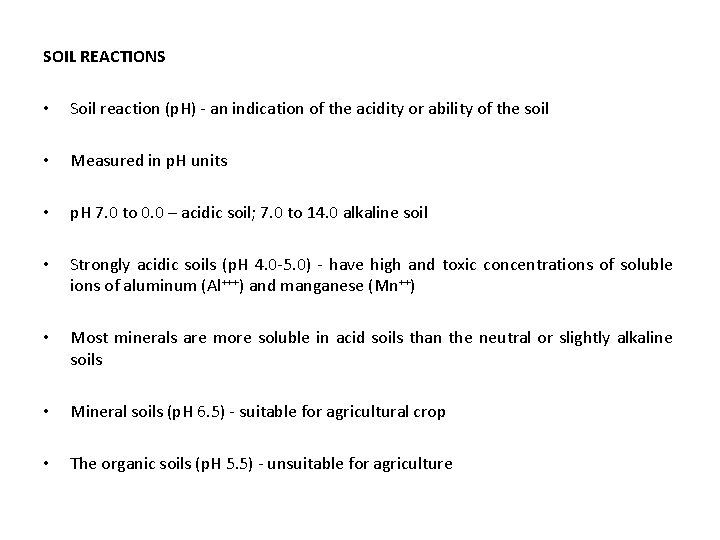
SOIL REACTIONS • Soil reaction (p. H) - an indication of the acidity or ability of the soil • Measured in p. H units • p. H 7. 0 to 0. 0 – acidic soil; 7. 0 to 14. 0 alkaline soil • Strongly acidic soils (p. H 4. 0 -5. 0) - have high and toxic concentrations of soluble ions of aluminum (Al+++) and manganese (Mn++) • Most minerals are more soluble in acid soils than the neutral or slightly alkaline soils • Mineral soils (p. H 6. 5) - suitable for agricultural crop • The organic soils (p. H 5. 5) - unsuitable for agriculture

• Soil become acidic even from basic parent materials by the leaching of the basic cat ion (Ca++, Mg++, K+ Na+) • Replacement of H+ ions from carbonic acid (H 2 CO 3 --) formed from water and dissolved carbon dioxide • Soils of higher p. H than 9. 0 have reduced growth of plants or even death • Increase of excess exchangeable sodium in the soil is removed by adding gypsum followed by irrigation to leach the exchangeable sodium ions • Leaching of basic cations is more in humid regions than arid regions in there soils

• Leaching is less in arid region, Ca. CO 3 and often bases accumulate near the surface and become neutral or slightly alkaline (p. H near 8. 0) • In extremely arid climates less rainfall to flush solvable salt, such soils are called saline soils • Acids - water solutions that certain more H+ cat ions than OH- anions • Bases - water solution containing more OH- anions than H+ cat ions • The concentration of OH- ions in solution determines the alkalinity • The acidity or alkalinity of a soil depends largely on the balance between the negatively charged anions (Cl-, SO 4 -, NO 3 -) and the positively charged basic cat ions (Ca++ , Mg++, K+, Na+)

• The p. H rises when the concentrations of bases increase and drops when the concentrations acidic cat ions increase • Aluminum toxicity can be avoided in the soil by maintaining soil p. H above 5. 0 • The dominant bases in cat ion exchange are calcium and magnesium • Calcium - 75 to 85% of exchangeable basis • Soil of p. H above 8. 0 has high percentage of sodium ions • Soils of p. H below 4. 0 contain sulfuric acid

SOIL FERTILITY • soil fertility and soil productivity - basic need • The nutrients essential for plant growth is called soil fertility • Factor limiting production- poor drainage, insects, drought etc • Organic matter and soil organisms immobilize the release of nutrients

Soil Productivity • Productivity - capacity of soil to produce crops and be expressed in terms of yield • Plant needs soil for mechanical support, nutrients, water, air and heat

Essential Nutrients • • • Carbon (CO 3 --, HCO 3 -) Hydrogen (H+) Oxygen (O-) Nitrogen (NH 4+, N- N 03 -) Phosphorus (HP 04 --, H 2 P 04 -) Potassium (K+) Calcium (Ca++) Magnesium (Mg++) Sulfur (SO 3 -SO 4) Iron (Fe+++) • • • Manganese (Mn++); Boron (B 03 --) Molybdenum (MOO 4) Zinc++), Copper (Cu+ Cu++) Chlorine (Cl-) In addition sodium (Na+) Cobalt (CO+) Vanadium & Silicon (Sio 3) are essential

• Basic nutrients – carbon and oxygen - 96% of total dry matter of the plant • Carbon and oxygen - 45% each and hydrogen - 6% of the total tissue • These elements are abundant and supplied from the atmosphere - need not be applied and do not limit the production

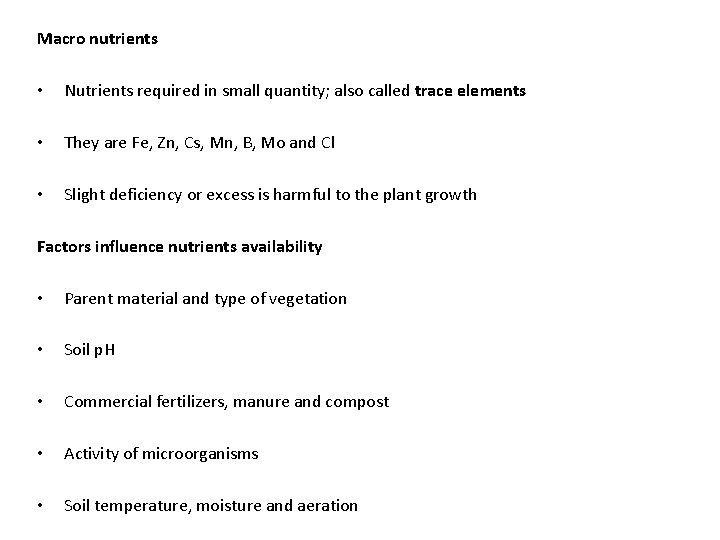
Macro nutrients • Nutrients required in small quantity; also called trace elements • They are Fe, Zn, Cs, Mn, B, Mo and Cl • Slight deficiency or excess is harmful to the plant growth Factors influence nutrients availability • Parent material and type of vegetation • Soil p. H • Commercial fertilizers, manure and compost • Activity of microorganisms • Soil temperature, moisture and aeration

ORGANIC CARBON • Organic matter - growth promoting substance; reservoir of nutrients • Contains considerable potential energy of which large proportion of which is readily transferable and used by plants • Carbon - common constituent of all organic matter

• Much of the energy acquired by the fauna and flora with in the soil comes from the oxidation of carbon • Organic matter - influences physical and chemical properties of soil • Also supplies energy and body building constituents for microorganisms • The sources of soil organic matter - plant tissue and animals

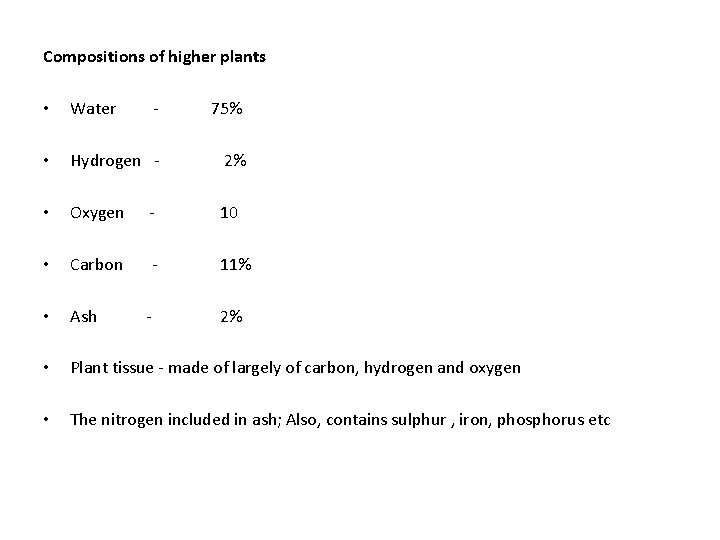
Compositions of higher plants • Water - 75% • Hydrogen - 2% • Oxygen - 10 • Carbon - 11% • Ash • Plant tissue - made of largely of carbon, hydrogen and oxygen • The nitrogen included in ash; Also, contains sulphur , iron, phosphorus etc - 2%

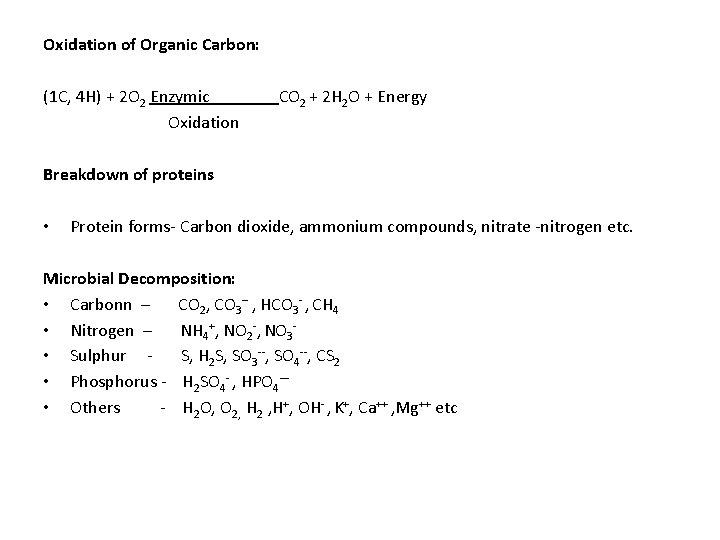
Oxidation of Organic Carbon: (1 C, 4 H) + 2 O 2 Enzymic Oxidation CO 2 + 2 H 2 O + Energy Breakdown of proteins • Protein forms- Carbon dioxide, ammonium compounds, nitrate -nitrogen etc. Microbial Decomposition: • Carbonn – CO 2, CO 3 -- , HCO 3 - , CH 4 • Nitrogen – NH 4+, NO 2 -, NO 3 • Sulphur S, H 2 S, SO 3 --, SO 4 --, CS 2 • Phosphorus - H 2 SO 4 - , HPO 4— • Others - H 2 O, O 2, H 2 , H+, OH- , K+, Ca++ , Mg++ etc

HUMUS • Humus - mixture of complex compounds • The compounds are either resistant or compound synthesized with in microbial tissues • Humus - complex and rather resistant mixture of brown or dark brown amorphous and colloidal substances modified from the original tissues or synthesized by the various organisms

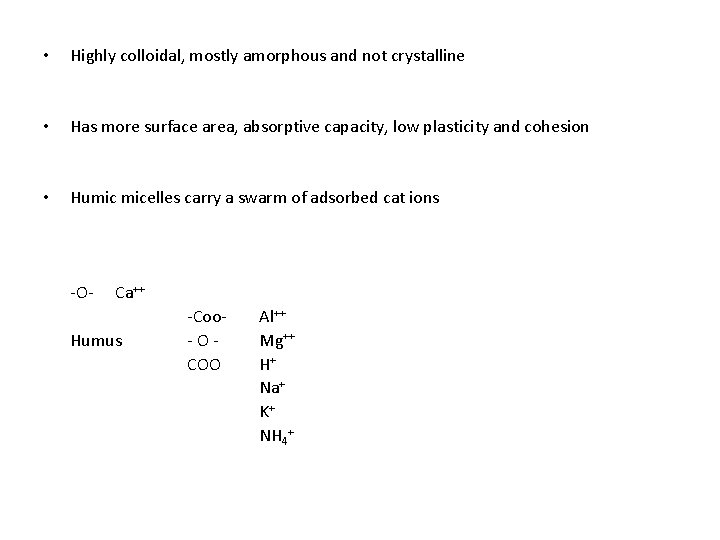
• Highly colloidal, mostly amorphous and not crystalline • Has more surface area, absorptive capacity, low plasticity and cohesion • Humic micelles carry a swarm of adsorbed cat ions -O- Ca++ Humus -Coo-OCOO Al++ Mg++ H+ Na+ K+ NH 4+

Importance of soil organic matter • Physical properties – granulation, plasticity, cohesion reduced • Water holding capacity - increased • High cat ion absorption capacity of nutrients – 2 to 30 times and adsorbing capacity for 30 -90% • Supply and availability of nutrients – replaceable cat ions, N, P and S and extraction of elements from humus

CARBON: NITROGEN RATIO • Carbon makes up a large and rather definite proportion of organic matter • Not surprising that the carbon nitrogen ratio of soils is fairly constant • carbon to nitrogen ratio in the organic matter of arable soil - ranges from 8: 1 to 15: 1; medium being between 10 and 12 to 1 • C: N ratio tends to be low in soils of arid regions than humid regions

• C: N ratio is narrower for sub-soils than surface soils • The ratio of C: N of plant material ranged from 20 to 30 to 1 for legumes and farm manure to as high as 90 to 1 • The available nitrogen results where residuals have high C: N ratio • Due to constancy of C. N ration Nitrogen do not become Nutrient

CONDUCTVITY OF SOIL • The cat ions and anions in soil carry current and important in conductivity of charges • Higher concentration of ions in solution - more electric conductance • Thus, the measurement of EC can be directly related to the soluble salts concentration • Electrical conductivity provides a rapid and reasonable accurate estimate of solute courses • Conductance is the reciprocal of the resistance and the unit of measure is the reciprocal of ohm designated as mho or Siemens (s)

REDOX POTENTIAL: • The value of redox potential indicates the state of oxidation and reduction process in soil or water • A lower value of redox potential indicates high reduction while a high value of it indicates high oxidation • For better aerobic treatment, waste water should be high (+200 to +600 mv) • For anaerobic treatment it should be below -100 to – 200 mv
