Seizing the Opportunity Treating newonset epilepsy in geriatric














- Slides: 41

Seizing the Opportunity Treating new-onset epilepsy in geriatric patients with dementia Karin Chisholm 2016 -17 Island Health Pharmacy Resident BC Wide Case Presentation Jan 13, 2017

Learning Objectives • Understand epilepsy epidemiology & pathophysiology as it applies to geriatric patients • List factors important to consider when selecting drug therapy for a geriatric patient • Review the evidence surrounding antiepileptic drugs (AEDs) in geriatric patients with dementia • Apply the evidence to a patient case

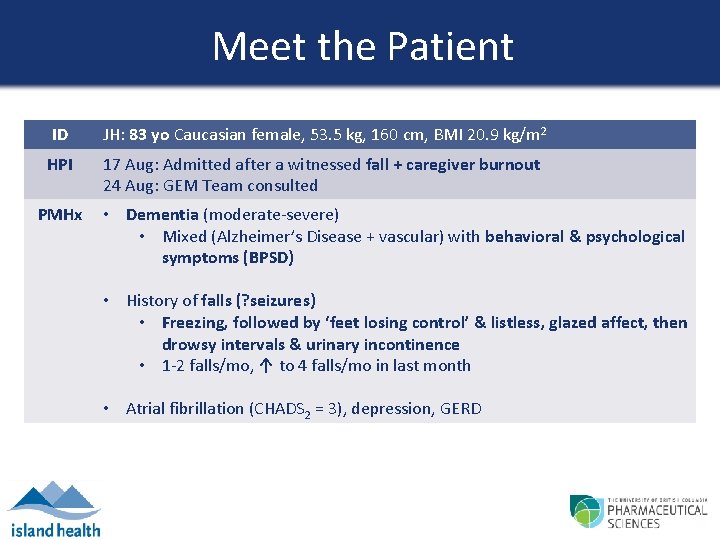
Meet the Patient ID JH: 83 yo Caucasian female, 53. 5 kg, 160 cm, BMI 20. 9 kg/m 2 HPI 17 Aug: Admitted after a witnessed fall + caregiver burnout 24 Aug: GEM Team consulted PMHx • Dementia (moderate-severe) • Mixed (Alzheimer’s Disease + vascular) with behavioral & psychological symptoms (BPSD) • History of falls (? seizures) • Freezing, followed by ‘feet losing control’ & listless, glazed affect, then drowsy intervals & urinary incontinence • 1 -2 falls/mo, ↑ to 4 falls/mo in last month • Atrial fibrillation (CHADS 2 = 3), depression, GERD

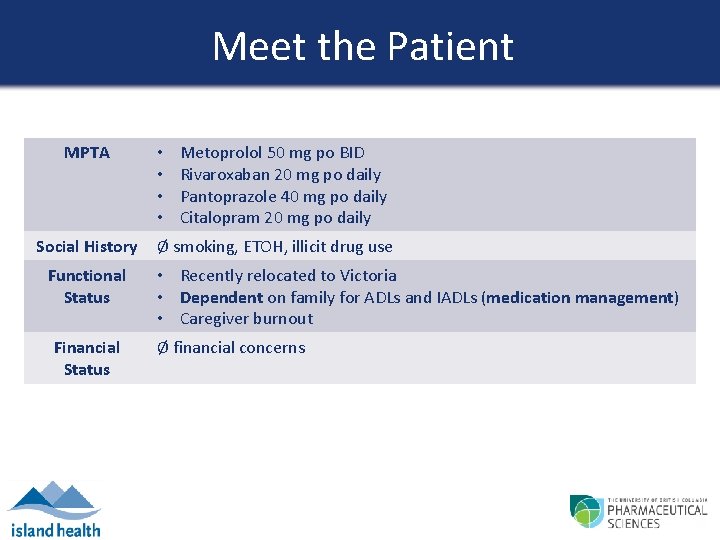
Meet the Patient MPTA Social History Functional Status Financial Status • • Metoprolol 50 mg po BID Rivaroxaban 20 mg po daily Pantoprazole 40 mg po daily Citalopram 20 mg po daily Ø smoking, ETOH, illicit drug use • Recently relocated to Victoria • Dependent on family for ADLs and IADLs (medication management) • Caregiver burnout Ø financial concerns

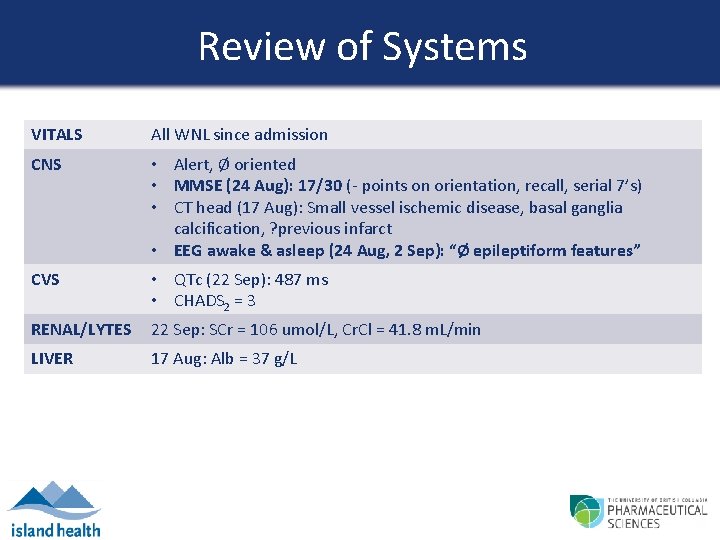
Review of Systems VITALS All WNL since admission CNS • Alert, Ø oriented • MMSE (24 Aug): 17/30 (- points on orientation, recall, serial 7’s) • CT head (17 Aug): Small vessel ischemic disease, basal ganglia calcification, ? previous infarct • EEG awake & asleep (24 Aug, 2 Sep): “Ø epileptiform features” CVS • QTc (22 Sep): 487 ms • CHADS 2 = 3 RENAL/LYTES 22 Sep: SCr = 106 umol/L, Cr. Cl = 41. 8 m. L/min LIVER 17 Aug: Alb = 37 g/L

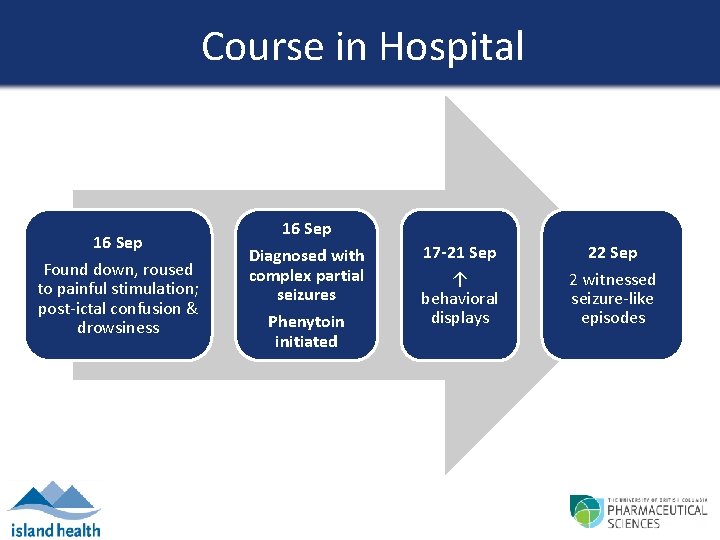
Course in Hospital 16 Sep Found down, roused to painful stimulation; post-ictal confusion & drowsiness 16 Sep Diagnosed with complex partial seizures Phenytoin initiated 17 -21 Sep ↑ behavioral displays 22 Sep 2 witnessed seizure-like episodes

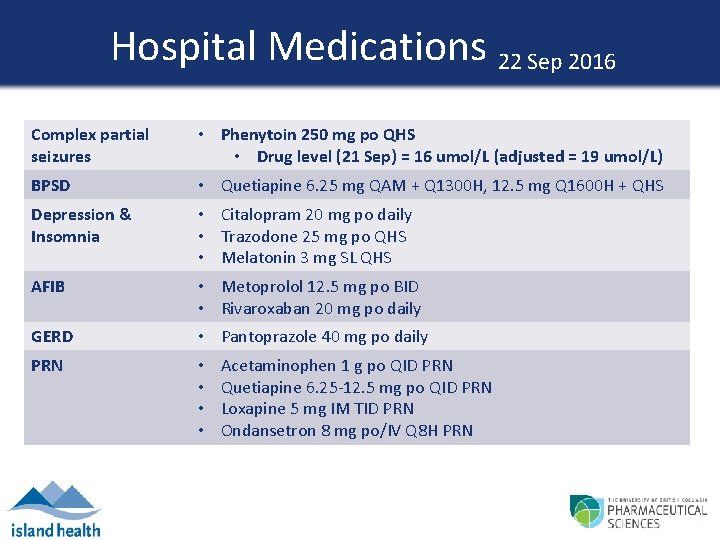
Hospital Medications 22 Sep 2016 Complex partial seizures • Phenytoin 250 mg po QHS • Drug level (21 Sep) = 16 umol/L (adjusted = 19 umol/L) BPSD • Quetiapine 6. 25 mg QAM + Q 1300 H, 12. 5 mg Q 1600 H + QHS Depression & Insomnia • Citalopram 20 mg po daily • Trazodone 25 mg po QHS • Melatonin 3 mg SL QHS AFIB • Metoprolol 12. 5 mg po BID • Rivaroxaban 20 mg po daily GERD • Pantoprazole 40 mg po daily PRN • • Acetaminophen 1 g po QID PRN Quetiapine 6. 25 -12. 5 mg po QID PRN Loxapine 5 mg IM TID PRN Ondansetron 8 mg po/IV Q 8 H PRN

Drug Therapy Problems Experiencing recurrence of ? seizures due to sub-therapeutic dose of phenytoin At risk of thromboembolic event (e. g. stroke) due to rivaroxaban-phenytoin interaction (↓ rivaroxaban levels) Experiencing increased behavioral symptoms due to quetiapine-phenytoin interaction (↓ quetiapine levels) At risk of mood lability due to citalopram-phenytoin interaction (↓ citalopram levels) Increased risk of Td. P & cardiac arrhythmias with use of multiple QTc prolonging medications

Goals of Therapy • Prevent recurrence of seizures & complications – Reduce frequency of falls and risk of fractures – Reduce recurrence of hospitalizations – Ease caregiver burden & distress • Minimize adverse effects (including worsening of behavioral symptoms and cognition) and drug interactions • Improve patient and caregiver quality of life

Epilepsy in the Elderly Epidemiology & Presentation Etiology • Bimodal incidence rates (highest during 1 st and after 60 th year of life) • Dementia or dementing illness associated with 5 -10 fold ↑ risk • Nearly all seizures are of partial (focal) onset • Monotherapy effective in majority • Presentation: • Convulsions & auras rare • Post-ictal confusion + hyperactivity, wandering, incontinence Jenssen S et al. Am J Alzheimers Dis Other Demen 2010; 25(1): 18 -26. Mendez MF et al. Drugs & Aging 2003; 20(11): 791 -803.

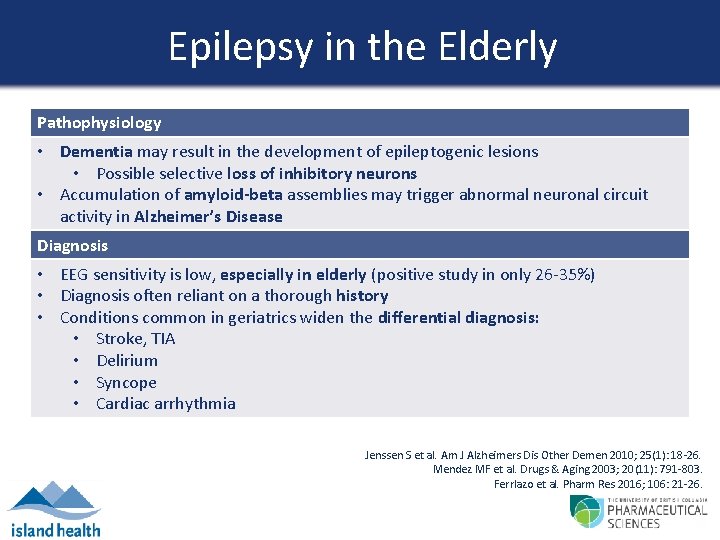
Epilepsy in the Elderly Pathophysiology • Dementia may result in the development of epileptogenic lesions • Possible selective loss of inhibitory neurons • Accumulation of amyloid-beta assemblies may trigger abnormal neuronal circuit activity in Alzheimer’s Disease Diagnosis • EEG sensitivity is low, especially in elderly (positive study in only 26 -35%) • Diagnosis often reliant on a thorough history • Conditions common in geriatrics widen the differential diagnosis: • Stroke, TIA • Delirium • Syncope • Cardiac arrhythmia Jenssen S et al. Am J Alzheimers Dis Other Demen 2010; 25(1): 18 -26. Mendez MF et al. Drugs & Aging 2003; 20(11): 791 -803. Ferrlazo et al. Pharm Res 2016; 106: 21 -26.

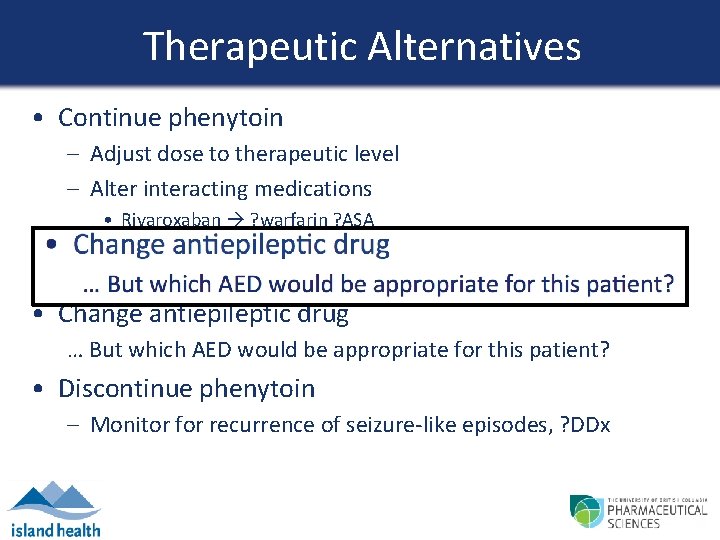
Therapeutic Alternatives • Continue phenytoin – Adjust dose to therapeutic level – Alter interacting medications • Rivaroxaban ? warfarin ? ASA • Quetiapine ? risperidone • Citalopram ? alternate antidepressant • Change antiepileptic drug … But which AED would be appropriate for this patient? • Discontinue phenytoin – Monitor for recurrence of seizure-like episodes, ? DDx

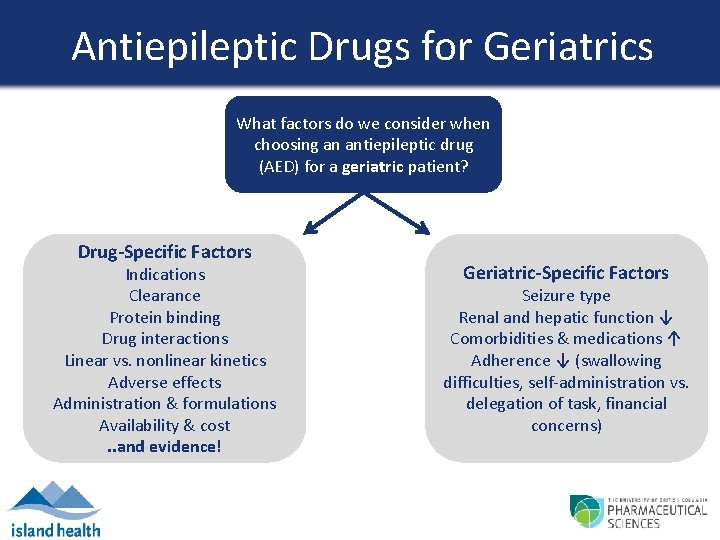
Antiepileptic Drugs for Geriatrics What factors do we consider when choosing an antiepileptic drug (AED) for a geriatric patient? Drug-Specific Factors Indications Clearance Protein binding Drug interactions Linear vs. nonlinear kinetics Adverse effects Administration & formulations Availability & cost. . and evidence! Geriatric-Specific Factors Seizure type Renal and hepatic function ↓ Seizure type Comorbidities & medications ↑ Renal and hepatic function Adherence ↓ (swallowing Comorbidities & medications difficulties, self-administration vs. Adherence concerns delegation of task, financial concerns) Patient-Specific Factors

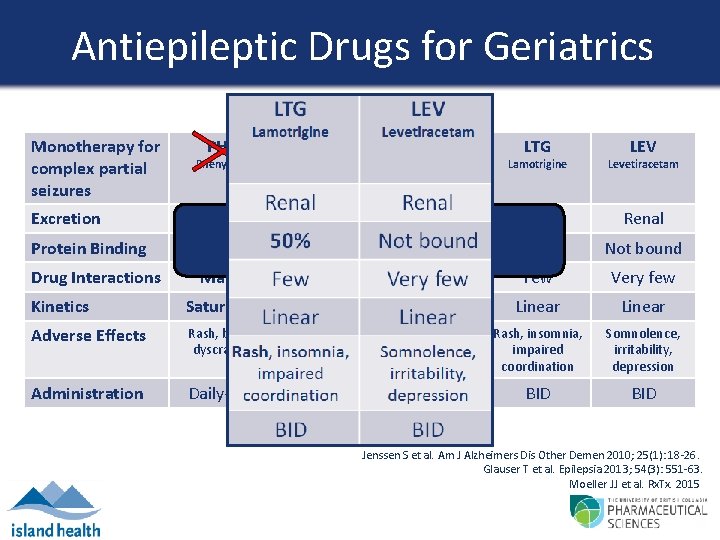
Antiepileptic Drugs for Geriatrics Monotherapy for complex partial seizures Phenytoin Carbamazepine Valproic Acid Lamotrigine Levetiracetam Excretion Biliary Renal Protein Binding 80 -95% 50% Not bound Drug Interactions PHT CBZ VPA What 75% is the evidence? 75 -90% LTG LEV Many Few Very few Kinetics Saturable Linear Adverse Effects Rash, blood dyscrasias Nausea, tremor, blood dyscrasias Rash, insomnia, impaired coordination Somnolence, irritability, depression Administration Daily-BID BID-TID BID Jenssen S et al. Am J Alzheimers Dis Other Demen 2010; 25(1): 18 -26. Glauser T et al. Epilepsia 2013; 54(3): 551 -63. Moeller JJ et al. Rx. Tx. 2015

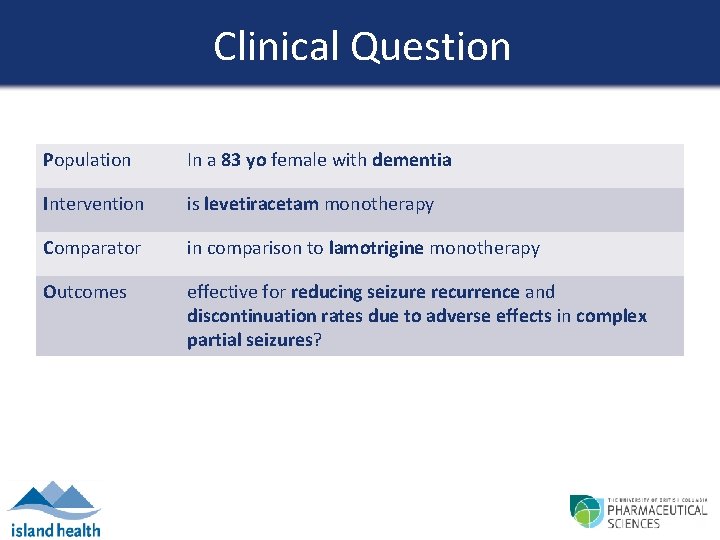
Clinical Question Population In a 83 yo female with dementia Intervention is levetiracetam monotherapy Comparator in comparison to lamotrigine monotherapy Outcomes effective for reducing seizure recurrence and discontinuation rates due to adverse effects in complex partial seizures?

Search Strategy + Results Databases Pub. Med, EMBASE, Medline, Google Scholar Search Terms (levetiracetam) AND (lamotrigine) AND (seizure* OR epilep*) AND (elderly OR geriatric OR aged) AND (dement*) Results 2 RCTs 3 Observational studies 2 Retrospective reviews Most Relevant Evidence Cumbo et al, 2010 Werhahn et al, 2015

Cumbo et al 2010 D Randomized, three-arm, case-control trial 4 week dose titration, 12 month follow-up P 60 -90 yo with mild-mod Alzheimer’s Disease (AD) (MMSE=10 -26) and partial epilepsy Key Exclusion: Concomitant antidepressant or neuroleptic, previous epilepsy therapy I Group 1: Levetiracetam (LEV)* Group 2: Lamotrigine (LTG)* Group 3: Phenobarbital (PB)* *titrated to target dose & individually adjusted C 3 groups compared to each other and to age-, sex-, & AD severity-matched control group O 1 : Efficacy of LEV compared to LTG & PB • % seizure-free or > 50% ↓ in seizure frequency 2 : Cognitive effects of LEV, LTG & PB compared to case-control group • Δ in cognitive function (MMSE score, ADAS-Cog score) • Δ in mood (Cornell scale score) Adverse effects of LEV, LTG, PB

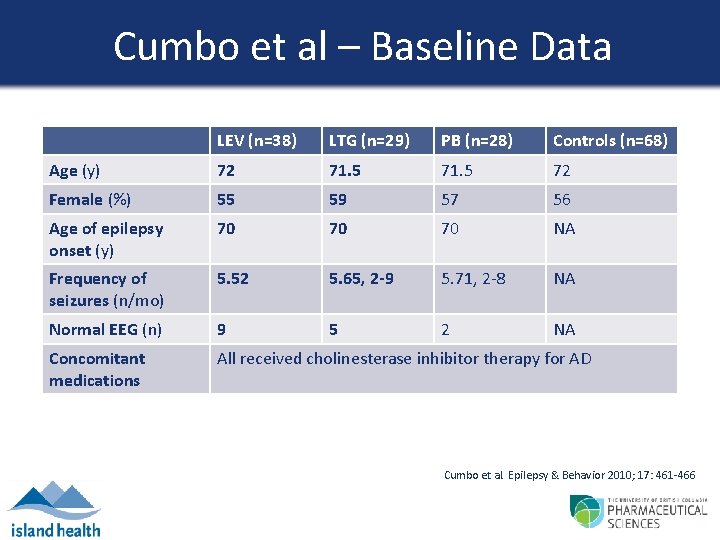
Cumbo et al – Baseline Data LEV (n=38) LTG (n=29) PB (n=28) Controls (n=68) Age (y) 72 71. 5 72 Female (%) 55 59 57 56 Age of epilepsy onset (y) 70 70 70 NA Frequency of seizures (n/mo) 5. 52 5. 65, 2 -9 5. 71, 2 -8 NA Normal EEG (n) 9 5 2 NA Concomitant medications All received cholinesterase inhibitor therapy for AD Cumbo et al. Epilepsy & Behavior 2010; 17: 461 -466

Cumbo et al – Results LEV (n=38) LTG (n=29) PB (n=28) Dose/d (mean, range) 955 (500 -2000) 57. 5 (25 -100) 90 (50 -100) Seizure-freedom (n, %) 11, 28. 9% 7, 24. 1% 8, 28. 5% Seizure reduction by 50 - 16, 42. 1% 99% (n, %) 10, 34. 5% 10, 35. 7% Responders (n, %) 27, 71. 1% 17, 58. 6% 18, 64. 2% Increased seizures 0 0 0 NSS difference in response rate between the groups Cumbo et al. Epilepsy & Behavior 2010; 17: 461 -466

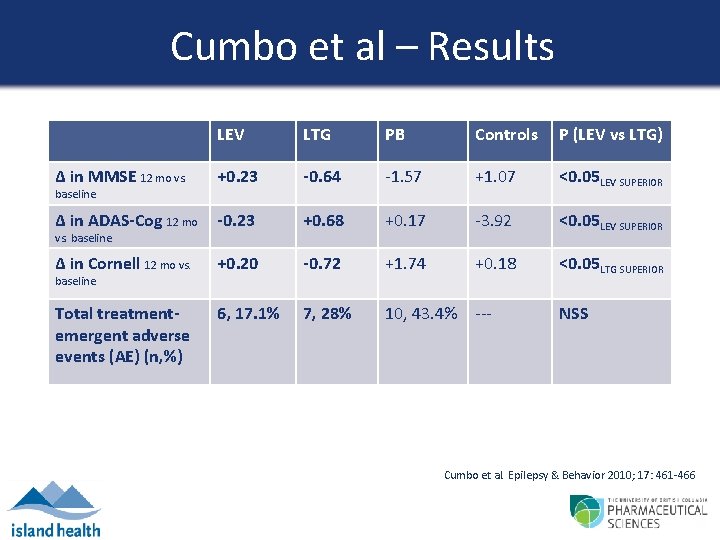
Cumbo et al – Results LEV LTG PB Controls P (LEV vs LTG) Δ in MMSE 12 mo vs. +0. 23 -0. 64 -1. 57 +1. 07 <0. 05 LEV SUPERIOR Δ in ADAS-Cog 12 mo -0. 23 +0. 68 +0. 17 -3. 92 <0. 05 LEV SUPERIOR Δ in Cornell 12 mo vs. +0. 20 -0. 72 +1. 74 +0. 18 <0. 05 LTG SUPERIOR Total treatmentemergent adverse events (AE) (n, %) 6, 17. 1% 7, 28% 10, 43. 4% --- NSS baseline vs. baseline Cumbo et al. Epilepsy & Behavior 2010; 17: 461 -466

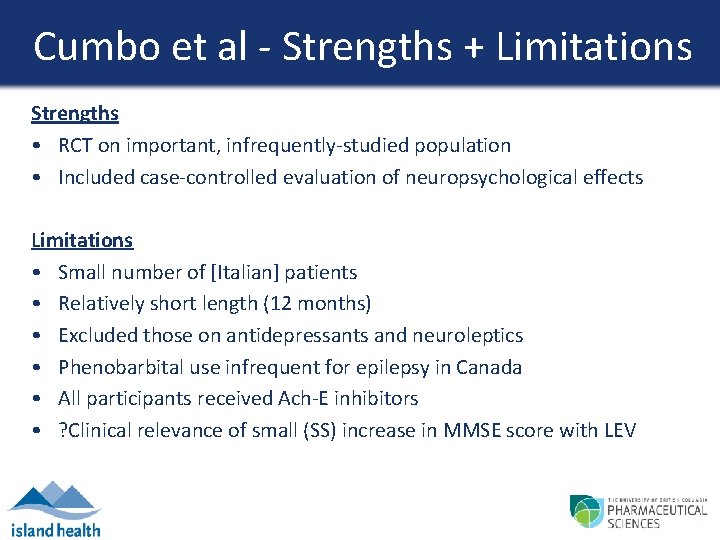
Cumbo et al - Strengths + Limitations Strengths • RCT on important, infrequently-studied population • Included case-controlled evaluation of neuropsychological effects Limitations • Small number of [Italian] patients • Relatively short length (12 months) • Excluded those on antidepressants and neuroleptics • Phenobarbital use infrequent for epilepsy in Canada • All participants received Ach-E inhibitors • ? Clinical relevance of small (SS) increase in MMSE score with LEV

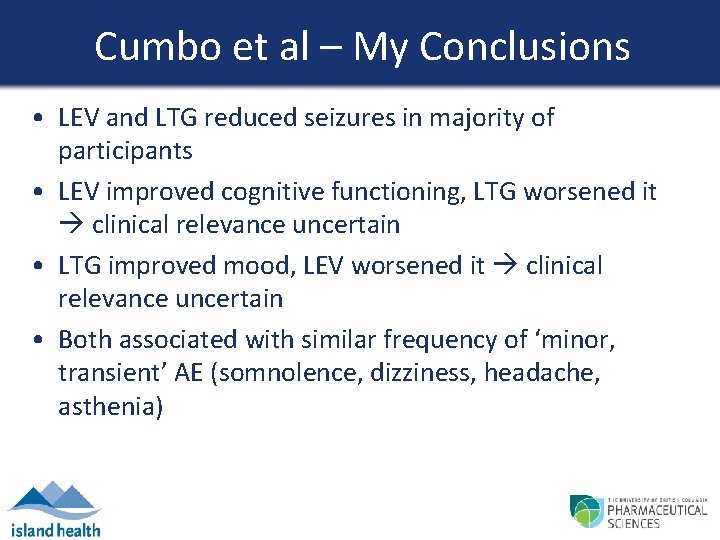
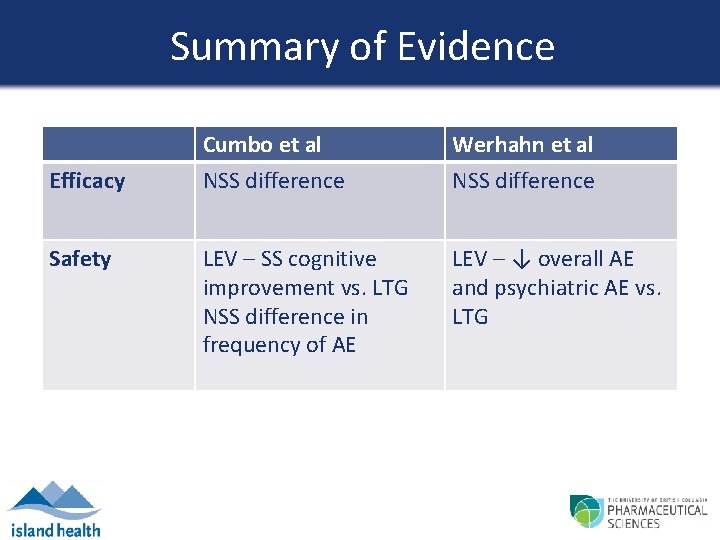
Cumbo et al – My Conclusions • LEV and LTG reduced seizures in majority of participants • LEV improved cognitive functioning, LTG worsened it clinical relevance uncertain • LTG improved mood, LEV worsened it clinical relevance uncertain • Both associated with similar frequency of ‘minor, transient’ AE (somnolence, dizziness, headache, asthenia)

Werhahn et al 2015 D Multicenter, double-blind, active comparator, randomized trial 6 week dose titration, 52 week follow-up P ≥ 60 yo with new-onset focal epilepsy; 0 -4 weeks previous AED treatment Key Exclusion: Dementia, GFR <50 m. L/min, seizures <2 weeks post-acute cerebral insult I Group 1: Carbamazepine CR (CR-CBZ)* Group 2: Lamotrigine (LTG)* Group 3: Levetiracetam (LEV)* *titrated & maintained depending on seizure relapse or tolerability C 3 groups compared to each other O 1 : Retention to treatment at 58 weeks 2 : Seizure-freedom, time to first seizure, time to first drug-related adverse event (AE) Safety and tolerability outcomes (AE, hematology, vital signs, physical & neurological exam)

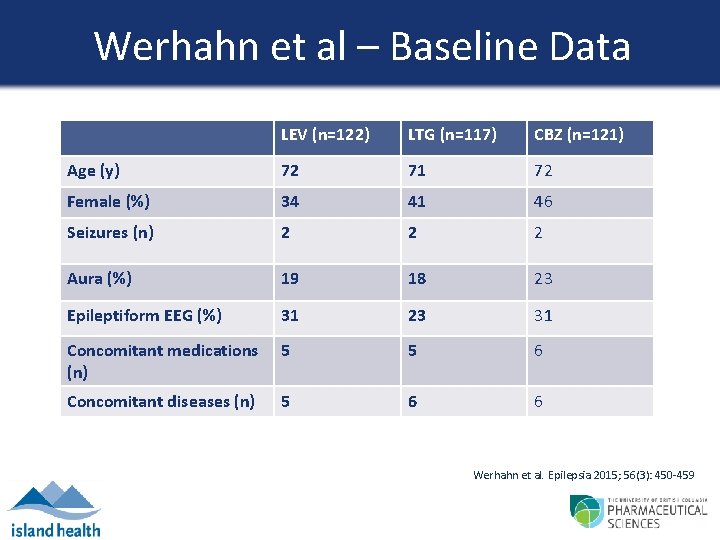
Werhahn et al – Baseline Data LEV (n=122) LTG (n=117) CBZ (n=121) Age (y) 72 71 72 Female (%) 34 41 46 Seizures (n) 2 2 2 Aura (%) 19 18 23 Epileptiform EEG (%) 31 23 31 Concomitant medications (n) 5 5 6 Concomitant diseases (n) 5 6 6 Werhahn et al. Epilepsia 2015; 56(3): 450 -459

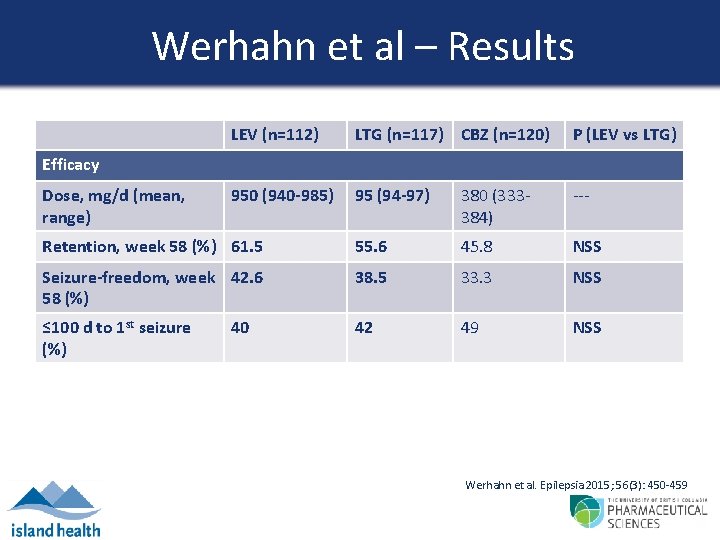
Werhahn et al – Results LEV (n=112) LTG (n=117) CBZ (n=120) P (LEV vs LTG) 950 (940 -985) 95 (94 -97) 380 (333384) --- Retention, week 58 (%) 61. 5 55. 6 45. 8 NSS Seizure-freedom, week 42. 6 58 (%) 38. 5 33. 3 NSS ≤ 100 d to 1 st seizure (%) 42 49 NSS Efficacy Dose, mg/d (mean, range) 40 Werhahn et al. Epilepsia 2015; 56(3): 450 -459

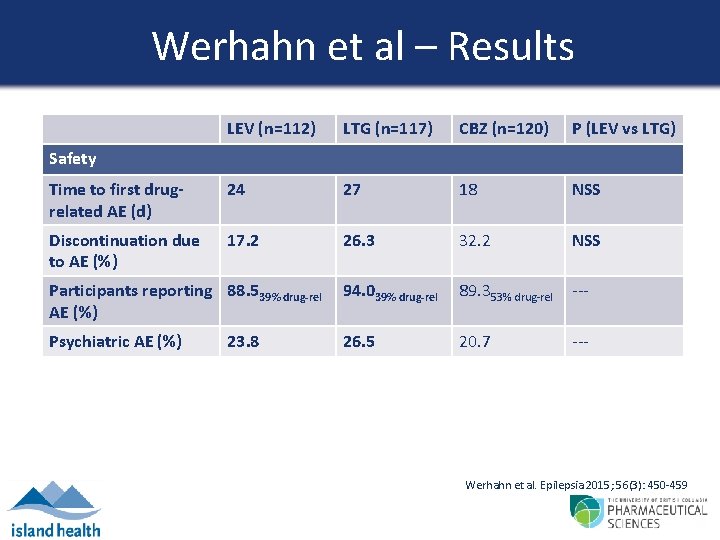
Werhahn et al – Results LEV (n=112) LTG (n=117) CBZ (n=120) P (LEV vs LTG) Time to first drugrelated AE (d) 24 27 18 NSS Discontinuation due to AE (%) 17. 2 26. 3 32. 2 NSS Participants reporting 88. 539% drug-rel AE (%) 94. 039% drug-rel 89. 353% drug-rel --- Psychiatric AE (%) 26. 5 20. 7 --- Safety 23. 8 Werhahn et al. Epilepsia 2015; 56(3): 450 -459

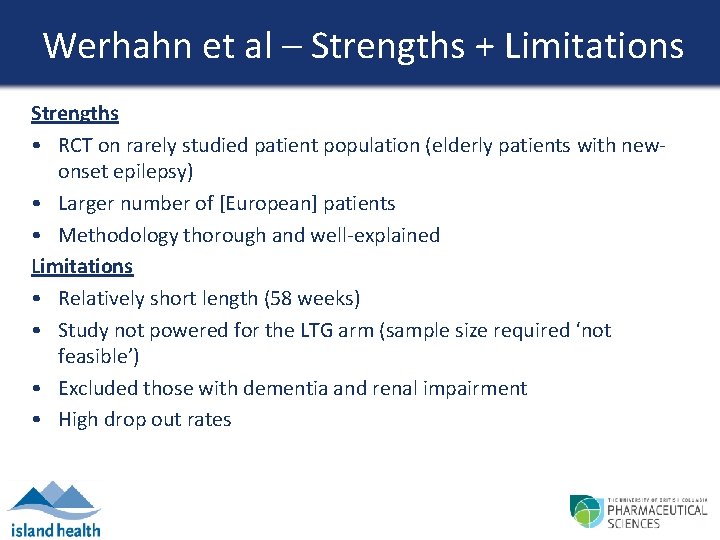
Werhahn et al – Strengths + Limitations Strengths • RCT on rarely studied patient population (elderly patients with newonset epilepsy) • Larger number of [European] patients • Methodology thorough and well-explained Limitations • Relatively short length (58 weeks) • Study not powered for the LTG arm (sample size required ‘not feasible’) • Excluded those with dementia and renal impairment • High drop out rates

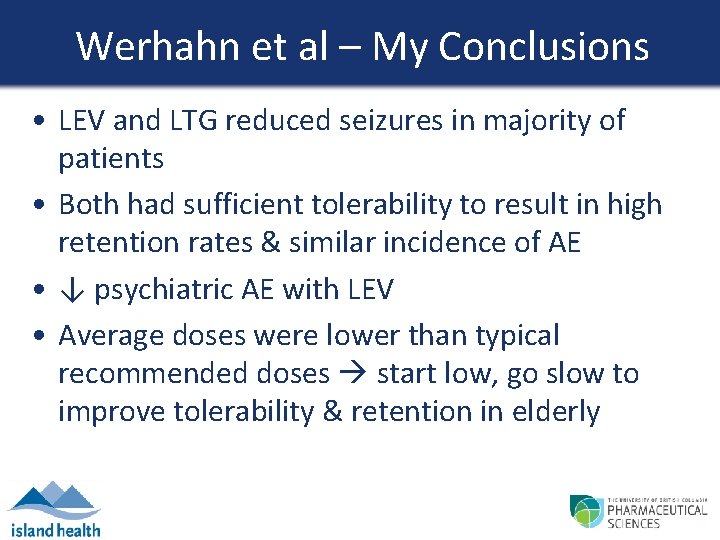
Werhahn et al – My Conclusions • LEV and LTG reduced seizures in majority of patients • Both had sufficient tolerability to result in high retention rates & similar incidence of AE • ↓ psychiatric AE with LEV • Average doses were lower than typical recommended doses start low, go slow to improve tolerability & retention in elderly

Summary of Evidence Efficacy Safety Cumbo et al NSS difference Werhahn et al NSS difference LEV – SS cognitive improvement vs. LTG NSS difference in frequency of AE LEV – ↓ overall AE and psychiatric AE vs. LTG

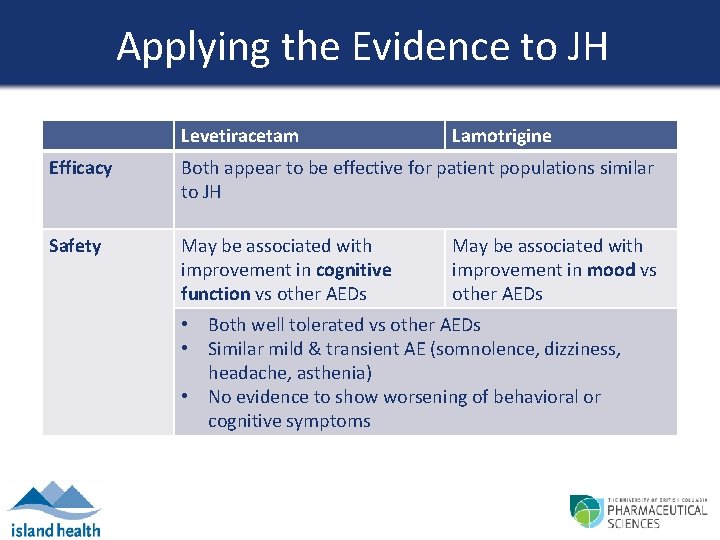
Applying the Evidence to JH Levetiracetam Lamotrigine Efficacy Both appear to be effective for patient populations similar to JH Safety May be associated with improvement in cognitive function vs other AEDs May be associated with improvement in mood vs other AEDs • Both well tolerated vs other AEDs • Similar mild & transient AE (somnolence, dizziness, headache, asthenia) • No evidence to show worsening of behavioral or cognitive symptoms

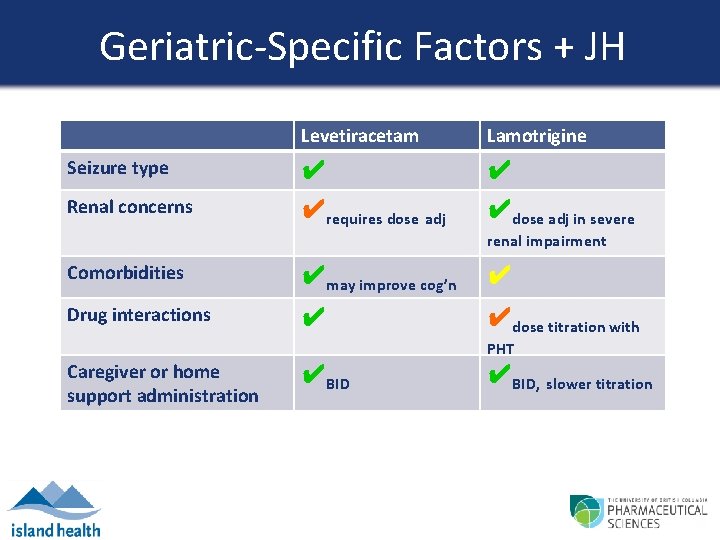
Geriatric-Specific Factors + JH Levetiracetam Lamotrigine Seizure type ✔ ✔ Renal concerns ✔requires dose adj ✔dose adj in severe renal impairment Comorbidities ✔may improve cog’n ✔ Drug interactions ✔ ✔dose titration with PHT Caregiver or home support administration ✔BID, slower titration

Recommendations 1. Discontinue phenytoin 250 mg po QHS 2. Initiate levetiracetam 250 mg po BID, increase by 250 mg/week to target 500 mg po BID 3. Discontinue ondansetron PRN

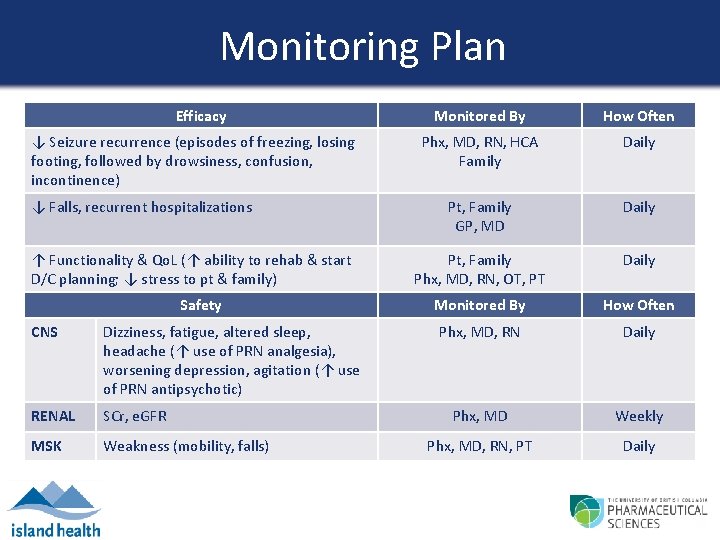
Monitoring Plan Efficacy ↓ Seizure recurrence (episodes of freezing, losing footing, followed by drowsiness, confusion, incontinence) ↓ Falls, recurrent hospitalizations ↑ Functionality & Qo. L (↑ ability to rehab & start D/C planning; ↓ stress to pt & family) Safety CNS Dizziness, fatigue, altered sleep, headache (↑ use of PRN analgesia), worsening depression, agitation (↑ use of PRN antipsychotic) RENAL SCr, e. GFR MSK Weakness (mobility, falls) Monitored By How Often Phx, MD, RN, HCA Family Daily Pt, Family GP, MD Daily Pt, Family Phx, MD, RN, OT, PT Daily Monitored By How Often Phx, MD, RN Daily Phx, MD Weekly Phx, MD, RN, PT Daily

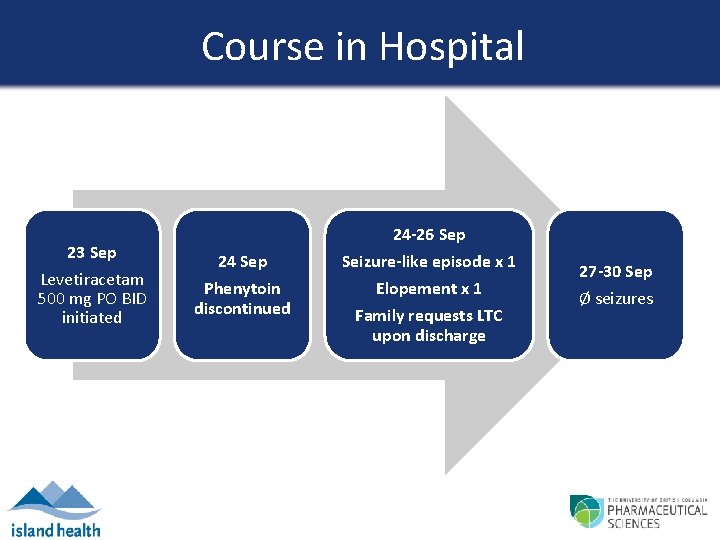
Course in Hospital 23 Sep Levetiracetam 500 mg PO BID initiated 24 Sep Phenytoin discontinued 24 -26 Sep Seizure-like episode x 1 Elopement x 1 Family requests LTC upon discharge 27 -30 Sep Ø seizures

COMMENTS?

References 1. Jenssen S, Schere D. Treatment and management of epilepsy in the elderly demented patient. Am J Alzheimers Dis Other Demen 2010; 25(1): 18 -26. 2. Mendez MF, Lim GT. Seizures in elderly patients with dementia. Drugs & Aging 2003; 20(11): 791 -803. 3. Moeller JJ, Sadler RM. Seizures and Epilepsy [Internet]. Rx. Tx. 2015 [cited 2016 Sep 26]. Available from: http: //www. e-therapeutics. ca. 4. Glauser T, Ben-Menachem E, Bourgeois B, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2013; 54(3): 551 -63. 5. Cumbo E, Ligori LD. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease. Epilepsy & Behavior 2010; 17: 461 -466. 6. Werhahn KJ, Trinka E, Dobesberger J, et al. A randomized, double-blind comparison of antiepileptic drug treatment in the elderly with new-onset focal epilepsy. Epilepsia 2015; 56(3): 450 -459. 7. Repchinsky C, editor. Keppra monograph. Compendium of Pharmaceuticals and Specialties [Internet]. Ottawa: Canadian Pharmacists Association; 2016 [cited 2016 Sep 25]. Available from: http: //www. etherapeutics. ca. 8. Ferrlazo E, Sueri C, Gasparini S, et al. Challenges in the pharmacological management of epilepsy and its cause in the elderly. Pharmacological Research 2016; 106: 21 -26.

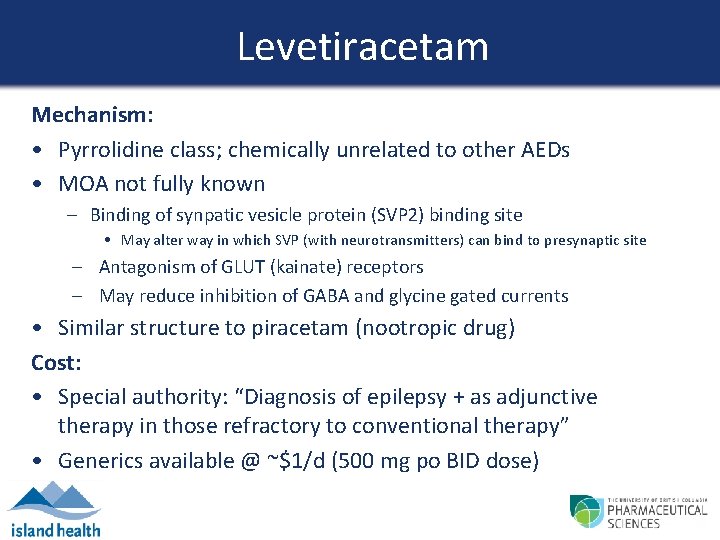
Levetiracetam Mechanism: • Pyrrolidine class; chemically unrelated to other AEDs • MOA not fully known – Binding of synpatic vesicle protein (SVP 2) binding site • May alter way in which SVP (with neurotransmitters) can bind to presynaptic site – Antagonism of GLUT (kainate) receptors – May reduce inhibition of GABA and glycine gated currents • Similar structure to piracetam (nootropic drug) Cost: • Special authority: “Diagnosis of epilepsy + as adjunctive therapy in those refractory to conventional therapy” • Generics available @ ~$1/d (500 mg po BID dose)

Lamotrigine Mechanism: • Blocks V-gated Na+ channels at high firing frequencies and antagonizes kainate receptors to decrease GLUT release Cost: • BC Pharmacare coverage • Special authority: Plan G with diagnosis of bipolar disorder • Generics available @ ~$0. 40 -0. 80/d (50 -100 mg po BID dose)

Werhahn et al – Titration • 6 week titration period – 6 week chosen to accommodate slower titration requirements with LTG • Target doses (selected based on observational studies in elderly) – LEV: 1000 mg/day (given as BID) – LTG: 100 mg/day (given as BID) – CBZ: 400 mg/day (given as BID) • Capsules: LEV 250 mg, LTG 25 mg, CBZ 100 mg • Schedule – 1 cap x 2 weeks, then 2 caps x 2 weeks, then 3 caps x 1 week, then 4 caps thereafter

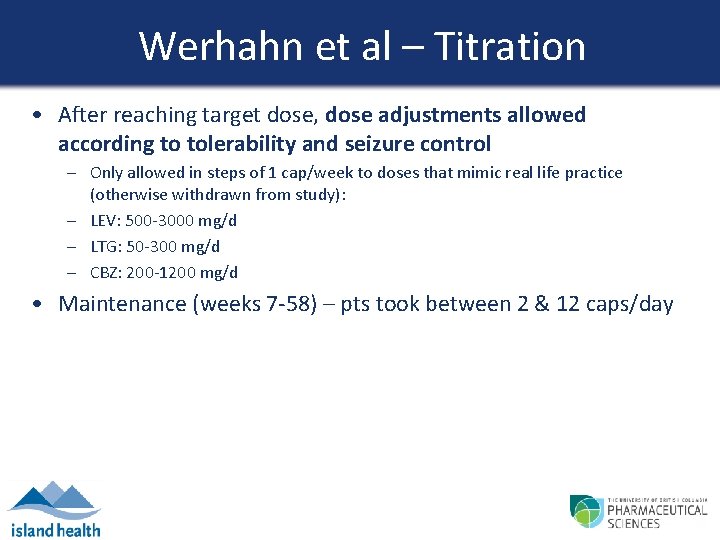
Werhahn et al – Titration • After reaching target dose, dose adjustments allowed according to tolerability and seizure control – Only allowed in steps of 1 cap/week to doses that mimic real life practice (otherwise withdrawn from study): – LEV: 500 -3000 mg/d – LTG: 50 -300 mg/d – CBZ: 200 -1200 mg/d • Maintenance (weeks 7 -58) – pts took between 2 & 12 caps/day

Werhahn et al – Exit Criteria • • Patient request Clinically significant change in medical condition Terminal illness ICU admission Artificial ventilation use Status epilepticus Benzo use x ≥ 2 for control of cluster seizures Non compliance
 Best practices for seizing electronic evidence
Best practices for seizing electronic evidence Invertebrate digestive system
Invertebrate digestive system Ddo seizing the dawn
Ddo seizing the dawn Opportunity assessment plan sample
Opportunity assessment plan sample Chapter 4 business ethics and social responsibility
Chapter 4 business ethics and social responsibility Chapter 24 lesson 2 preventing and treating stds
Chapter 24 lesson 2 preventing and treating stds Behaviourist approach to explaining phobias
Behaviourist approach to explaining phobias Tumor treating fields mechanism of action
Tumor treating fields mechanism of action Treating customers fairly training
Treating customers fairly training Cognitive approach to treating depression
Cognitive approach to treating depression Bulk heat treatment
Bulk heat treatment Ethical issues in treating lgbt patients
Ethical issues in treating lgbt patients Gems diamond geriatric
Gems diamond geriatric Community geriatric psychiatry
Community geriatric psychiatry Geriatric giant
Geriatric giant Gems assessment
Gems assessment Geriatric giant
Geriatric giant Centrum geriatric
Centrum geriatric Geriatric case presentation
Geriatric case presentation Frailty syndrome
Frailty syndrome Objectives of elderly care
Objectives of elderly care Geriatric giants
Geriatric giants Palmerston north geriatric
Palmerston north geriatric Geriatric giants mnemonic
Geriatric giants mnemonic Geriatric nutrition
Geriatric nutrition Gems diamond geriatric
Gems diamond geriatric Va geriatric scholars program
Va geriatric scholars program Hannah seymour
Hannah seymour Sarc f
Sarc f Geriatric hat trick
Geriatric hat trick What is geriatric syndromes
What is geriatric syndromes Geriatric competency assessment
Geriatric competency assessment 10:5 meeting the needs of the elderly
10:5 meeting the needs of the elderly Geriatric syndrome
Geriatric syndrome Geriatric psychiatry definition
Geriatric psychiatry definition Migraine vs epilepsy
Migraine vs epilepsy Epilepsy society of southern ny
Epilepsy society of southern ny Ne regional epilepsy group
Ne regional epilepsy group Rolandic epilepsy icd 10
Rolandic epilepsy icd 10 Convagran
Convagran Vanderbilt neurology residents
Vanderbilt neurology residents Epilepsy
Epilepsy
































































