Selecting a study population for clinical trials Dr





























- Slides: 46

+ Selecting a study population for clinical trials Dr Greg Fox University of Sydney, Australia

+ Overview n Selecting a suitable study population to answer our research question: where? who’s in? who’s out? n Minimising biases in randomized trials n Case study: selecting a study population for a randomized study of LTBI treatment

+ Part I: Selecting a suitable study population

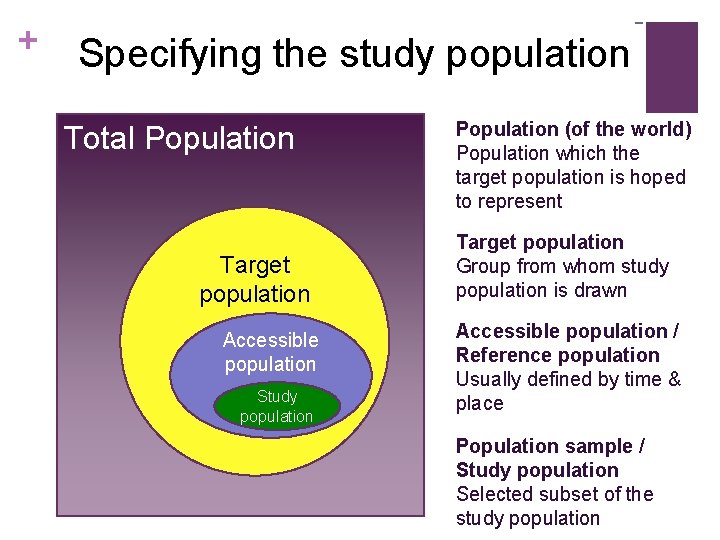
+ Specifying the study population Total Population Target population Accessible population Study population Population (of the world) Population which the target population is hoped to represent Target population Group from whom study population is drawn Accessible population / Reference population Usually defined by time & place Population sample / Study population Selected subset of the study population

+ Who’s in and who’s out? Selecting a study population n Why select ? Rarely possible to study all target population n Criteria for selection: relevant to the study objectives; practicality (accessible); usually defined by time & place n Sources of study population: Community, workplace, school, hospital; 5

+ Choosing the accessible population n Common options are: n Clinic based n Population based n Hospital based n Each setting has its problems n How might biases affect your results? 6

+ Study population: select how? n Requires enumeration of population n Random sampling: n n Random: Each person (unit) has an equal chance Stratified random: Random samples from specified sub groups n Systematic sampling: Use regular interval to sample (every 5 th person) n Cluster: random sample of groups (households) n Convenience (‘grab’): easily accessed but not random 7

+ 8 How can you be sure your study sample is representative of the target population?

+ Selecting the study setting and study population n Selecting a suitable study setting n n Setting is often determined by available clinical links or existing collaborations Some study questions may not be answerable in some settings (e. g. treatment for DR-LTBI in a low-prevalence setting, complex interventions in weak health care setting) Single site or multi-site recruitment ? Defining a suitable study population to answer the research question n n Define study subjects precisely and unambiguously Consider whether to choose broad vs narrow selection criteria n Desired target population (e. g. household vs all ‘close’ contacts) n Intended generalisability (e. g. adults vs all ages) n High risk groups of particular clinical importance (e. g. solid organ transplant recipients, PLHIV, children) n Consider efficiency of recruitment (e. g. TST positive only vs all contacts)

+ Case study 1 : MDR-TB prevention among household contacts We will illustrate the issues relating to selecting study populations through the design of a clinical trial of levofloxacin as treatment for latent TB infection among contacts of MDR-TB patients

Case study 1: MDR-TB prevention among household contacts + Background to V-QUIN MDR trial n Treating active MDR-TB is complex, toxic and costly n Close contacts of people with MDR-TB have a high risk of developing TB 1 n Preventive therapy is routinely offered to infected contacts of people with drug-susceptible TB (isoniazid, rifampicin, isoniazid+rifapentine…) n There is not yet evidence from RCTs to determine whether preventive therapy may be effective in MDR -TB contacts n “There is an urgent need for a multicenter, randomized, controlled trial”… of preventive therapy 2 1 – Kritski 1996; 2 - Schaaf et al, Paediatrics, 2002

Case study 1: MDR-TB prevention among household contacts + Research question n “What is the effectiveness of levofloxacin given for 6 months, compared to placebo, in the prevention of active TB among close contacts of patients with MDR-TB who have latent tuberculosis infection? ” Which settings and study populations could best answer this question?

Case study 1: MDR-TB prevention among household contacts + Study setting: requirements n Availability of a sufficiently large target population for recruitment n Capacity of health system to implement the study n Effective local engagement in research n Sufficient infrastructure to support technical aspects of the trial

+ Choosing study eligibility criteria n n If criteria are too narrow: n Unable to reach recruitment targets n Results not generalizable to other important patient populations n Recruitment process too complex If criteria are too broad: n May reduce average effect size (choosing some who may not actually benefit) n May include individuals less likely to comply (reducing follow-up) n Proportion of eligible subjects recruited may be lower, with potential for selection bias Choosing a balance between 1. Internal validity (ability to identify what is ‘true’ in the study population) and 2. Generalizability (external validity – an extension of the observed results to a larger population)

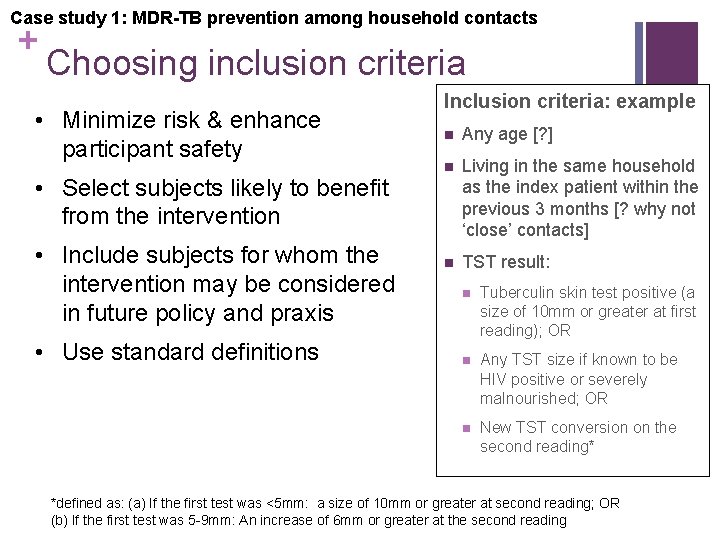
Case study 1: MDR-TB prevention among household contacts + Choosing inclusion criteria • Minimize risk & enhance participant safety • Select subjects likely to benefit from the intervention • Include subjects for whom the intervention may be considered in future policy and praxis • Use standard definitions Inclusion criteria: example n Any age [? ] n Living in the same household as the index patient within the previous 3 months [? why not ‘close’ contacts] n TST result: n Tuberculin skin test positive (a size of 10 mm or greater at first reading); OR n Any TST size if known to be HIV positive or severely malnourished; OR n New TST conversion on the second reading* *defined as: (a) If the first test was <5 mm: a size of 10 mm or greater at second reading; OR (b) If the first test was 5 -9 mm: An increase of 6 mm or greater at the second reading

Case study 1: MDR-TB prevention among household contacts + Choosing exclusion criteria Consider excluding: • Those with clear, recognised contraindications to the intervention • Those highly unlikely to comply with trial protocol • Those in whom the intervention may not be effective, and/or ethically justifiable However, avoid unnecessary complexity and narrow criteria Exclusion criteria: example n A diagnosis of current active TB disease made during initial assessment [? how] n Known to be pregnant n Unable to take oral medication n Documented previous treatment for MDR-TB n Dialysis-dependent chronic kidney disease n etc.

+ An aside: Including children in TB trials n Barriers to including children in clinical trials for TB include n n n Lack of pharmacokinetic and pharmacodynamic data Lack of appropriate drug formulations Concerns by IRBs and clinicians Lack of funding (2% of total TB drug research funding, 25% of need)1 Parental concerns Consensus statement on child TB trials: n « Children should be included in studies at the early phases of drug development and be an integral part of the clinical development plan, rather than after approval » 1 1 Nachman et al, Towards early inclusion of children in tuberculosis drugs trials: a consensus statement. Lancet ID 2015

+ Case study 2: MDR prevention among liver transplant candidates Torre-Cisneros, CID 2015

Case study 2: MDR-TB prevention among liver transplant candidates + Study design n Multi-centre, prospective, non-inferiority RCT comparing isonazid with levofloxacin in treatment of LTBI in patients eligible for liver transplantation n n 500 mg daily levofloxacin for 9 months vs 300 mg isoniazid for 9 months Target sample size 870 subjects to be randomized Torre-Cisneros, CID 2015

Case study 2: MDR-TB prevention among liver transplant candidates + Inclusion criteria n On the waiting list for solid organ transplant within a network of Spanish hospitals n Aged ≥ 18 years n No evidence of active TB n One of: n Latent TB infection (TST ≥ 5 mm or positive IGRA); or n History of ‘improperly treated TB’, or n Recent TB contact, or n Xray changes consistent with old TB (apical nodules, calcified lymph nodes, pleural thickening) Torre-Cisneros, CID 2015

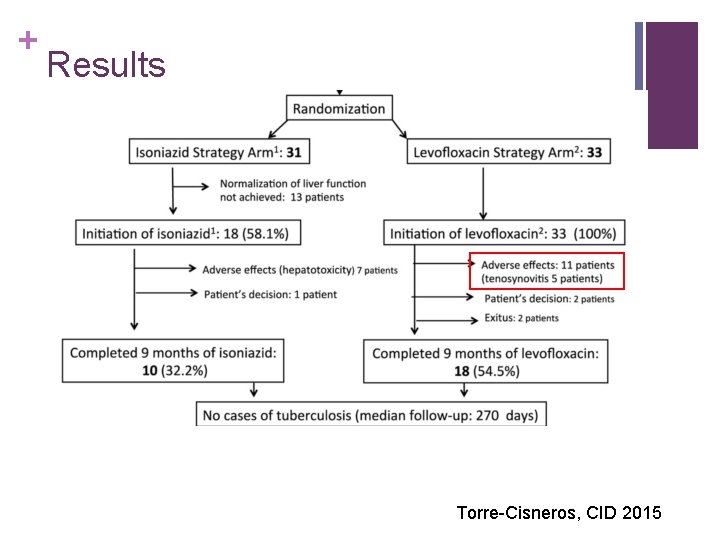
+ Results Torre-Cisneros, CID 2015

+ Results n 33/33 LEV and 27/31 INH patients took steroids n 2/33 LEV patients (6%) and 7/18 INH patients (38. 9%) developed severe hepatotoxicity n 6/33 LEV patients (18. 2%) developed tenosynovitis, affecting knee in 5 and achilles tendon in 1, permanently discontinued in 5 n Study terminated early: “Due to high frequency and intensity of this unexpected side effect the trial was definitively stopped” (? pre-defined stopping rules) Torre-Cisneros, CID 2015

+ Considerations in selecting study populations n Study population affects interpretation of findings n Generalizability of findings from Torre-Cisneros ? – determined by participant characteristics n Ensure the research question can be addressed within the intended study population n Clearly specify inclusion and exclusion criteria in detail n Consider generalizability of findings n Consider advantages vs disadvantages of multiple sites

+ Part II: Minimising bias in clinical trials

+ Minimising bias in clinical trials

+ Bias in clinical trials n Our goal in conducting clinical trials is to obtain valid (‘truthful’) and precise (‘accurate’) estimates of the relationship between an intervention and outcome n The main threats to validity are caused by bias: a tendency of an estimate to deviate in one direction from a true value Leading to underestimation or overestimation of the effect of the intervention n It is impossible to know for sure whether a clinical study is biased, as we cannot know ‘the truth’ n

+ Important forms of bias n Key forms of bias in clinical trials include: Confounding (an ‘imbalance’ between groups that may be systematic, or by chance)* n Selection bias (selection for an intervention is based upon the outcome) n Information bias (measurement error in the exposure, outcome or covariates = ‘misclassification’**) n How can we minimise biases in clinical trials? *Confounders can be describe as variables that are: (a) Independently predictive of disease, within strata of exposure, (b) Associated with the exposure, (c) Not an intermediate in the causal pathway between exposure and outcome ** Misclassification bias for categorical variables

+ Randomization is the random allocation of an individual or group to an intervention • Each individual theoretically has the same opportunity to be assigned to each of the study groups • If done properly, randomization can ensure study groups are balanced - for both measured and unmeasured factors (confounders) • Randomization can satisfy assumptions required by statistical methods (e. g. independence between observations, no unmeasured confounding)

+ Viera, Fam Med 2007

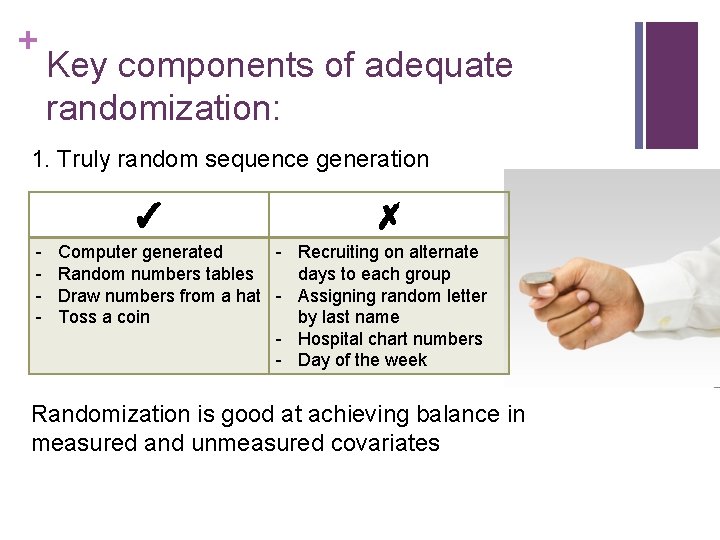
+ Key components of adequate randomization: 1. Truly random sequence generation ✓ - ✗ Computer generated - Recruiting on alternate Random numbers tables days to each group Draw numbers from a hat - Assigning random letter Toss a coin by last name - Hospital chart numbers - Day of the week Randomization is good at achieving balance in measured and unmeasured covariates


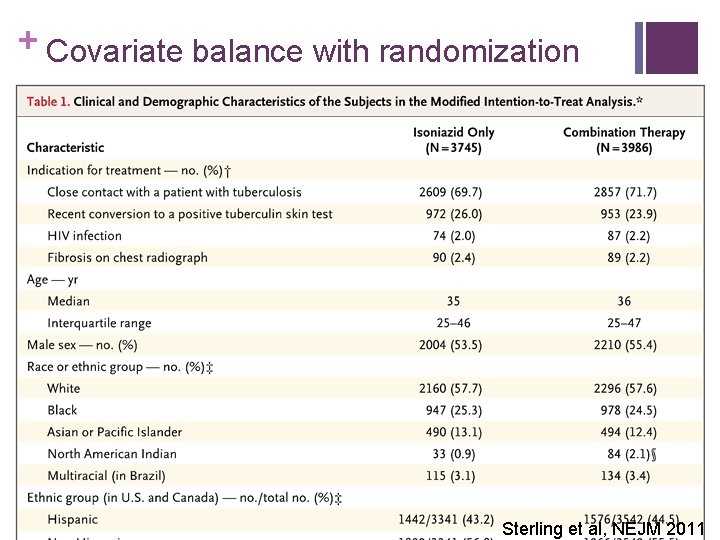
+ Covariate balance with randomization Sterling et al, NEJM 2011

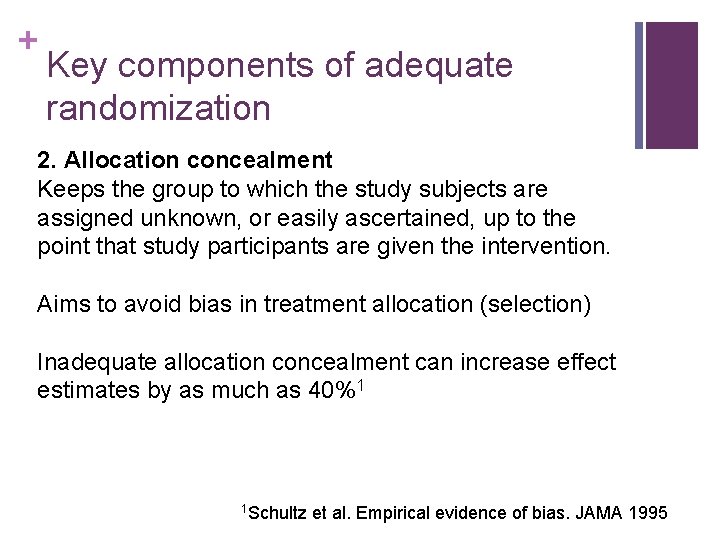
+ Key components of adequate randomization 2. Allocation concealment Keeps the group to which the study subjects are assigned unknown, or easily ascertained, up to the point that study participants are given the intervention. Aims to avoid bias in treatment allocation (selection) Inadequate allocation concealment can increase effect estimates by as much as 40%1 1 Schultz et al. Empirical evidence of bias. JAMA 1995

+ Blinding

+ Key components of adequate randomization: 3. Blinding • Blinding all concerned to the intervention group can reduce ascertainment bias 1. • The best way to reduce ascertainment bias is to keep all participants and investigators in the study blinded as long as possible. • Blinding is not always possible, by nature of the intervention (e. g. surgery for MDR-TB – although sham surgery possible) 1 Ascertainment bias occurs when the results or conclusions of a trial are systematically distorted by knowledge of which intervention each participant is receiving. 2 Schultz et al. Empirical evidence of bias. JAMA 1995

+ Levels of blinding n Single Blind: Subject is not aware of group allocation n Double Blind: Neither the subjects nor treating staff know group allocation n Triple-Blind: Neither subjects, investigators nor data analysts and monitoring committee know group allocation

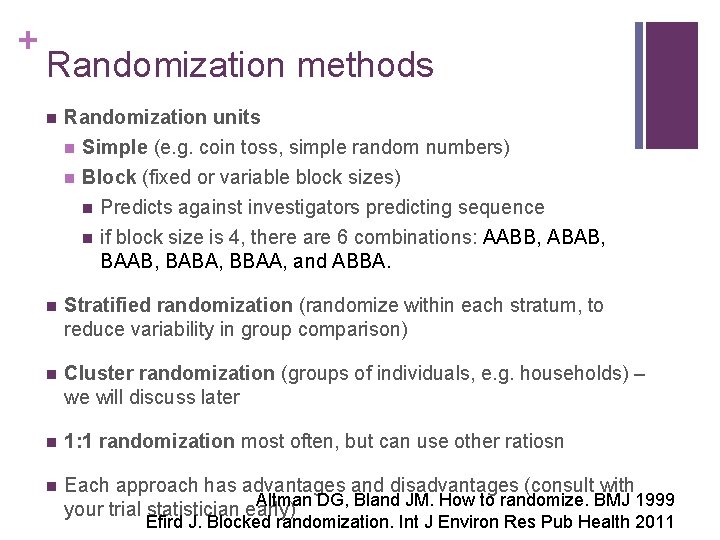
+ Randomization methods n Randomization units n Simple (e. g. coin toss, simple random numbers) n Block (fixed or variable block sizes) n Predicts against investigators predicting sequence n if block size is 4, there are 6 combinations: AABB, ABAB, BAAB, BABA, BBAA, and ABBA. n Stratified randomization (randomize within each stratum, to reduce variability in group comparison) n Cluster randomization (groups of individuals, e. g. households) – we will discuss later n 1: 1 randomization most often, but can use other ratiosn n Each approach has advantages and disadvantages (consult with Altman DG, Bland JM. How to randomize. BMJ 1999 your trial statistician early) Efird J. Blocked randomization. Int J Environ Res Pub Health 2011

+ Part III: Example of choosing appropriate sample size

+ Sample size calculations n The sample size is the expected number of participants required to adequately answer the research question n Sample size is clinically and ethically important n Too few subjects: may prevent valid and precise determination of the treatment effect; may incur excessive cost and time n Too many subjects: may expose more individuals to risk n Before embarking upon the sample size calculation, you need to determine the planned primary outcome measure and measures of interest. n What is the clinically important difference?

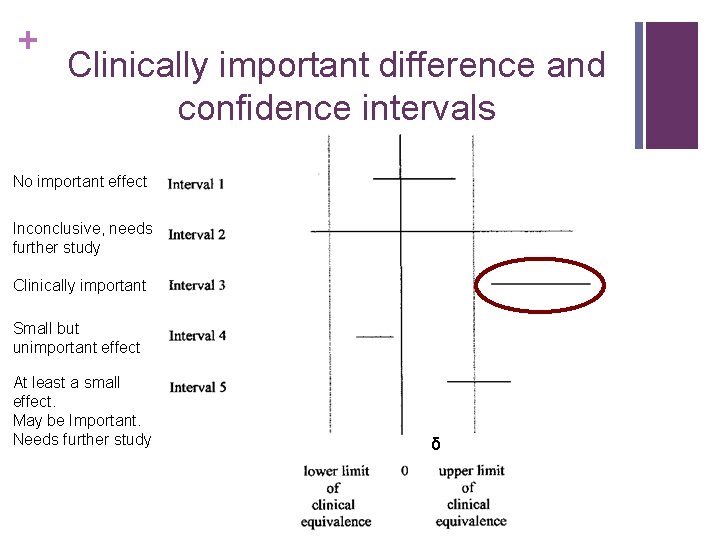
+ Clinically important difference and confidence intervals No important effect Inconclusive, needs further study Clinically important Small but unimportant effect At least a small effect. May be Important. Needs further study δ

+ Example: sample size for V-QUIN trial n Sample size calculations require explicit decisions, including: n Study design (e. g. superiority / non-inferiority; individual or cluster randomization; stratified effects required) n Statistical analytic method planned (usually frequentist; could use Bayesian methods) n Outcome measures (relative risk, risk difference etc) n Thresholds for type I (e. g. α = 0. 05) and type II (1 - β = 0. 8) errors n Minimum clinically important difference (δ) (prior slide) n Precision of the estimates (standard deviations in each group, σ) n Expected event rates (e. g. TB incidence) based on other studies n Expected recruitment and drop-out rates

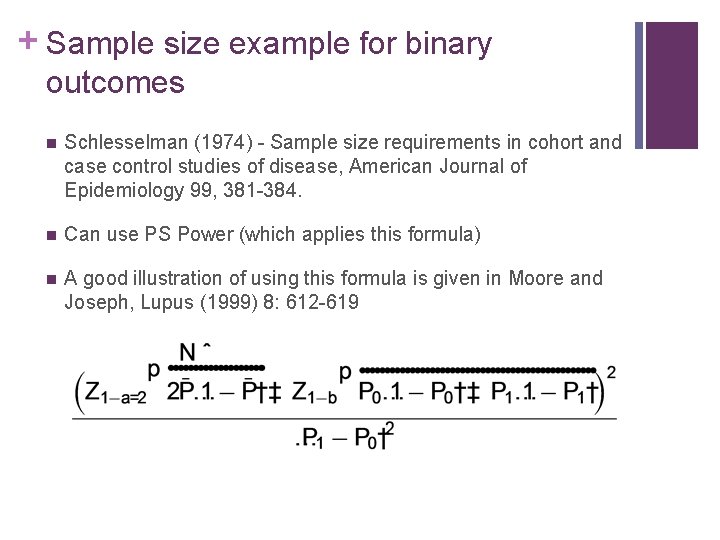
+ Sample size example for binary outcomes n Schlesselman (1974) - Sample size requirements in cohort and case control studies of disease, American Journal of Epidemiology 99, 381 -384. n Can use PS Power (which applies this formula) n A good illustration of using this formula is given in Moore and Joseph, Lupus (1999) 8: 612 -619

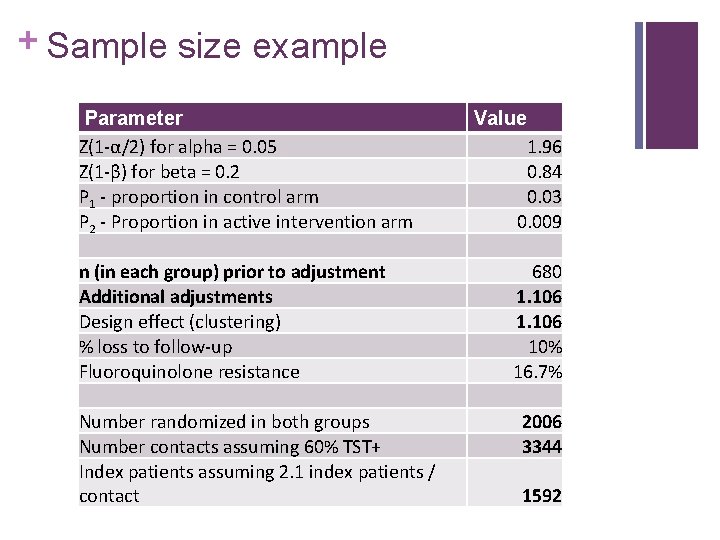
+ Sample size example Parameter Z(1 -α/2) for alpha = 0. 05 Z(1 -β) for beta = 0. 2 P 1 - proportion in control arm P 2 - Proportion in active intervention arm n (in each group) prior to adjustment Additional adjustments Design effect (clustering) % loss to follow-up Fluoroquinolone resistance Number randomized in both groups Number contacts assuming 60% TST+ Index patients assuming 2. 1 index patients / contact Value 1. 96 0. 84 0. 03 0. 009 680 1. 106 10% 16. 7% 2006 3344 1592

+ Sample size calculations Other considerations: n Cost n n Event rate Feasibility Sample size calculations are covered well in many places: n Moore AD, Joseph L. Sample size considerations for superiority trials in systemic lupus erythematosus. Lupus, 1999. n Joseph L. Bayesian and mixed Bayesian likelihood criteria for sample size determination. Stat Med 1997. n Zou KH, Normand S-L T. On determination of sample size in hierarchical binomial models. Stat Med 2001.


+ Acknowledgements Vietnam National Tuberculosis Program A/Prof Nguyen Viet Nhung A/Prof Dinh Ngoc Sy Pham Ngoc Thach Hospital An Giang, Binh Dinh, Ca Mau Can Tho, Da Nang, Ha Noi, Tien Giang, Ho Chi Minh City, Vinh Phuc Tuberculosis Programs Australian National Health and Medical Research Council (NHMRC) Woolcock Institute of Medical Research, Sydney Dr Carol Armour, Director and staff Woolcock Institute of Medical Research, Vietnam Dr Nguyen Thu Anh And, most importantly, the people of the participating provinces