SEDATIVE HYPNOTICS Introduction Sedatives Drugs that have an


- Slides: 36

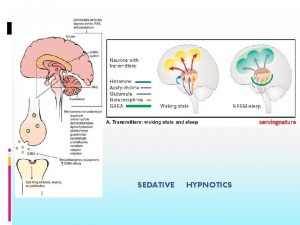
SEDATIVE HYPNOTICS




Introduction Sedatives Drugs that have an inhibitory effect on the CNS to the degree that they reduce: Nervousness Excitability Irritability without causing sleep Hypnotics Calm or soothe CNS to the point that they cause sleep A sedative can become a hypnotic if it is given in large enough doses

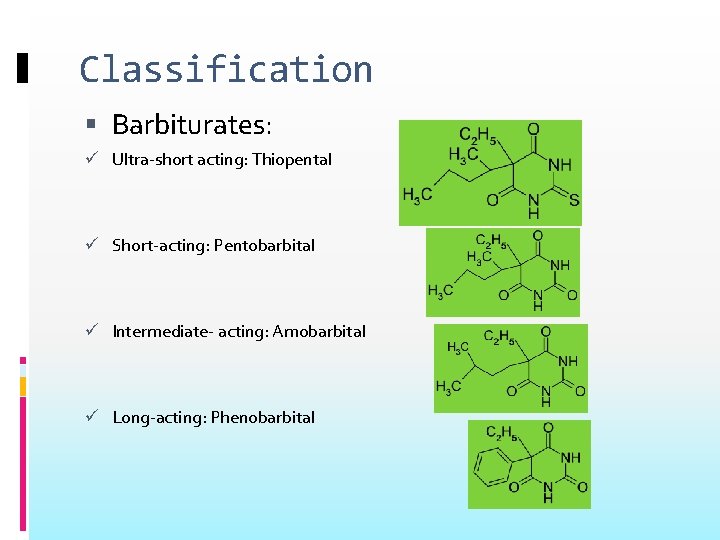
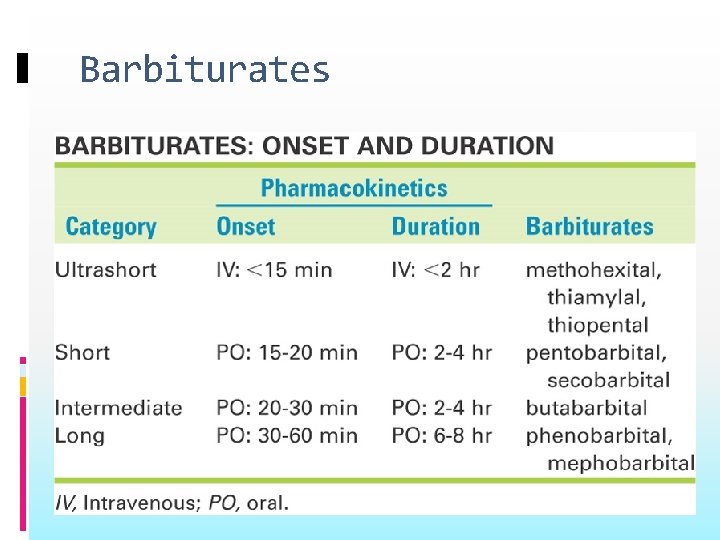
Classification Barbiturates: Ultra-short acting: Thiopental Short-acting: Pentobarbital Intermediate- acting: Amobarbital Long-acting: Phenobarbital

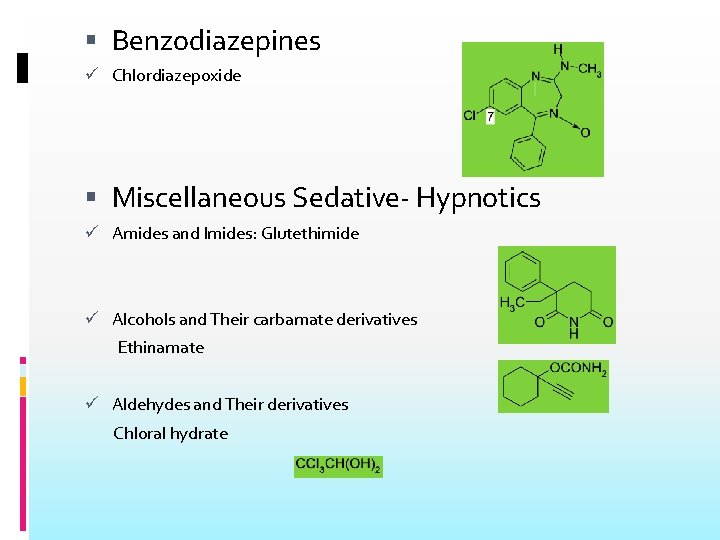
Benzodiazepines Chlordiazepoxide Miscellaneous Sedative- Hypnotics Amides and Imides: Glutethimide Alcohols and Their carbamate derivatives Ethinamate Aldehydes and Their derivatives Chloral hydrate

Benzodiazepine Indication Drug effect Sedation Calming effect on the CNS Sleep induction Useful in controlling agitation and anxiety Skeletal muscle relaxation Anxiety relief Reduce excessive sensory stimulation, inducing sleep Treatment of alcohol withdrawal Induce skeletal muscle relaxation Agitation Depression Epilepsy Balanced anesthesia

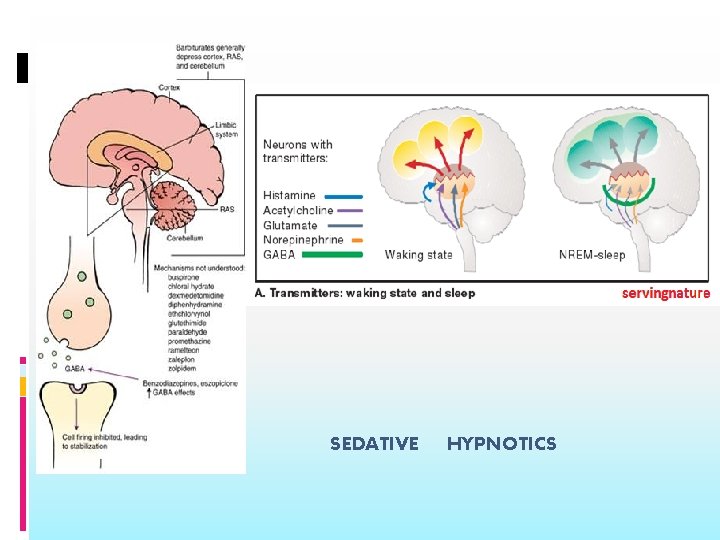
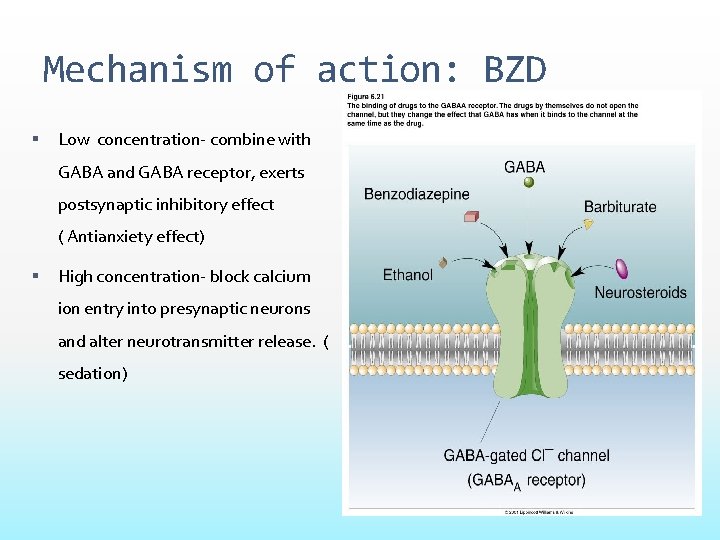
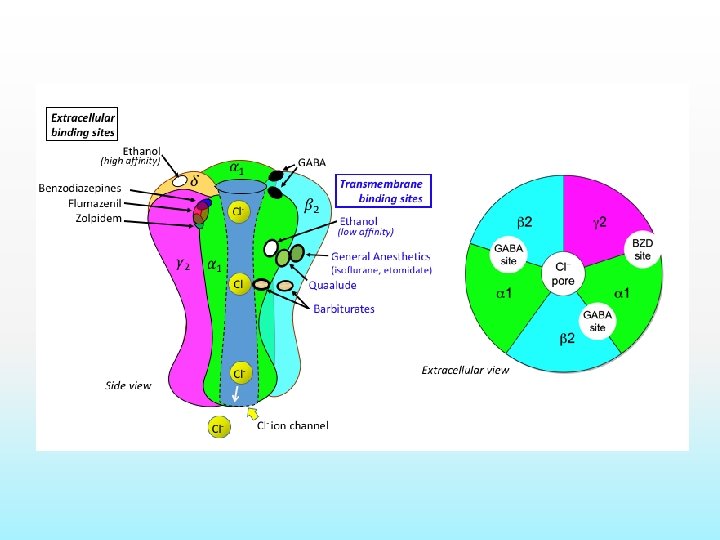
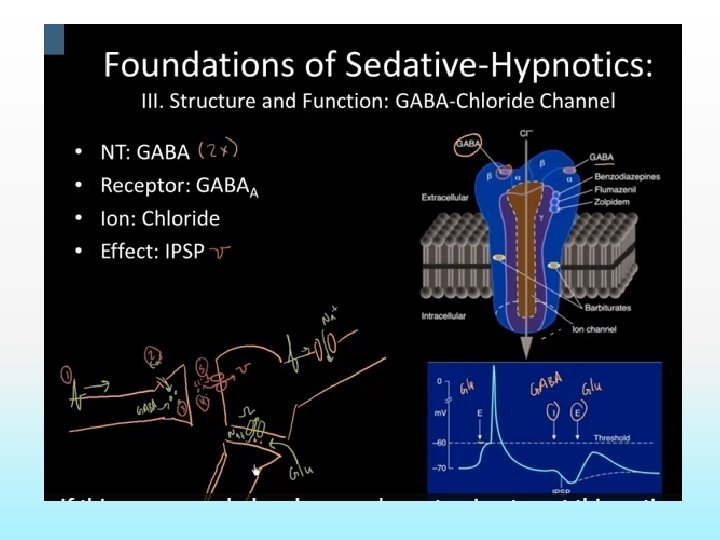
Mechanism of action: BZD Low concentration- combine with GABA and GABA receptor, exerts postsynaptic inhibitory effect ( Antianxiety effect) High concentration- block calcium ion entry into presynaptic neurons and alter neurotransmitter release. ( sedation)



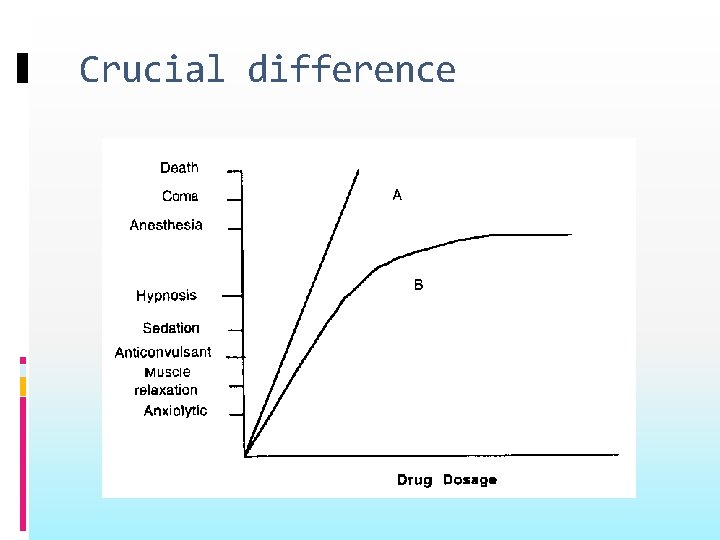
Crucial difference

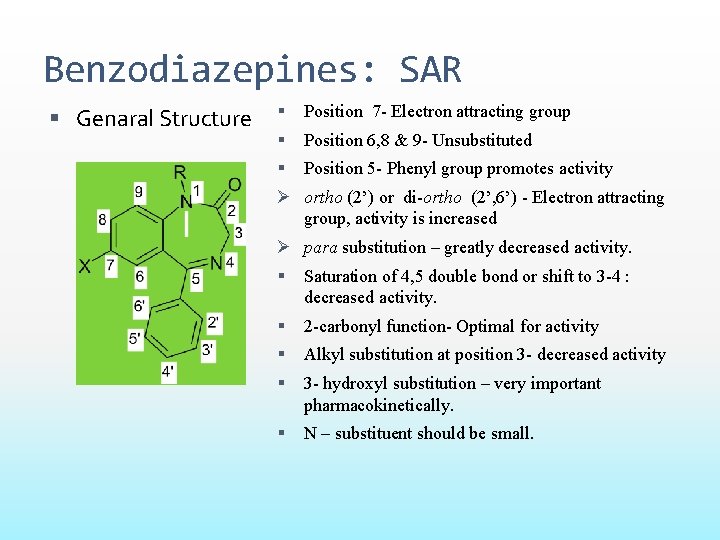
Benzodiazepines: SAR Genaral Structure Position 7 - Electron attracting group Position 6, 8 & 9 - Unsubstituted Position 5 - Phenyl group promotes activity Ø ortho (2’) or di-ortho (2’, 6’) - Electron attracting group, activity is increased Ø para substitution – greatly decreased activity. Saturation of 4, 5 double bond or shift to 3 -4 : decreased activity. 2 -carbonyl function- Optimal for activity Alkyl substitution at position 3 - decreased activity 3 - hydroxyl substitution – very important pharmacokinetically. N – substituent should be small.

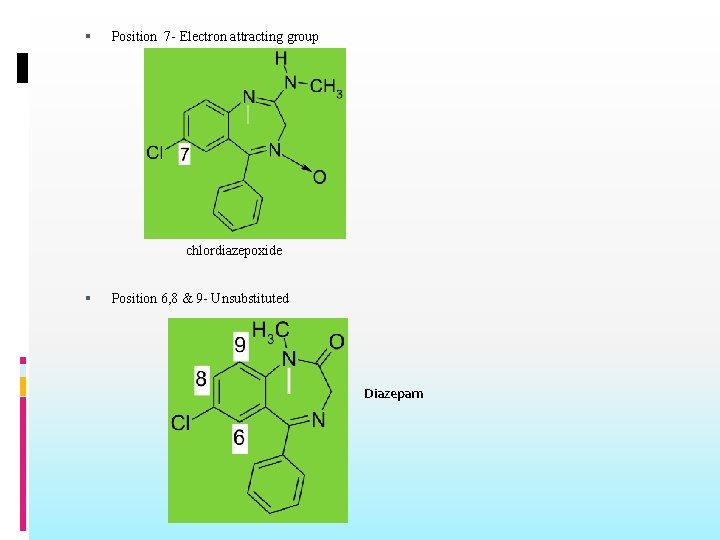
Position 7 - Electron attracting group chlordiazepoxide Position 6, 8 & 9 - Unsubstituted Diazepam

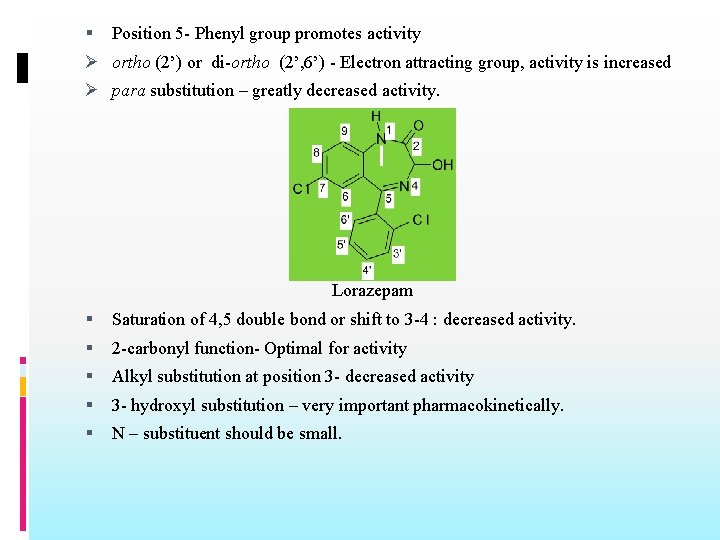
Position 5 - Phenyl group promotes activity Ø ortho (2’) or di-ortho (2’, 6’) - Electron attracting group, activity is increased Ø para substitution – greatly decreased activity. Lorazepam Saturation of 4, 5 double bond or shift to 3 -4 : decreased activity. 2 -carbonyl function- Optimal for activity Alkyl substitution at position 3 - decreased activity 3 - hydroxyl substitution – very important pharmacokinetically. N – substituent should be small.

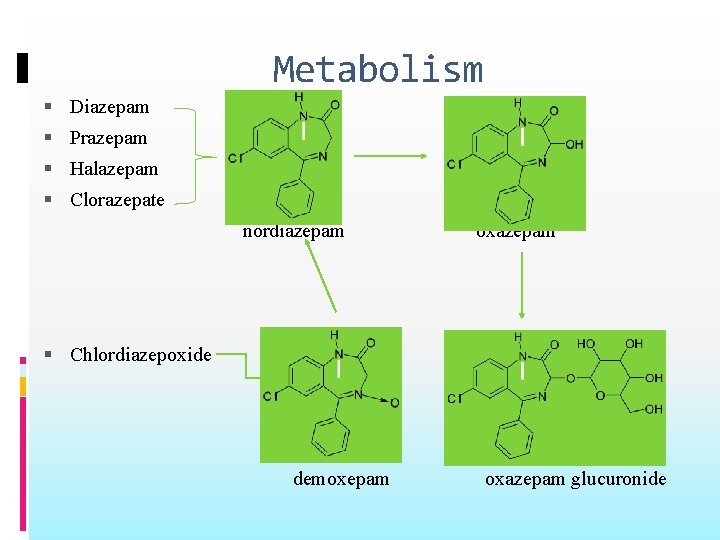
Metabolism Diazepam Prazepam Halazepam Clorazepate nordiazepam oxazepam Chlordiazepoxide demoxepam oxazepam glucuronide

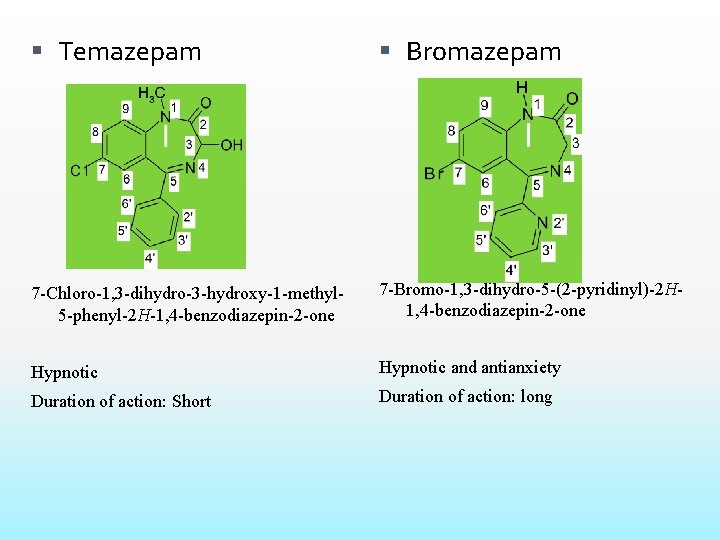
Temazepam Bromazepam 7 -Chloro-1, 3 -dihydro-3 -hydroxy-1 -methyl 5 -phenyl-2 H-1, 4 -benzodiazepin-2 -one 7 -Bromo-1, 3 -dihydro-5 -(2 -pyridinyl)-2 H 1, 4 -benzodiazepin-2 -one Hypnotic and antianxiety Duration of action: Short Duration of action: long

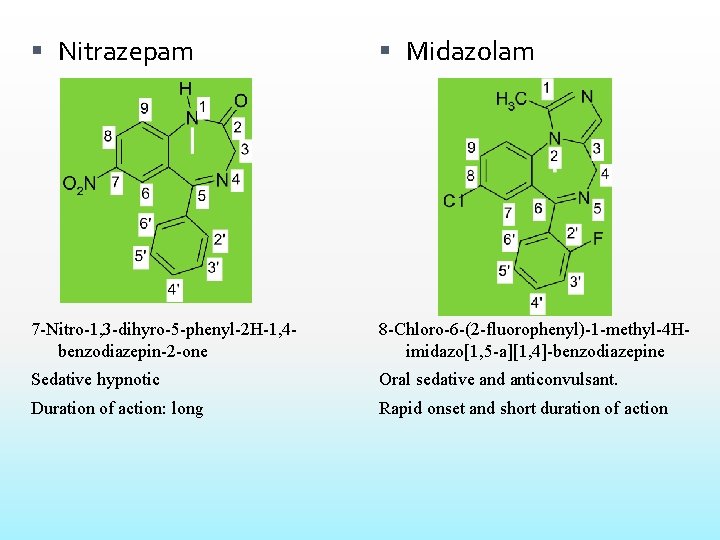
Nitrazepam Midazolam 7 -Nitro-1, 3 -dihyro-5 -phenyl-2 H-1, 4 benzodiazepin-2 -one 8 -Chloro-6 -(2 -fluorophenyl)-1 -methyl-4 Himidazo[1, 5 -a][1, 4]-benzodiazepine Sedative hypnotic Oral sedative and anticonvulsant. Duration of action: long Rapid onset and short duration of action

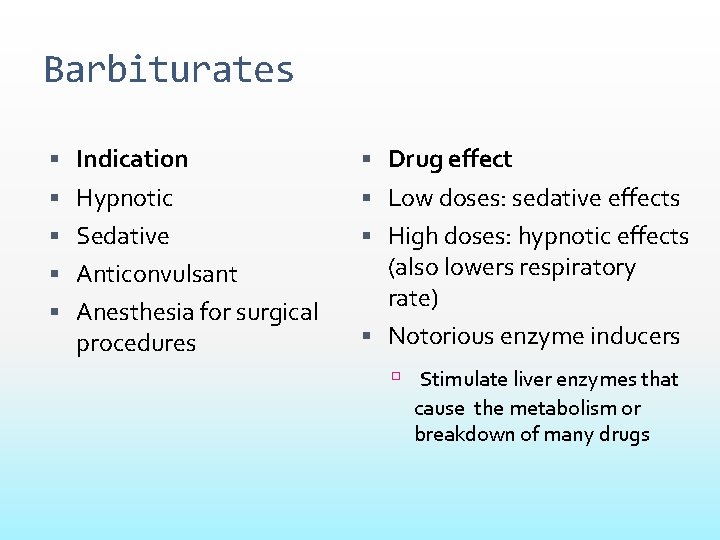
Barbiturates Indication Drug effect Hypnotic Low doses: sedative effects Sedative High doses: hypnotic effects (also lowers respiratory rate) Anticonvulsant Anesthesia for surgical procedures Notorious enzyme inducers Stimulate liver enzymes that cause the metabolism or breakdown of many drugs

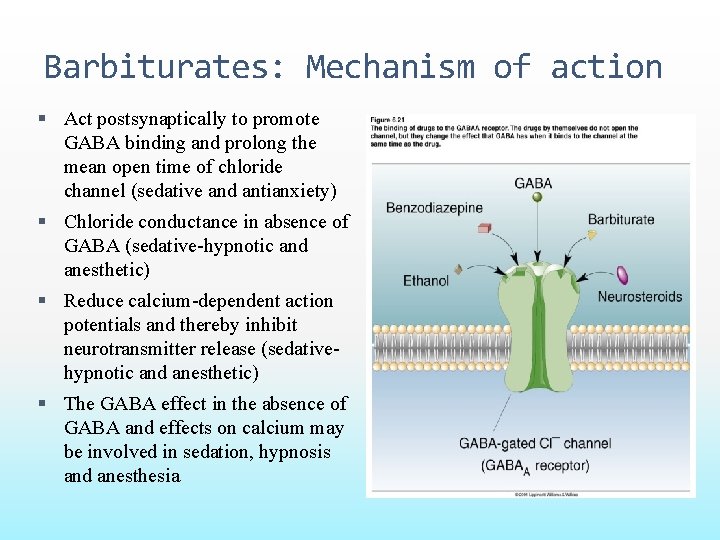
Barbiturates: Mechanism of action Act postsynaptically to promote GABA binding and prolong the mean open time of chloride channel (sedative and antianxiety) Chloride conductance in absence of GABA (sedative-hypnotic and anesthetic) Reduce calcium-dependent action potentials and thereby inhibit neurotransmitter release (sedativehypnotic and anesthetic) The GABA effect in the absence of GABA and effects on calcium may be involved in sedation, hypnosis and anesthesia

Barbiturates

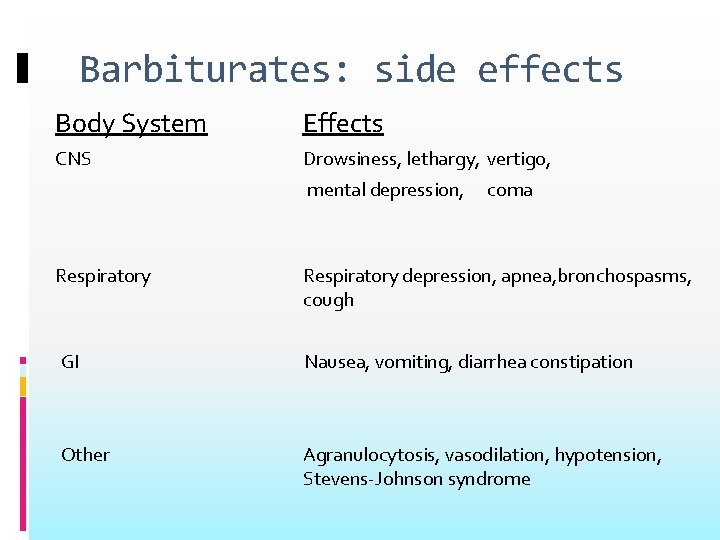
Barbiturates: side effects Body System Effects CNS Drowsiness, lethargy, vertigo, mental depression, coma Respiratory depression, apnea, bronchospasms, cough GI Nausea, vomiting, diarrhea constipation Other Agranulocytosis, vasodilation, hypotension, Stevens-Johnson syndrome

Barbiturates: Toxicity and Overdose frequently leads to respiratory depression, and subsequently, respiratory arrest Overdose produces CNS depression (sleep to coma and death) Can be therapeutic Anesthesia induction Uncontrollable seizures: “phenobarbital coma”

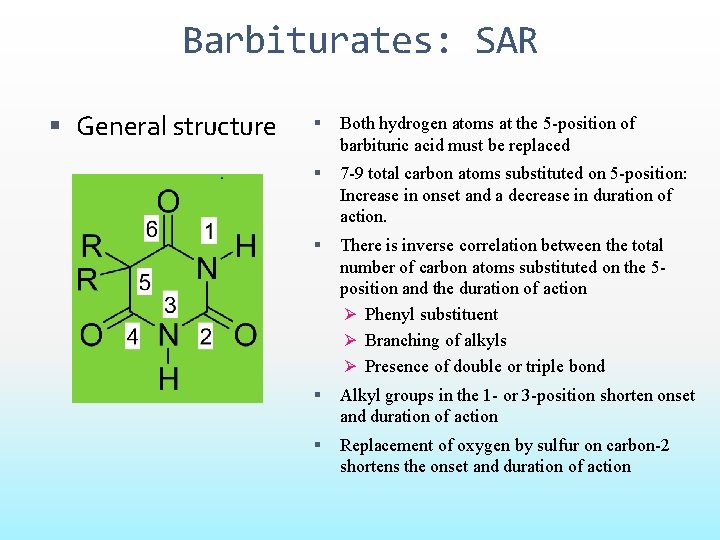
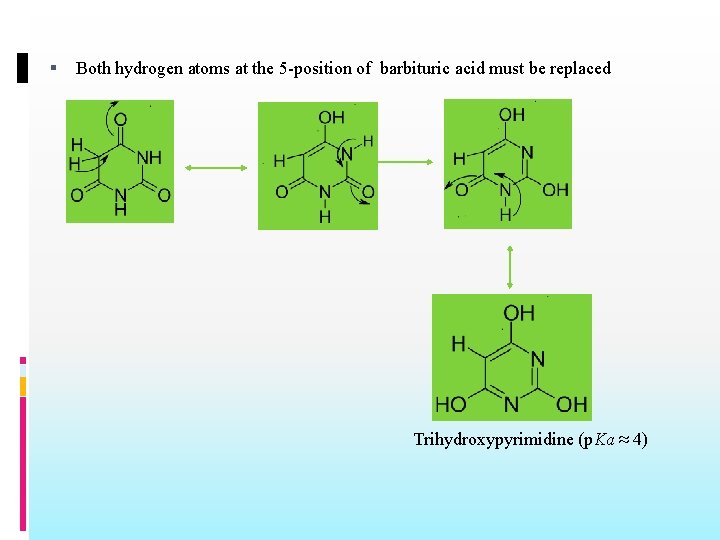
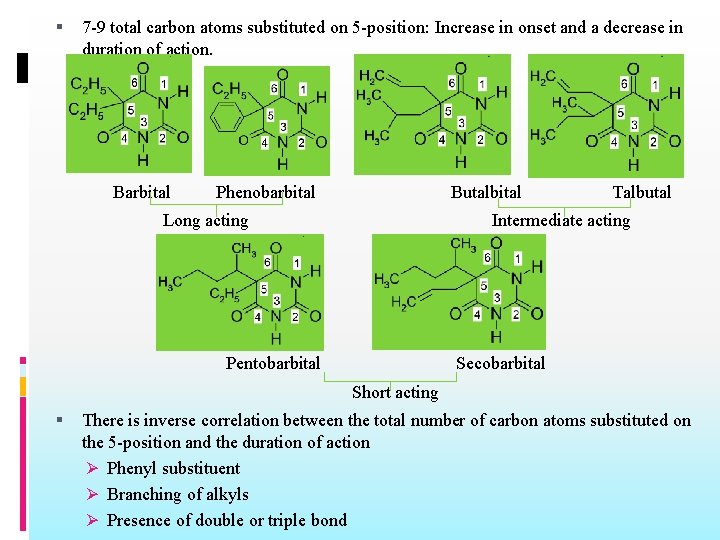
Barbiturates: SAR General structure Both hydrogen atoms at the 5 -position of barbituric acid must be replaced 7 -9 total carbon atoms substituted on 5 -position: Increase in onset and a decrease in duration of action. There is inverse correlation between the total number of carbon atoms substituted on the 5 position and the duration of action Ø Phenyl substituent Ø Branching of alkyls Ø Presence of double or triple bond Alkyl groups in the 1 - or 3 -position shorten onset and duration of action Replacement of oxygen by sulfur on carbon-2 shortens the onset and duration of action

Both hydrogen atoms at the 5 -position of barbituric acid must be replaced Trihydroxypyrimidine (p. Ka ≈ 4)

7 -9 total carbon atoms substituted on 5 -position: Increase in onset and a decrease in duration of action. Barbital Phenobarbital Butalbital Long acting Talbutal Intermediate acting Pentobarbital Secobarbital Short acting There is inverse correlation between the total number of carbon atoms substituted on the 5 -position and the duration of action Ø Phenyl substituent Ø Branching of alkyls Ø Presence of double or triple bond

Alkyl groups in the 1 - or 3 -position shorten onset and duration of action Metharbital Hexobarbital Replacement of oxygen by sulfur on carbon-2 shortens the onset and duration of action Ultra short acting Thiopental

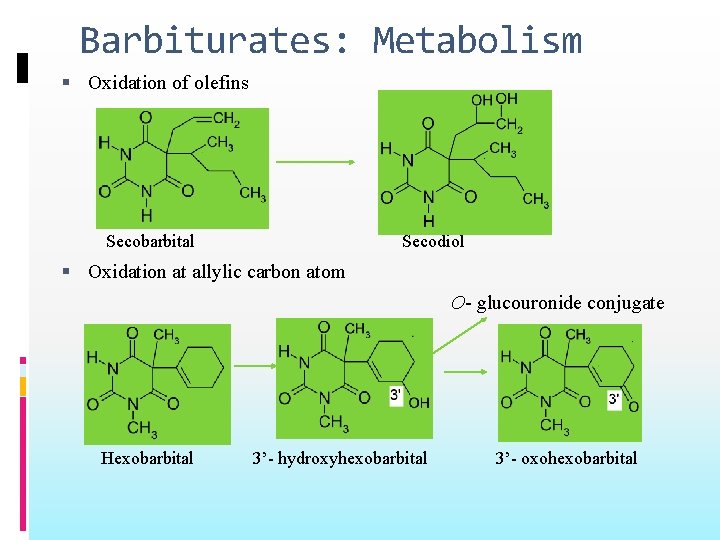
Barbiturates: Metabolism Oxidation of olefins Secobarbital Secodiol Oxidation at allylic carbon atom O- glucouronide conjugate Hexobarbital 3’- hydroxyhexobarbital 3’- oxohexobarbital

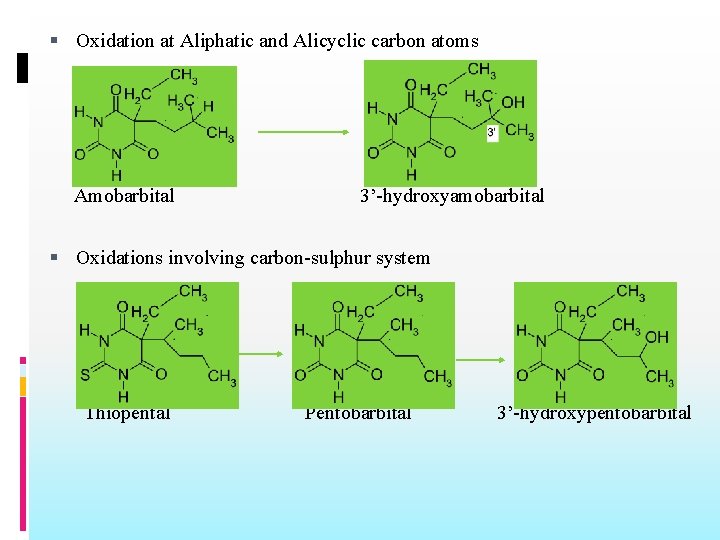
Oxidation at Aliphatic and Alicyclic carbon atoms Amobarbital 3’-hydroxyamobarbital Oxidations involving carbon-sulphur system Thiopental Pentobarbital 3’-hydroxypentobarbital

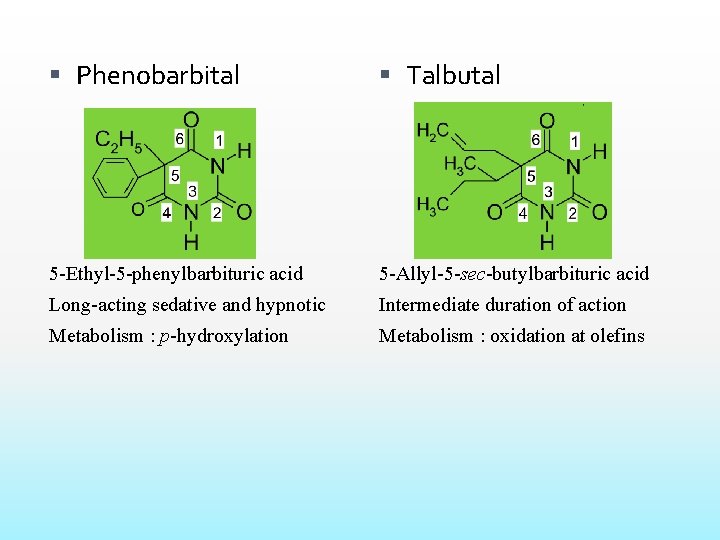
Phenobarbital Talbutal 5 -Ethyl-5 -phenylbarbituric acid 5 -Allyl-5 -sec-butylbarbituric acid Long-acting sedative and hypnotic Intermediate duration of action Metabolism : p-hydroxylation Metabolism : oxidation at olefins

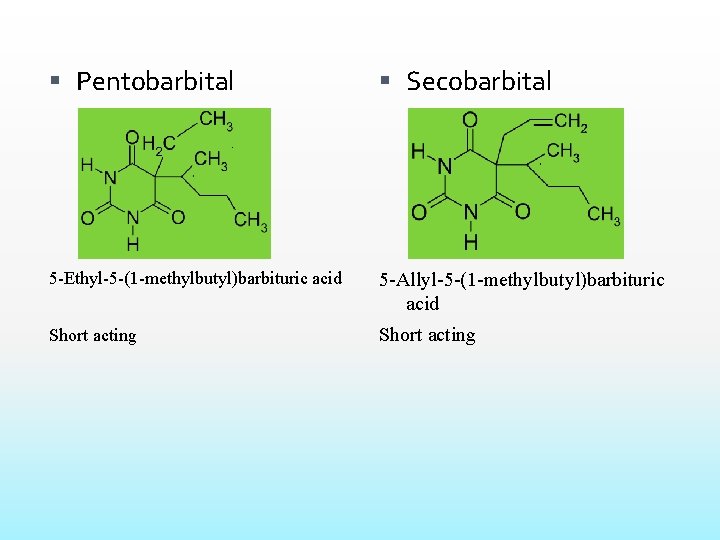
Pentobarbital Secobarbital 5 -Ethyl-5 -(1 -methylbutyl)barbituric acid 5 -Allyl-5 -(1 -methylbutyl)barbituric acid Short acting

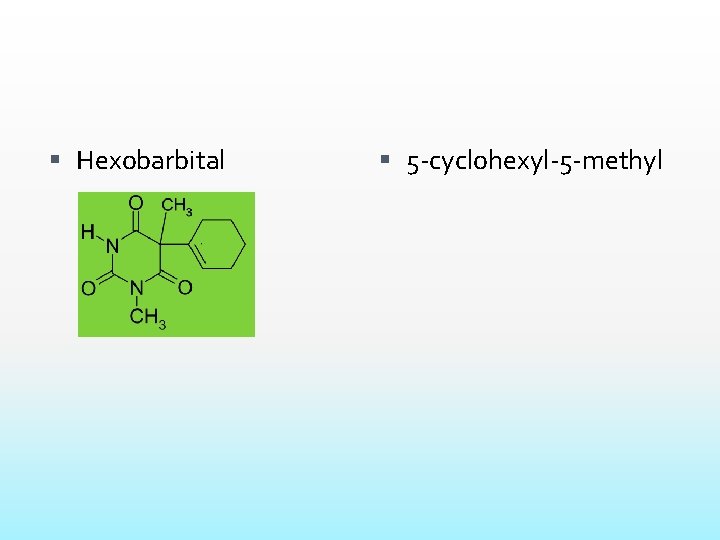
Hexobarbital 5 -cyclohexyl-5 -methyl

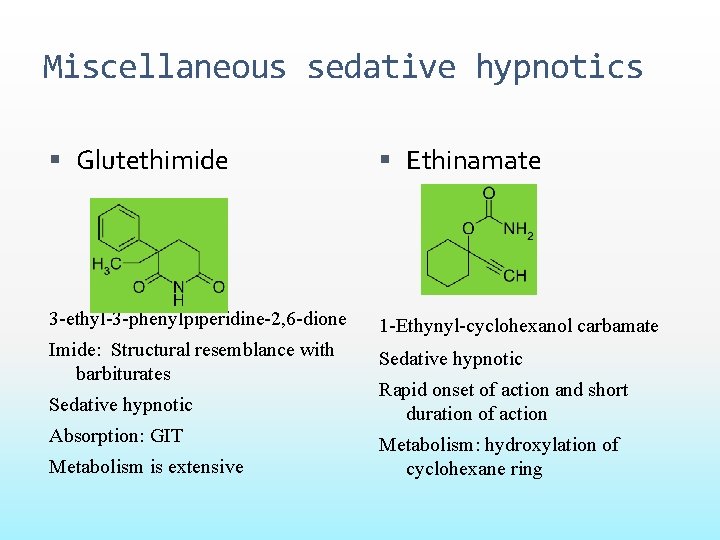
Miscellaneous sedative hypnotics Glutethimide Ethinamate 3 -ethyl-3 -phenylpiperidine-2, 6 -dione 1 -Ethynyl-cyclohexanol carbamate Imide: Structural resemblance with barbiturates Sedative hypnotic Absorption: GIT Metabolism is extensive Rapid onset of action and short duration of action Metabolism: hydroxylation of cyclohexane ring

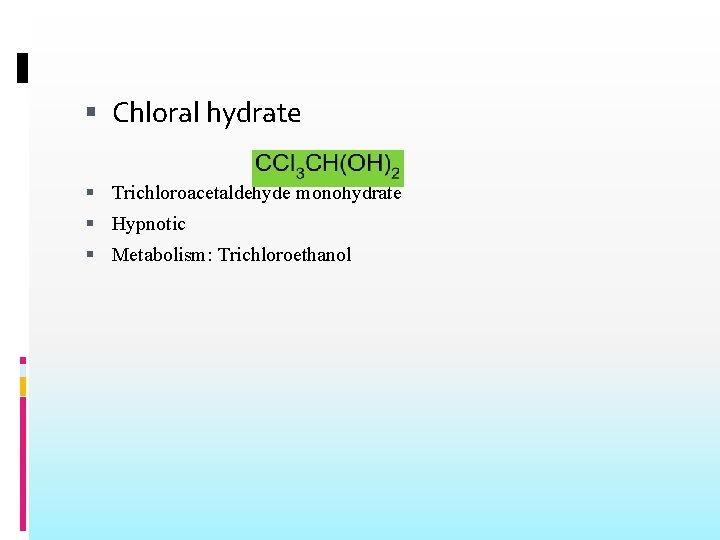
Chloral hydrate Trichloroacetaldehyde monohydrate Hypnotic Metabolism: Trichloroethanol

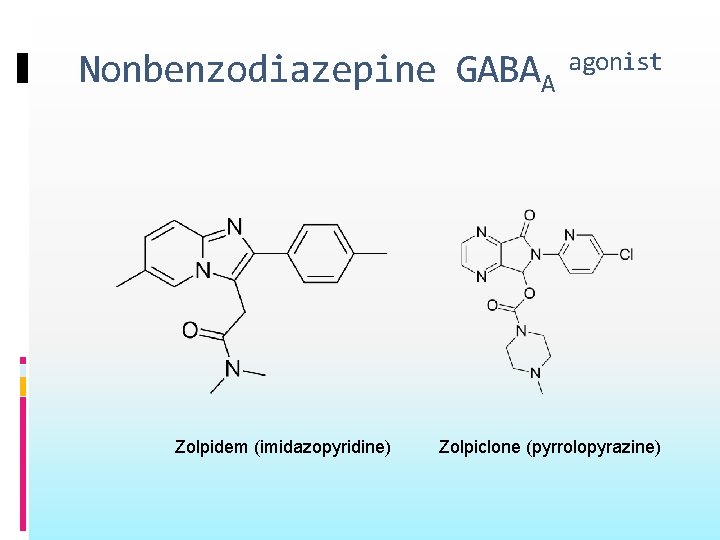
Nonbenzodiazepine GABAA Zolpidem (imidazopyridine) agonist Zolpiclone (pyrrolopyrazine)

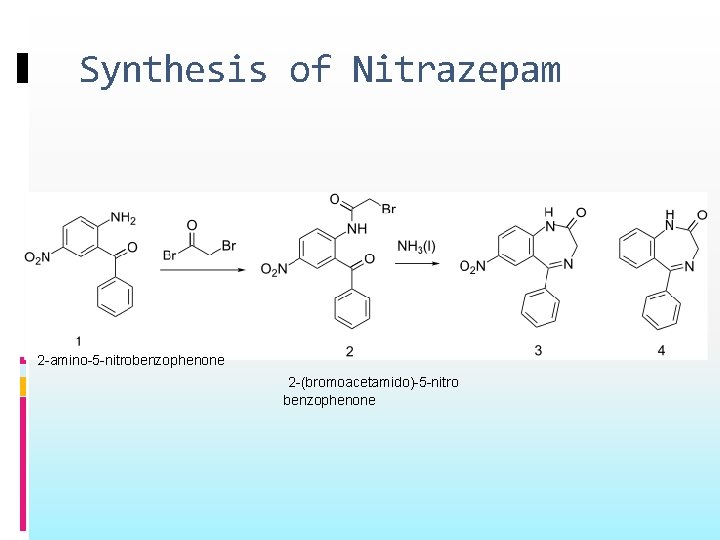
Synthesis of Nitrazepam 2 -amino-5 -nitrobenzophenone 2 -(bromoacetamido)-5 -nitro benzophenone
 Sedative drugs
Sedative drugs Bloodborne sedative
Bloodborne sedative Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Block xoang nhĩ độ 2 type 1
Block xoang nhĩ độ 2 type 1 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt It has 6 square faces 12 edges and vertices
It has 6 square faces 12 edges and vertices Words have meaning and names have power
Words have meaning and names have power Does congress have the power to stop mail on saturdays
Does congress have the power to stop mail on saturdays Making suggestions with modals + verbs
Making suggestions with modals + verbs It is not you they are rejecting but me
It is not you they are rejecting but me I have decided i have resolved
I have decided i have resolved Ideas have consequences bad ideas have victims
Ideas have consequences bad ideas have victims Past simple
Past simple Some animals are dangerous *
Some animals are dangerous * Modal + be + past participle
Modal + be + past participle Echinoderm means spiny skin
Echinoderm means spiny skin I have a dream introduction
I have a dream introduction Seed paragraph
Seed paragraph Body paragraph
Body paragraph Ccna drugs
Ccna drugs Schedule 1 drugs
Schedule 1 drugs Liothyronine
Liothyronine Fibrinolytic drugs classification
Fibrinolytic drugs classification Fibrinolytic agents
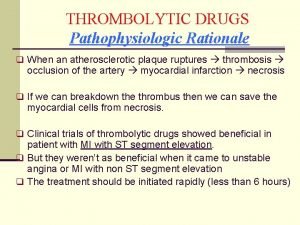
Fibrinolytic agents Thrombolytic drugs
Thrombolytic drugs Thrombolytic drugs mechanism of action
Thrombolytic drugs mechanism of action Fibrinolytic drugs
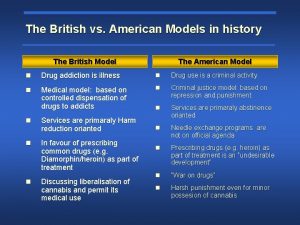
Fibrinolytic drugs British model involved with drugs
British model involved with drugs Difference between catecholamines and noncatecholamines
Difference between catecholamines and noncatecholamines Sar of anticholinergic drugs
Sar of anticholinergic drugs