SIGNIFICANCE OF GIT IN CRITICALLY ILL Prof Mehdi





























- Slides: 51

SIGNIFICANCE OF GIT IN CRITICALLY ILL Prof. Mehdi Hasan Mumtaz

ANATOMY & HISTORY OF GUT

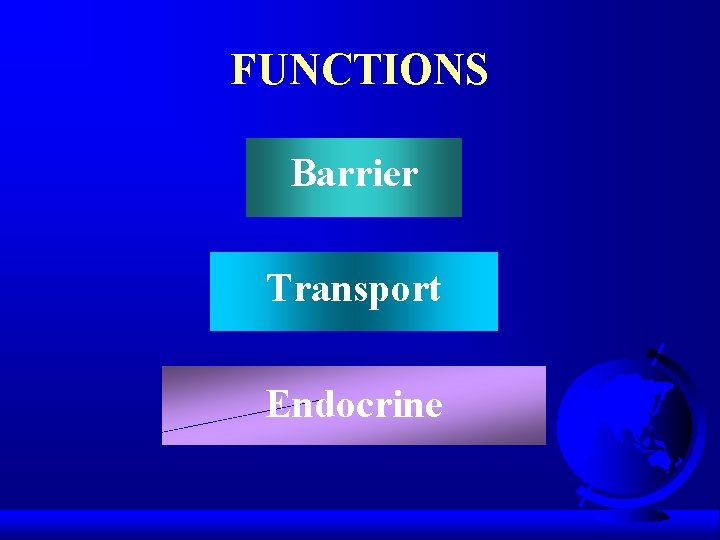
FUNCTIONS Barrier Transport Endocrine

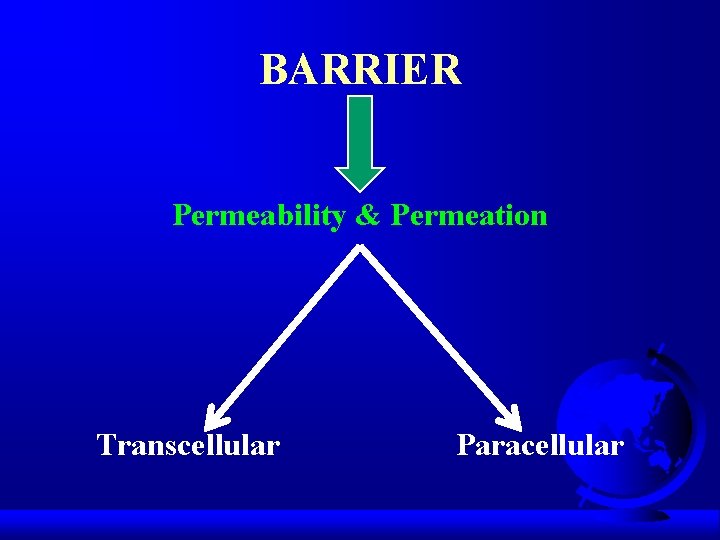
BARRIER Permeability & Permeation Transcellular Paracellular

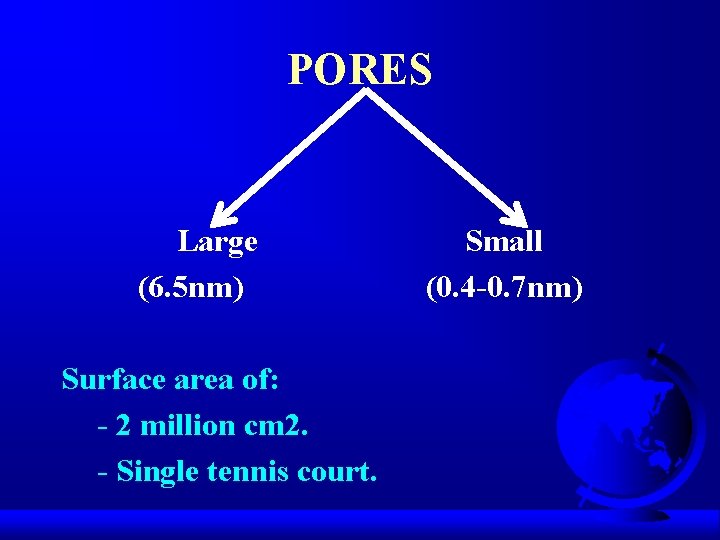
PORES Large (6. 5 nm) Surface area of: - 2 million cm 2. - Single tennis court. Small (0. 4 -0. 7 nm)

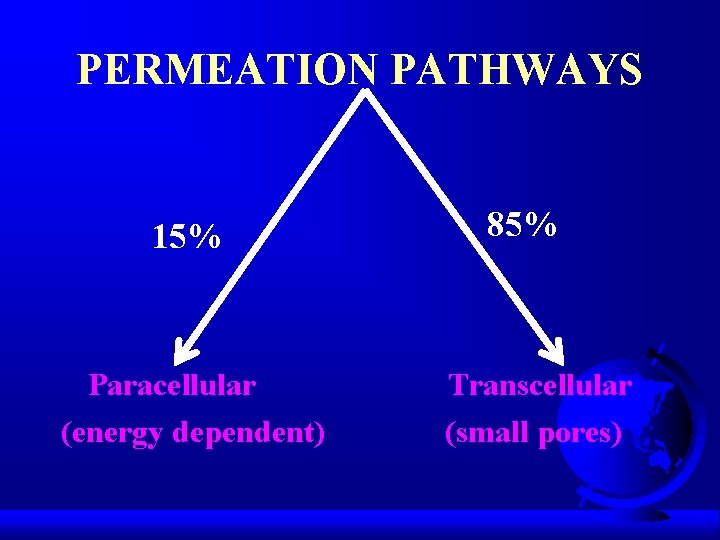
PERMEATION PATHWAYS 15% Paracellular (energy dependent) 85% Transcellular (small pores)

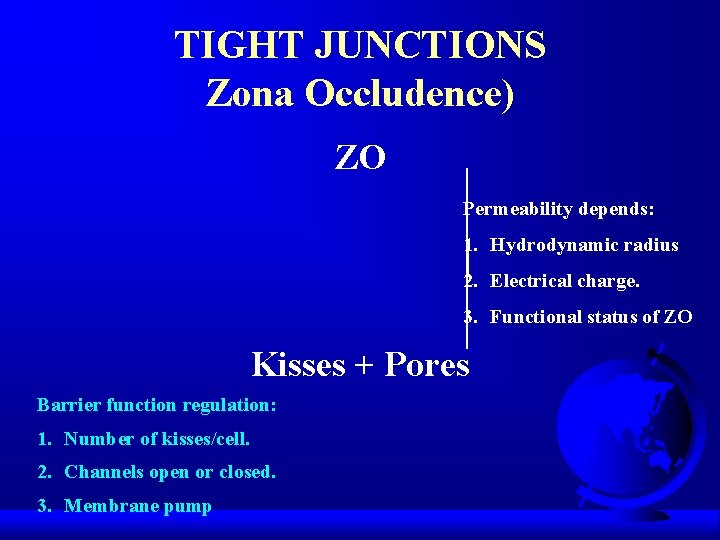
TIGHT JUNCTIONS Zona Occludence) ZO Permeability depends: 1. Hydrodynamic radius 2. Electrical charge. 3. Functional status of ZO Kisses + Pores Barrier function regulation: 1. Number of kisses/cell. 2. Channels open or closed. 3. Membrane pump

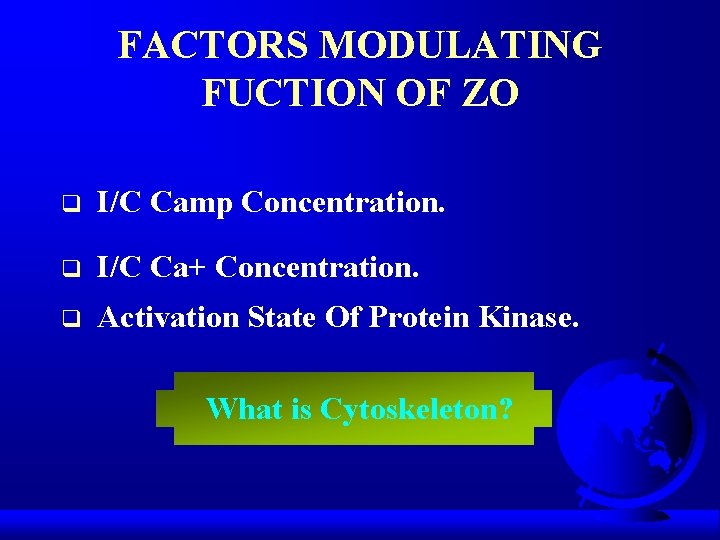
FACTORS MODULATING FUCTION OF ZO q I/C Camp Concentration. q I/C Ca+ Concentration. q Activation State Of Protein Kinase. What is Cytoskeleton?

TRANSLOCATION DEFINITION

CAUSES q q q q Non Occlusive Intestinal Gangrene. Neutropenia. Colon Cancer. Penumatosis Intestinals. Necrtising Enterocolitis. Ionizing Radiation. Cytotoxic Drugs.

CAUSES q q q q Cytokine Release Syndrome. Crohns Disease. Ulcerative Colitis. Haemorrhagic Shock. Severe Trauma Burn Injury. Leukaemia.

FACTORS 1. 2. luminal microbial density. Damage to eipthelium. – Irradiation. – Cytotoxic drugs. – Irritants. – Cytomegatovirus. – Mucosal disease. – Bowel manipulation. – Obstruction. – Free O 2 radicals. 3. 4. Diminished blood flow. – Haemorrhagic shock. – Burn. – Inflammtory agent. – Endotoxins. – M. occlusion. – Hypoxia. – Fever. Immunosuppressant. – Corticosteroids in high dosage. – Blood transfusion.

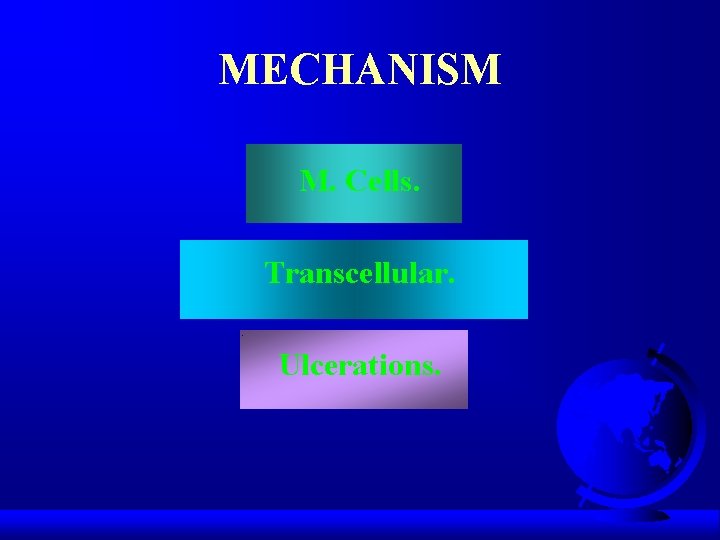
MECHANISM M. Cells. Transcellular. Ulcerations.

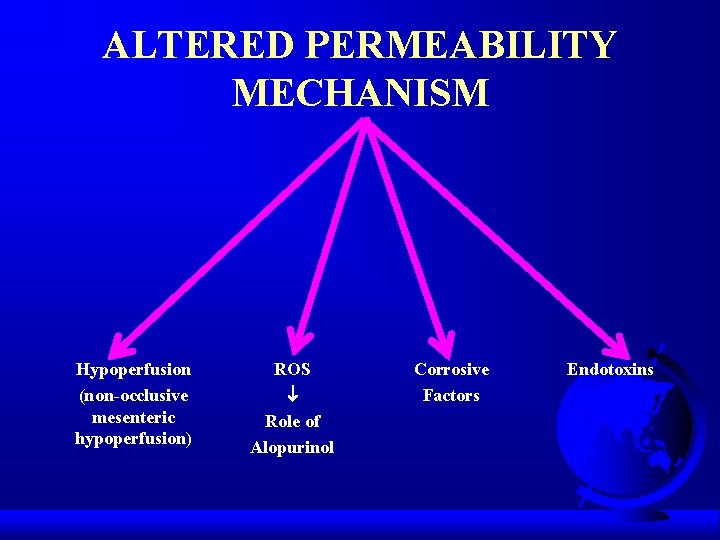
ALTERED PERMEABILITY MECHANISM Hypoperfusion (non-occlusive mesenteric hypoperfusion) ROS Role of Alopurinol Corrosive Factors Endotoxins

NON-OCCLUSIVE HYPOPERFUSION q Hypovolaemia. q Cardiogenic. q Septic shock.

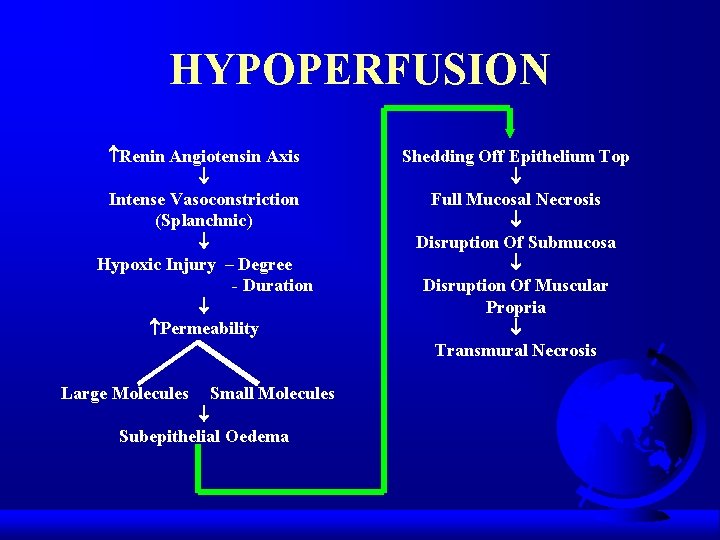
HYPOPERFUSION Renin Angiotensin Axis Intense Vasoconstriction (Splanchnic) Hypoxic Injury – Degree - Duration Permeability Large Molecules Small Molecules Subepithelial Oedema Shedding Off Epithelium Top Full Mucosal Necrosis Disruption Of Submucosa Disruption Of Muscular Propria Transmural Necrosis

ROS Role of Allopurinal

CORROSIVE FACTORS q q q Hydrochloric acid. Bile salts. Bacterial toxins. Proteases. Digestive enzymes.

ENDOTOXINS q q Ischaemia. Direct injury. metabolic demand of GUT. Alteration of micro-circulation.

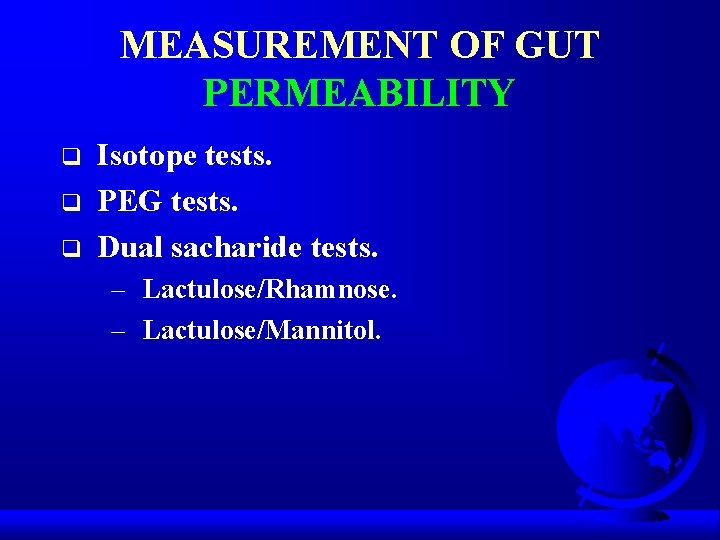
MEASUREMENT OF GUT PERMEABILITY q q q Isotope tests. PEG tests. Dual sacharide tests. – Lactulose/Rhamnose. – Lactulose/Mannitol.

NON MUCOSAL FACTORS q q q Gastric emptying. Intestinal transit. Dilution by secretion. Surface area available. Altered renal clearance.

TECHNIQUE FOR MEASUREMENT OF GUT PERMEABILITY USING LACTULOSE & L-RHAMNOSE. Stop nasogastric feed/nil by mouth for 6 h prior to the study. 2. Empty bladder & urinary collecting system. 3. Isotonic solution containing 5 g oflactulose and 1 g of Lrhamnose administred via the nasogastric tube. 4. All urine collected over 5 h. Total volume noted and a 20 ml sample frozen for future analysis. 5. Concentration of sugrs in urine quantified. 6. %recovery of each sugar calculated: Sugar concentration x urine volume %Recovery =--------------------------- x 100 Amount of sugar given enterally 7. %recovery lactulose to %recovery L-rhamnose ratio calculated. Normal range 0 -0. 08. 1.

IMMUNONUTRTION (Nutritional Paharmacology) Why Name Immunonutrition? q q Lipids -3, -6 Aminoacids – Arginine – Glutamine q q Ribonucleic acid Vitamins, E, C and A

LIPIDS q q q q q Production of free radicals. Inflammatory response. Ulcer formation. Hypersensitivity response. Altered renal vascular flow. Uterine contraction. Incidence of atherosclerosis. Incidence of heart attacks. Bleeding tendency. Haemorrhagic strokes.

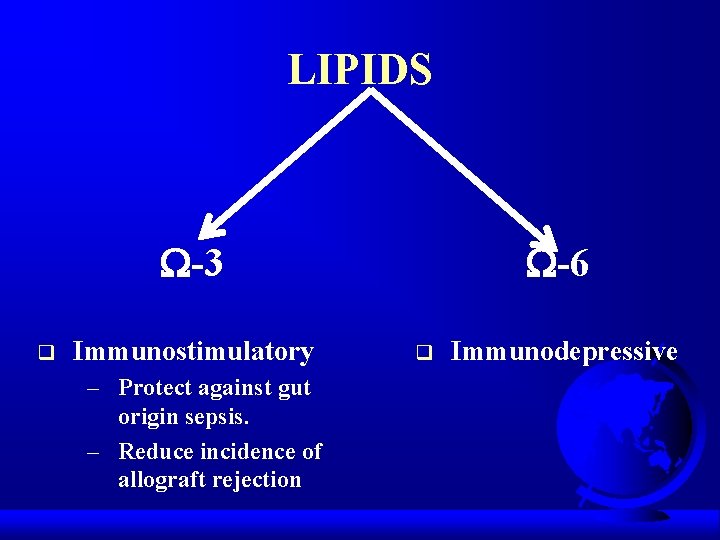
LIPIDS -3 q Immunostimulatory – Protect against gut origin sepsis. – Reduce incidence of allograft rejection -6 q Immunodepressive

VITAMINS, E, C, A q Control lipid peroxidation. q Regulate RO intermediates (macrophages).

ARGININE 1. Production and secretion. – – – – 2. Pitintary GH. Protaction. IGF-1. Glucagon. Somatostatin. Pancreatic polypeptide. Nor-epinephrin. Pre-cursor of growth factors. – Putrescine. – Spermidine.

ARGININE 3. 4. 5. 6. 7. 8. 9. 10. Produce NO. Resistance. T-cell immunity. Wound healing. Cancer growth. Protein content. Lymphocyte nitrogen & allogenic response. No effect on translocation.

GLUTANINE q q q Barrier function. T-cell function. Neutrophil function. Kills translocated bacteria. Hospital stay.

NUCLEOTIDES q Resistance. q Immune response.

EFFECT OF CRITICAL ILLNESS ON GIT q q q Starvation & Bowel rest. Metabolic stress. Entral/Parenteral nutrition. Sepsis. Shock.

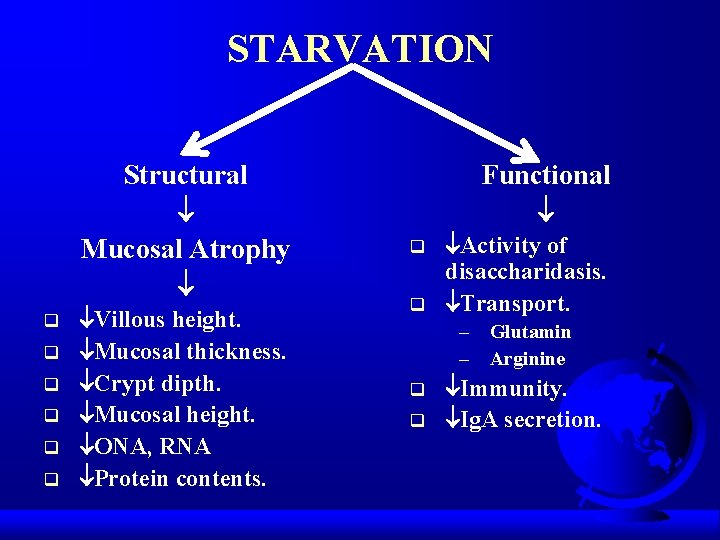
STARVATION Structural Mucosal Atrophy q q q Villous height. Mucosal thickness. Crypt dipth. Mucosal height. ONA, RNA Protein contents. Functional q q Activity of disaccharidasis. Transport. – Glutamin – Arginine q q Immunity. Ig. A secretion.

GIT IMMUNOLOGIC DEFENCE q q Ig. A. Lymphocyte macrophages & neutrophils. Lymph nodes. Kupffer cells in liver.

BOWEL REST q q q G. I. Mass. Small bowel mucosal weight. DNA content. Protein content. Villous height. Enzyme activity. Even if nitrogen balance is maintained & on TPN

PRESENCE OF LUMINAL NUTRIENTS NECESSARY FOR NORMAL GUT GROWTH & FUNCTION

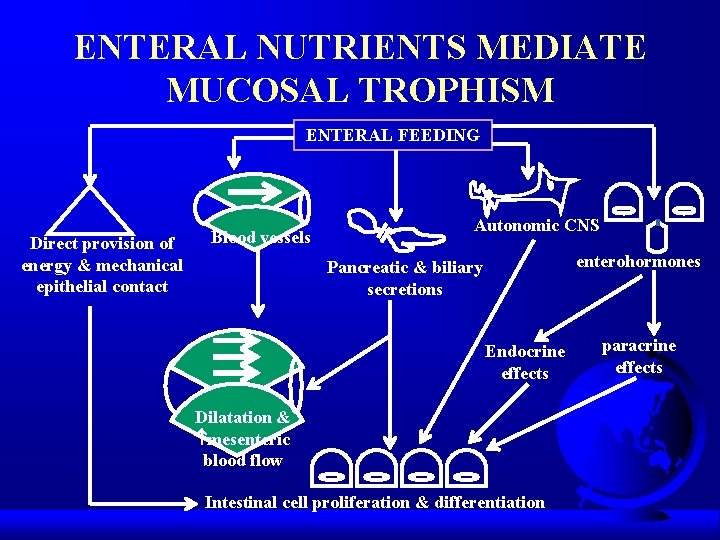
ENTERAL NUTRIENTS MEDIATE MUCOSAL TROPHISM ENTERAL FEEDING Direct provision of energy & mechanical epithelial contact Blood vessels Autonomic CNS enterohormones Pancreatic & biliary secretions Endocrine effects Dilatation & mesenteric blood flow Intestinal cell proliferation & differentiation paracrine effects

METABOLIC STRESS Starvation+Bowel Rest+Critical Illness, Shock, Hypovolaemia q q q q Mesenteric blood flow. Hypoxia. Production of intestinal mucous. Mucosal acidosis. Mucosal permeability. Epithelial necrosis. O 2 free radicals. Antibiotic. – – q Microflora. Colonization. Gastric acid colonization. Mucosal & immunologic impairment. Passage of intraduminal microbes & toxins intocirculation.

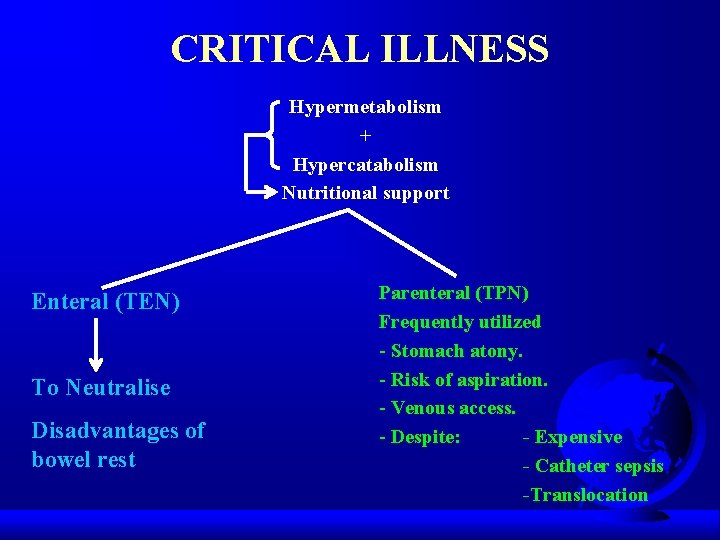
CRITICAL ILLNESS Hypermetabolism + Hypercatabolism Nutritional support Enteral (TEN) To Neutralise Disadvantages of bowel rest Parenteral (TPN) Frequently utilized - Stomach atony. - Risk of aspiration. - Venous access. - Despite: - Expensive - Catheter sepsis -Translocation

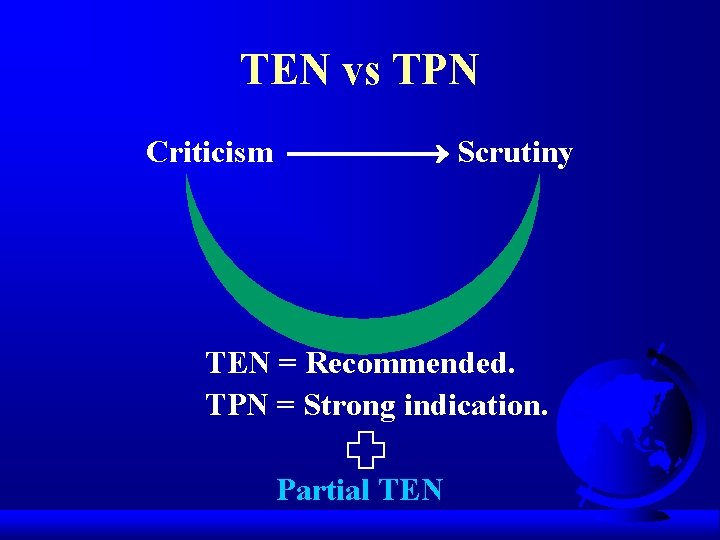
TEN vs TPN Criticism Scrutiny TEN = Recommended. TPN = Strong indication. Partial TEN

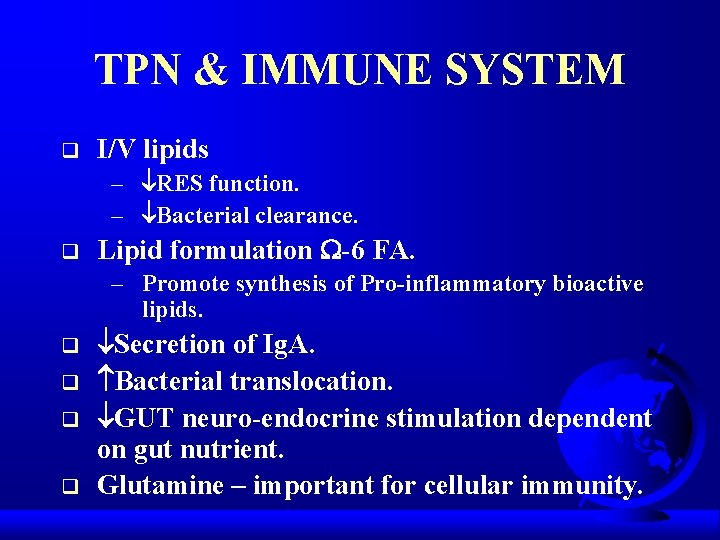
TPN & IMMUNE SYSTEM q I/V lipids – RES function. – Bacterial clearance. q Lipid formulation -6 FA. – Promote synthesis of Pro-inflammatory bioactive lipids. q q Secretion of Ig. A. Bacterial translocation. GUT neuro-endocrine stimulation dependent on gut nutrient. Glutamine – important for cellular immunity.

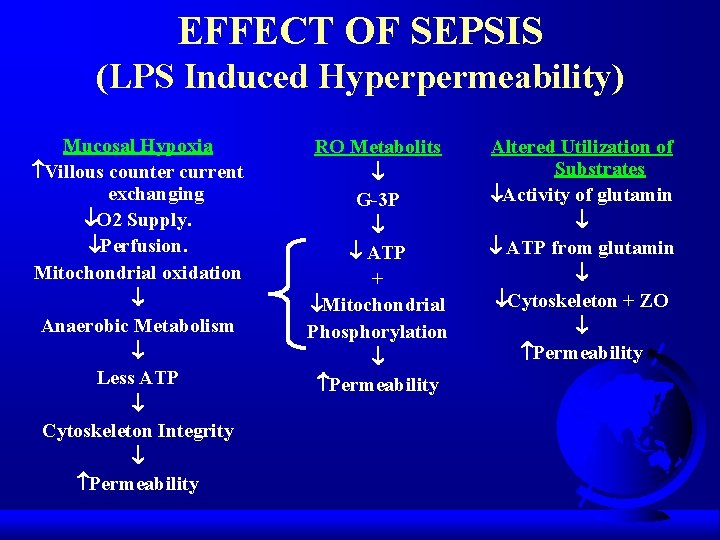
EFFECT OF SEPSIS (LPS Induced Hyperpermeability) Mucosal Hypoxia Villous counter current exchanging O 2 Supply. Perfusion. Mitochondrial oxidation Anaerobic Metabolism Less ATP Cytoskeleton Integrity Permeability RO Metabolits G-3 P ATP + Mitochondrial Phosphorylation Permeability Altered Utilization of Substrates Activity of glutamin ATP from glutamin Cytoskeleton + ZO Permeability

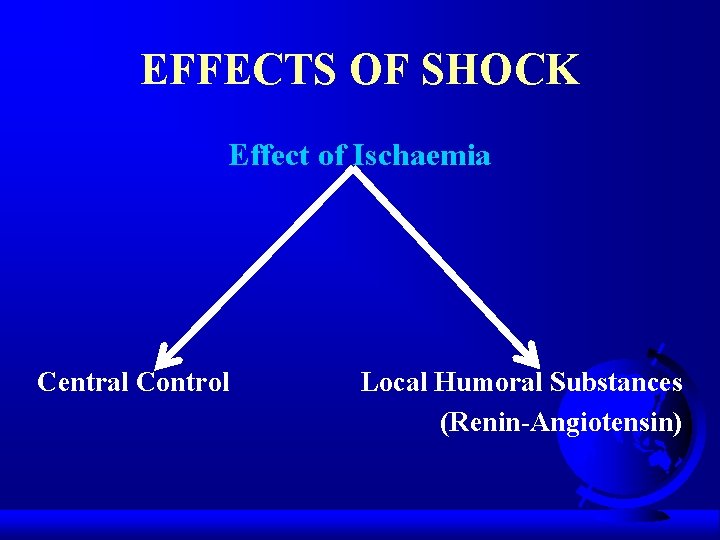
EFFECTS OF SHOCK Effect of Ischaemia Central Control Local Humoral Substances (Renin-Angiotensin)

THE CONTINUUM OF INTESTINAL ISCHAEMIC INJURY Normal Mucosa Capillar Permeability Mucosal Permeability Superficial Mucosal Injury Transmural Injury

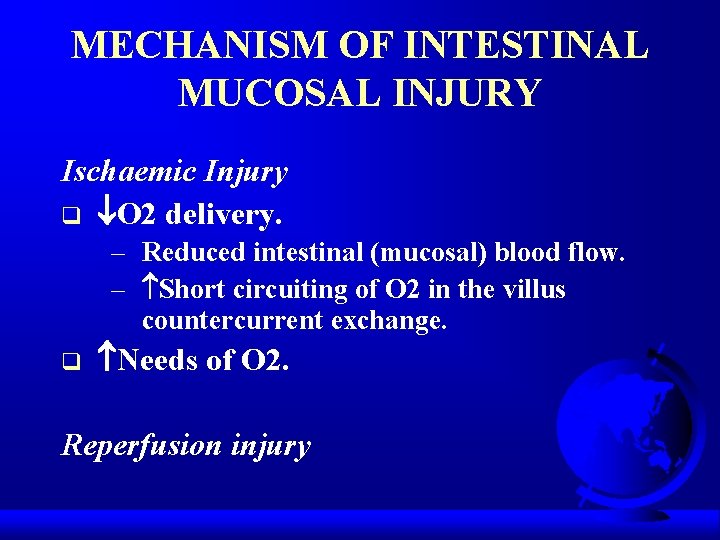
MECHANISM OF INTESTINAL MUCOSAL INJURY Ischaemic Injury q O 2 delivery. – Reduced intestinal (mucosal) blood flow. – Short circuiting of O 2 in the villus countercurrent exchange. q Needs of O 2. Reperfusion injury

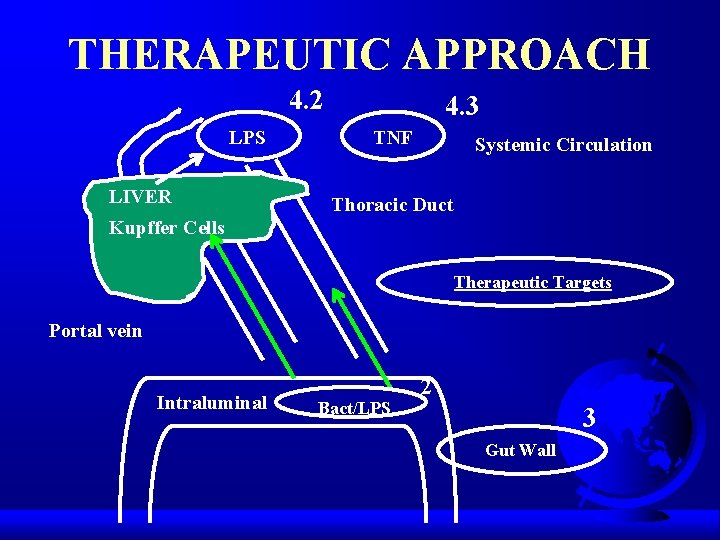
THERAPEUTIC APPROACH q Intraluminal therapeutic approach. q Maintenance of Gut Wall. q Intravasal therapeutic measures.

INTRALUMINAL THERAPEUTIC APPROACH q Peristaltic movement. – Fibre application. q q Bacterial adherence. Bacterial elimination. – SDD. q LPS Neutralization. – Bile acids. – Lactoferin. – Lactulose.

MAINTENANCE OF GUT WALL q Splanchnic perfusion. – Fluid support. – TXA 2 receptor blocker – Angiotensin blocker. q q Xanthin oxidase blockade. NO – donors. Metabolic support. Growth factors support.

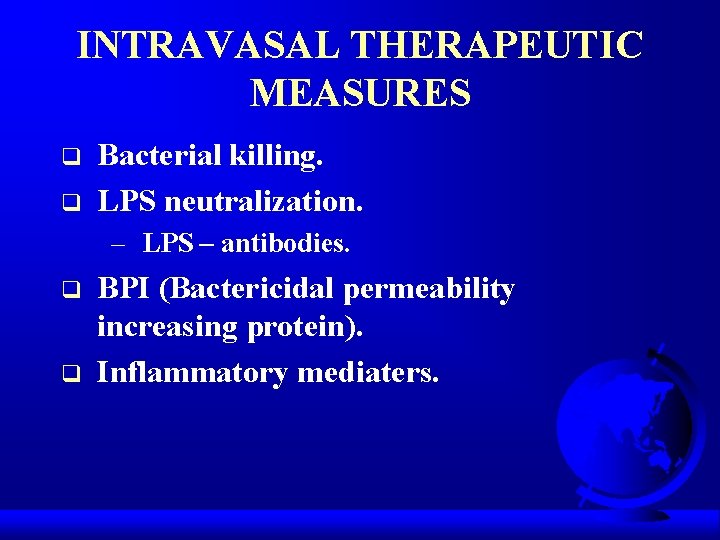
INTRAVASAL THERAPEUTIC MEASURES q q Bacterial killing. LPS neutralization. – LPS – antibodies. q q BPI (Bactericidal permeability increasing protein). Inflammatory mediaters.

THERAPEUTIC APPROACH 4. 2 LPS LIVER 4. 3 TNF Systemic Circulation Thoracic Duct Kupffer Cells Therapeutic Targets Portal vein Intraluminal Bact/LPS 2 3 Gut Wall

NEW & FUTURE THERAPIES q Metabolic intestinal fuels. – Glutamine. – Shot-chain fatty acids (SCFA). q q Intestinal growth factors. Immunomodulation. – Arginine. – -3 fatty acids. q Antioxidants.

SELECTIVE DECONTAMINATI ON OF DIGESTIVE TRACT
 Gpio
Gpio Care of the critically ill surgical patient
Care of the critically ill surgical patient Nasogastrio
Nasogastrio Mehdi nt
Mehdi nt Mehdi bouguerra
Mehdi bouguerra Mehdi
Mehdi Mehdi namazi
Mehdi namazi Azure certification poster
Azure certification poster Sodium correction formula
Sodium correction formula Mehdi khalighi
Mehdi khalighi R-tree java
R-tree java Mehdi bouguerra
Mehdi bouguerra Amir salek md
Amir salek md Ang namumuno sa bansang iran upang makamit ang kalayaan
Ang namumuno sa bansang iran upang makamit ang kalayaan Mehdi hamadani
Mehdi hamadani Mehdi salek md
Mehdi salek md Base deficit
Base deficit Dr. mehdi pain management
Dr. mehdi pain management Thinking critically
Thinking critically Thoughtful learning
Thoughtful learning Thinking critically with psychological science answer key
Thinking critically with psychological science answer key Thinking critically with psychological science
Thinking critically with psychological science Critical thinking example in nursing
Critical thinking example in nursing Listening critically
Listening critically Aseptic
Aseptic Sonnet 27 translation
Sonnet 27 translation Valid safeguards in antt
Valid safeguards in antt Thinking critically with psychological science
Thinking critically with psychological science Illusory correlations ______.
Illusory correlations ______. Negative issue
Negative issue Pico cat voorbeeld
Pico cat voorbeeld Where you go ill go
Where you go ill go Rising action of the sob sisters story
Rising action of the sob sisters story It is an immediate and temporary care
It is an immediate and temporary care Ill think
Ill think Scream and ill tell your mom
Scream and ill tell your mom Open conditional
Open conditional Rapid ill
Rapid ill Daughter and dad
Daughter and dad Suffix
Suffix Tell me and ill forget
Tell me and ill forget Objection ill allow it
Objection ill allow it Fabiana will not go to work today __ a bit ill
Fabiana will not go to work today __ a bit ill What is forensic psychiatry
What is forensic psychiatry Concepts of ill health
Concepts of ill health Ticls
Ticls Ill project management
Ill project management She worked hard. she made herself ill
She worked hard. she made herself ill Yesterday you met a friend of yours charlie
Yesterday you met a friend of yours charlie By doing nothing we learn to be ill
By doing nothing we learn to be ill Songs about waiting on god
Songs about waiting on god C ill
C ill