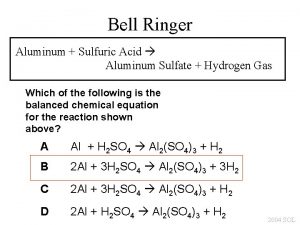
DO NOW What mass of aluminum hydroxide is









- Slides: 13

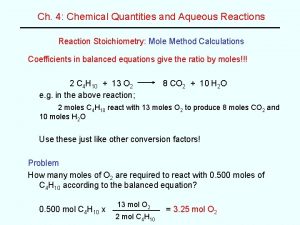
DO NOW: What mass of aluminum hydroxide is needed to decompose in order to produce aluminum oxide and 35 L of water at STP? Word equation: Formula equation:

After today you will be able to… • Calculate the limiting reactant using two mass-mass calculations

Limiting Reactant Calculations The limiting reactant (L. R. ) is the reactant which runs out first and limits the amount of product that can be made. • Two mass-mass calculations will be used. • The L. R. is the one that yields the smaller amount.

Limiting Reactant Example

A real-world example: Making Funfetti Cupcakes! 1 Ok… Time Mixes are the “limiting to relate reactant” because they are used up first! this back to chemistry… ___mix 3+ ___eggs + 2___oil ___pan of cupcakes 1 Let’s say you have… 3 mixes x 15 eggs x 8 tbsp oil x 1 pan 1 mix 1 pan 3 eggs 1 pan 2 tbsp oil = 3 pans = 5 pans = 4 pans

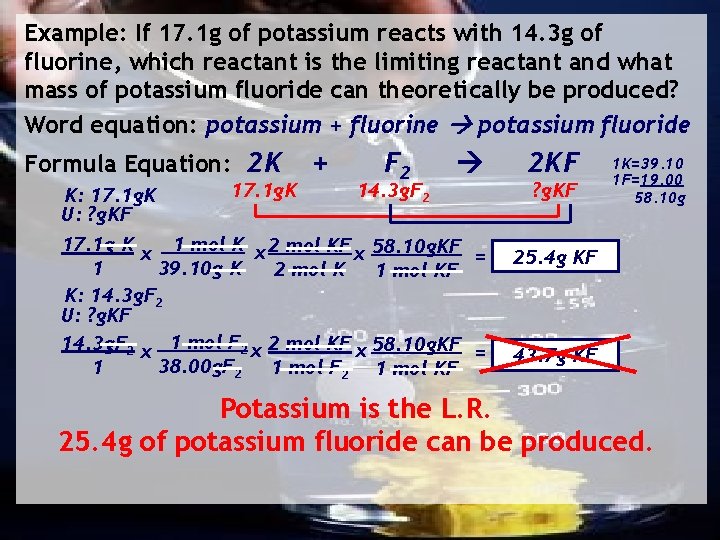
Example: If 17. 1 g of potassium reacts with 14. 3 g of fluorine, which reactant is the limiting reactant and what mass of potassium fluoride can theoretically be produced? Word equation: potassium + fluorine potassium fluoride Formula Equation: 2 K K: 17. 1 g. K U: ? g. KF 17. 1 g. K + F 2 14. 3 g. F 2 17. 1 g K x 1 mol K x 2 mol KF x 58. 10 g. KF = 39. 10 g K 1 2 mol K 1 mol KF K: 14. 3 g. F 2 U: ? g. KF 14. 3 g. F 2 x 1 mol F 2 x 2 mol KF x 58. 10 g. KF = 38. 00 g. F 2 1 mol KF 1 2 KF ? g. KF 1 K=39. 10 1 F=19. 00 58. 10 g 25. 4 g KF 43. 7 g KF Potassium is the L. R. 25. 4 g of potassium fluoride can be produced.

After today you will be able to… • Calculate the limiting reactant using other mole relationships • Calculate how much of the excess reactant reacts and how much is left over

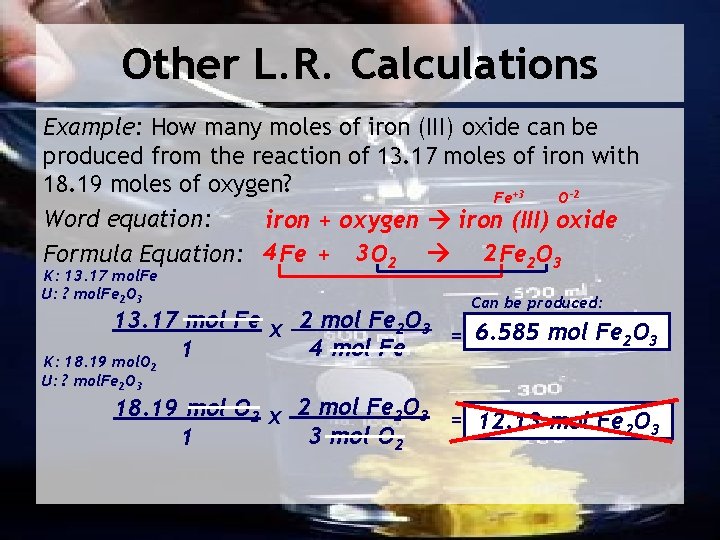
Other L. R. Calculations Example: How many moles of iron (III) oxide can be produced from the reaction of 13. 17 moles of iron with 18. 19 moles of oxygen? Fe+3 O-2 Word equation: iron + oxygen iron (III) oxide Formula Equation: 4 Fe + 3 O 2 2 Fe 2 O 3 K: 13. 17 mol. Fe U: ? mol. Fe 2 O 3 Can be produced: 13. 17 mol Fe x 2 mol Fe 2 O 3 = 6. 585 mol Fe 2 O 3 4 mol Fe 1 K: 18. 19 mol. O U: ? mol. Fe 2 O 3 2 18. 19 mol O 2 x 2 mol Fe 2 O 3 = 12. 13 mol Fe 2 O 3 3 mol O 1 2

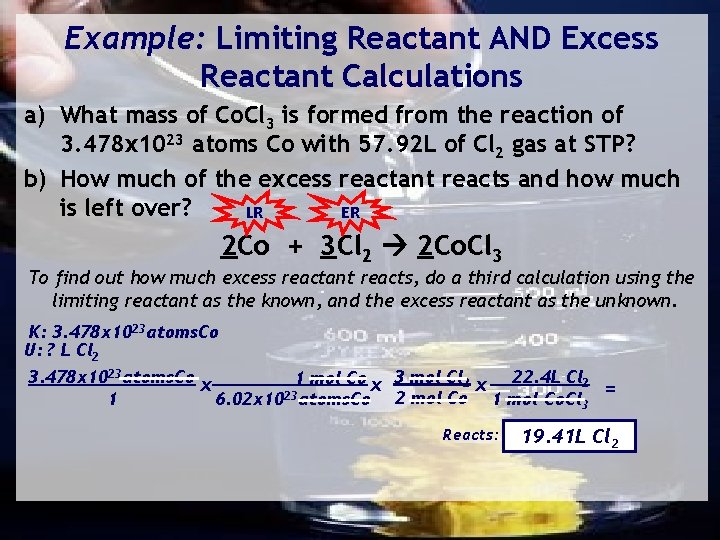
Example: Limiting Reactant AND Excess Reactant Calculations a) What mass of Co. Cl 3 is formed from the reaction of 3. 478 x 1023 atoms Co with 57. 92 L of Cl 2 gas at STP? b) How much of the excess reactant reacts and how much is left over? 1 Co=58. 78 K: 3. 478 x 1023 atoms. Co U: ? g. Co. Cl 3 LR 2 Co + 3 Cl 2 2 Co. Cl 3 3 Cl=106. 35 165. 13 g 1 mol Co x 2 mol Co. Cl 3 x 165. 13 g. Co. Cl 3 3. 478 x 1023 atoms. Co x = 1 mol Co. Cl 3 6. 02 x 1023 atoms. Co 2 mol Co 1 K: 57. 92 LCl 2 U: ? g. Co. Cl 3 ER Can be produced: 57. 92 L Cl 2 x 1 mol Cl 2 x 2 mol Co. Cl 3 x 165. 13 g. Co. Cl 3 = 22. 4 L Cl 2 3 mol Cl 2 1 1 mol Co. Cl 3 95. 40 g. Co. Cl 3 284. 7 g. Co. Cl 3

Example: Limiting Reactant AND Excess Reactant Calculations a) What mass of Co. Cl 3 is formed from the reaction of 3. 478 x 1023 atoms Co with 57. 92 L of Cl 2 gas at STP? b) How much of the excess reactant reacts and how much is left over? LR ER 2 Co + 3 Cl 2 2 Co. Cl 3 To find out how much excess reactant reacts, do a third calculation using the limiting reactant as the known, and the excess reactant as the unknown. K: 3. 478 x 1023 atoms. Co U: ? L Cl 2 3. 478 x 1023 atoms. Co x 1 mol Co x 3 mol Cl 2 x 22. 4 L Cl 2 = 1 6. 02 x 1023 atoms. Co 2 mol Co 1 mol Co. Cl 3 Reacts: 19. 41 L Cl 2

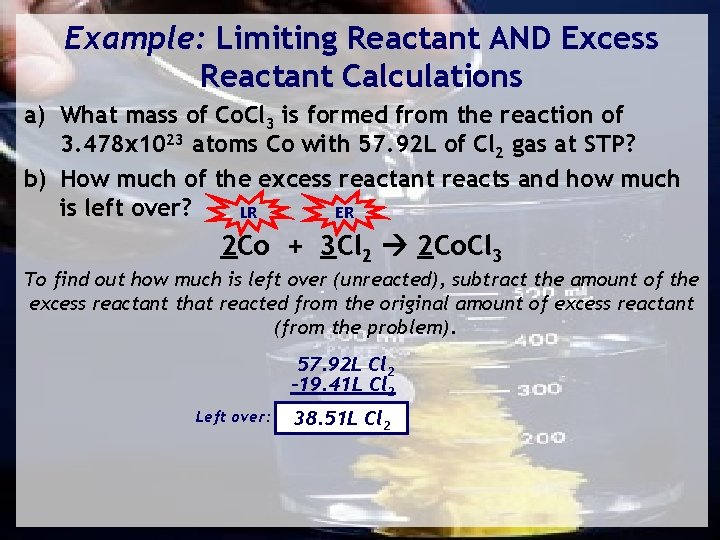
Example: Limiting Reactant AND Excess Reactant Calculations a) What mass of Co. Cl 3 is formed from the reaction of 3. 478 x 1023 atoms Co with 57. 92 L of Cl 2 gas at STP? b) How much of the excess reactant reacts and how much is left over? LR ER 2 Co + 3 Cl 2 2 Co. Cl 3 To find out how much is left over (unreacted), subtract the amount of the excess reactant that reacted from the original amount of excess reactant (from the problem). 57. 92 L Cl 2 -19. 41 L Cl 2 Left over: 38. 51 L Cl 2

Complete Worksheet!

 What mass of aluminum hydroxide are needed to decompose
What mass of aluminum hydroxide are needed to decompose Aluminum sulfate + calcium hydroxide
Aluminum sulfate + calcium hydroxide Kno3 oxidation number of n
Kno3 oxidation number of n Sulphuric acid + aluminium
Sulphuric acid + aluminium Molecular mass of naoh
Molecular mass of naoh Now i see it now you don't
Now i see it now you don't Types of dental bases
Types of dental bases Neutral ferric chloride test
Neutral ferric chloride test Humectants
Humectants Hydrochloric acid and potassium hydroxide
Hydrochloric acid and potassium hydroxide Double flat wrap perm
Double flat wrap perm 7 strong acid
7 strong acid Sodium hydroxide iupac id sodium oxidanide
Sodium hydroxide iupac id sodium oxidanide Sodium oxide word equation
Sodium oxide word equation