Sample Exercise 8 1 Identifying Conjugate Acids and
![Sample Exercise 8. 4 Calculating [H+] for Pure Water Calculate the values of [H+] Sample Exercise 8. 4 Calculating [H+] for Pure Water Calculate the values of [H+]](https://slidetodoc.com/presentation_image_h2/36223c3fd92aaa348d1bb831e941e8ba/image-4.jpg)
![Sample Exercise 8. 5 Calculating [H+] from [OH-] Calculate the concentration of H+(aq) in Sample Exercise 8. 5 Calculating [H+] from [OH-] Calculate the concentration of H+(aq) in](https://slidetodoc.com/presentation_image_h2/36223c3fd92aaa348d1bb831e941e8ba/image-5.jpg)
![Sample Exercise 8. 6 Calculating p. H from [H+] Calculate the p. H values Sample Exercise 8. 6 Calculating p. H from [H+] Calculate the p. H values](https://slidetodoc.com/presentation_image_h2/36223c3fd92aaa348d1bb831e941e8ba/image-6.jpg)
![Sample Exercise 8. 7 Calculating [H+] from p. H A sample of freshly pressed Sample Exercise 8. 7 Calculating [H+] from p. H A sample of freshly pressed](https://slidetodoc.com/presentation_image_h2/36223c3fd92aaa348d1bb831e941e8ba/image-7.jpg)










![Sample Exercise 8. 23 Preparing a Buffer Solution (Continued) [NH 4+] must equal 0. Sample Exercise 8. 23 Preparing a Buffer Solution (Continued) [NH 4+] must equal 0.](https://slidetodoc.com/presentation_image_h2/36223c3fd92aaa348d1bb831e941e8ba/image-39.jpg)





- Slides: 57

Sample Exercise 8. 1 Identifying Conjugate Acids and Bases (a) What is the conjugate base of each of the following acids: HCl. O 4, H 2 S, PH 4+, HCO 3–? (b) What is the conjugate acid of each of the following bases: CN–, SO 42–, H 2 O, HCO 3– ? Solution Analyze: We are asked to give the conjugate base for each of a series of species and to give the conjugate acid for each of another series of species. Plan: The conjugate base of a substance is simply the parent substance minus one proton, and the conjugate acid of a substance is the parent substance plus one proton. Solve: (a) HCl. O 4 less one proton (H+) is Cl. O 4–. The other conjugate bases are HS–, PH 3, and CO 32–. (b) CN– plus one proton (H+) is HCN. The other conjugate acids are HSO 4–, H 3 O+, and H 2 CO 3. Notice that the hydrogen carbonate ion (HCO 3–) is amphiprotic. It can act as either an acid or a base. Practice Exercise Write the formula for the conjugate acid of each of the following: HSO 3–, F– , PO 43–, CO. Answers: H 2 SO 3, HF, HPO 42–, HCO+ Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 2 Writing Equations for Proton-Transfer Reactions The hydrogen sulfite ion (HSO 3–) is amphiprotic. (a) Write an equation for the reaction of HSO 3– with water, in which the ion acts as an acid. (b) Write an equation for the reaction of HSO 3– with water, in which the ion acts as a base. In both cases identify the conjugate acid–base pairs. Solution Analyze and Plan: We are asked to write two equations representing reactions between HSO 3– and water, one in which HSO 3– should donate a proton to water, thereby acting as a Brønsted–Lowry acid, and one in which HSO 3– should accept a proton from water, thereby acting as a base. We are also asked to identify the conjugate pairs in each equation. Solve: The conjugate pairs in this equation are HSO 3– (acid) and SO 32– (conjugate base); and H 2 O (base) and H 3 O+ (conjugate acid). The conjugate pairs in this equation are H 2 O (acid) and OH– (conjugate base), and HSO 3– (base) and H 2 SO 3 (conjugate acid). Practice Exercise When lithium oxide (Li 2 O) is dissolved in water, the solution turns basic from the reaction of the oxide ion (O 2 –) with water. Write the reaction that occurs, and identify the conjugate acid–base pairs. Answer: O 2–(aq) + H 2 O(l) → OH–(aq) + OH–(aq). OH– is the conjugate acid of the base O 2–. OH– is also the conjugate base of the acid H 2 O. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 3 Predicting the Position of a Proton-Transfer Equilibrium For the following proton-transfer reaction, use Figure 16. 4 to predict whether the equilibrium lies predominantly to the left (that is, Kc < 1 ) or to the right (Kc > 1): Solution Analyze: We are asked to predict whether the equilibrium shown lies to the right, favoring products, or to the left, favoring reactants. Plan: This is a proton-transfer reaction, and the position of the equilibrium will favor the proton going to the stronger of two bases. The two bases in the equation are CO 32–, the base in the forward reaction as written, and SO 42– the conjugate base of HSO 4–. We can find the relative positions of these two bases in Figure 8. 1 to determine which is the stronger base. Solve: CO 32– appears lower in the right-hand column in Figure 16. 4 and is therefore a stronger base than SO 42–. CO 32–, therefore, will get the proton preferentially to become HCO 3–, while SO 42– will remain mostly unprotonated. The resulting equilibrium will lie to the right, favoring products (that is, Kc > 1 ). Comment: Of the two acids in the equation, HSO 4– and HCO 3–, the stronger one gives up a proton more readily while the weaker one tends to retain its proton. Thus, the equilibrium favors the direction in which the proton moves from the stronger acid and becomes bonded to the stronger base. Practice Exercise For each of the following reactions, use Figure 16. 4 to predict whether the equilibrium lies predominantly to the left or to the right: Answers: (a) left, (b) right Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
![Sample Exercise 8 4 Calculating H for Pure Water Calculate the values of H Sample Exercise 8. 4 Calculating [H+] for Pure Water Calculate the values of [H+]](https://slidetodoc.com/presentation_image_h2/36223c3fd92aaa348d1bb831e941e8ba/image-4.jpg)
Sample Exercise 8. 4 Calculating [H+] for Pure Water Calculate the values of [H+] and [OH-] in a neutral solution at 25 ºC. Solution Analyze: We are asked to determine the concentrations of H+ and OH– ions in a neutral solution at 25 ºC. Plan: We will use Equation 16. 16 and the fact that, by definition, [H+] = [OH–] in a neutral solution. Solve: We will represent the concentration of [H+] and [OH–] in neutral solution with x. This gives In an acid solution [H+] is greater than ; 1. 0 × 10– 7 M in a basic solution [H+] is less than 1. 0 × 10– 7 M. Practice Exercise Indicate whether solutions with each of the following ion concentrations are neutral, acidic, or basic: (a) [H+] = 4 × 10– 9 M ; (b) [H+] = 4 × 10– 9 M ; (c) [OH–] = 7 × 10– 13 M. Answers: (a) basic, (b) neutral, (c) acidic Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
![Sample Exercise 8 5 Calculating H from OH Calculate the concentration of Haq in Sample Exercise 8. 5 Calculating [H+] from [OH-] Calculate the concentration of H+(aq) in](https://slidetodoc.com/presentation_image_h2/36223c3fd92aaa348d1bb831e941e8ba/image-5.jpg)
Sample Exercise 8. 5 Calculating [H+] from [OH-] Calculate the concentration of H+(aq) in (a) a solution in which [OH–] is 0. 010 M, (b) a solution in which [OH –] is 1. 8 × 10– 9 M. Note: In this problem and all that follow, we assume, unless stated otherwise, that the temperature is 25 ºC. Solution Analyze: We are asked to calculate the hydronium ion concentration in an aqueous solution where the hydroxide concentration is known. Plan: We can use the equilibrium-constant expression for the autoionization of water and the value of Kw to solve for each unknown concentration. Solve: a) Using Equation 16. 16, we have: This solution is basic because (b) In this instance This solution is acidic because Practice Exercise Calculate the concentration of OH–(aq) in a solution in which (a) [H+] = 2 × 10– 6 M; (b) [H+] = [OH–]; (c) [H+] = 100× [OH–]. Answers: (a) 5 × 10– 9 M, (b) 1. 0 × 10– 7 M, (c) 1. 0 × 10– 8 M Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
![Sample Exercise 8 6 Calculating p H from H Calculate the p H values Sample Exercise 8. 6 Calculating p. H from [H+] Calculate the p. H values](https://slidetodoc.com/presentation_image_h2/36223c3fd92aaa348d1bb831e941e8ba/image-6.jpg)
Sample Exercise 8. 6 Calculating p. H from [H+] Calculate the p. H values for the two solutions described in Sample Exercise 8. 5. Solution Analyze: We are asked to determine the p. H of aqueous solutions for which we have already calculated [H +]. Plan: We can calculate p. H using its defining equation, Equation 16. 17. Solve: (a) In the first instance we found [H+]. to be 1. 0 × 10– 12 M. Because 1. 0 × 10– 12 has two significant figures, the p. H has two decimal places, 12. 00. (b) For the second solution, [H+] = 5. 6 × 10– 6 M. Before performing the calculation, it is helpful to estimate the p. H. To do so, we note that [H+] lies between 1 × 10– 6 and 1 × 10– 5 Thus, we expect the p. H to lie between 6. 0 and 5. 0. We use Equation 16. 17 to calculate the p. H. Check: After calculating a p. H, it is useful to compare it to your prior estimate. In this case the p. H, as we predicted, falls between 6 and 5. Had the calculated p. H and the estimate not agreed, we should have reconsidered our calculation or estimate or both. Practice Exercise (a) In a sample of lemon juice [H+] is 3. 8 × 10– 4 M. What is the p. H? (b) A commonly available windowcleaning solution has [OH–] = 1. 9 × 10– 6 M. What is the p. H? Answers: (a) 3. 42, (b) [H+] = 5. 3 × 10– 9 M, so p. H = 8. 28 Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
![Sample Exercise 8 7 Calculating H from p H A sample of freshly pressed Sample Exercise 8. 7 Calculating [H+] from p. H A sample of freshly pressed](https://slidetodoc.com/presentation_image_h2/36223c3fd92aaa348d1bb831e941e8ba/image-7.jpg)
Sample Exercise 8. 7 Calculating [H+] from p. H A sample of freshly pressed apple juice has a p. H of 3. 76. Calculate [H+]. Solution Analyze: We need to calculate [H+] from p. H. Plan: We will use Equation 16. 17, p. H = –log[H+], for the calculation. Solve: From Equation 16. 17, we have Thus, To find [H+] , we need to determine the antilog of – 3. 76. Scientific calculators have an antilog function (sometimes labeled INV log or 10 x) that allows us to perform the calculation: Comment: Consult the user’s manual for your calculator to find out how to perform the antilog operation. The number of significant figures in [H+] is two because the number of decimal places in the p. H is two. Check: Because the p. H is between 3. 0 and 4. 0, we know that [H+] will be between 1 × 10– 3 and 1 × 10– 4 M. Our calculated [H+] falls within this estimated range. Practice Exercise A solution formed by dissolving an antacid tablet has a p. H of 9. 18. Calculate [H+]. Answer: [H+] = 6. 6× 10– 10 M Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 8 Calculating the p. H of a Strong Acid What is the p. H of a 0. 040 M solution of HCl. O 4? Solution Analyze and Plan: Because HCl. O 4 is a strong acid, it is completely ionized, giving [H+] = [Cl. O 4 -] = 0. 040 M Solve: The p. H of the solution is given by p. H = –log(0. 040) = 1. 40. Check: Because [H+] lies between 1 × 10– 2 and 1 × 10– 1, the p. H will be between 2. 0 and 1. 0. Our calculated p. H falls within the estimated range. Furthermore, because the concentration has two significant figures, the p. H has two decimal places. Practice Exercise An aqueous solution of HNO 3 has a p. H of 2. 34. What is the concentration of the acid? Answer: 0. 0046 M Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 9 Calculating the p. H of a Strong Base What is the p. H of (a) a 0. 028 M solution of Na. OH, (b) a 0. 0011 M solution of Ca(OH)2? Solution Analyze: We are asked to calculate the p. H of two solutions of strong bases. Plan: We can calculate each p. H by either of two equivalent methods. First, we could use Equation 16. 16 to calculate [H+] and then use Equation 16. 17 to calculate the p. H. Alternatively, we could use [OH–] to calculate p. OH and then use Equation 16. 20 to calculate the p. H. Solve: (a) Na. OH dissociates in water to give one OH– ion per formula unit. Therefore, the OH– concentration for the solution in (a) equals the stated concentration of Na. OH, namely 0. 028 M. (b) Ca(OH)2 is a strong base that dissociates in water to give two OH– ions per formula unit. Thus, the concentration of OH–(aq) for the solution in part (b) is 2 × (0. 0011 M) = 0. 0022 M Practice Exercise What is the concentration of a solution of (a) KOH for which the p. H is 11. 89; (b) Ca(OH)2 for which the p. H is 11. 68? Answers: (a) 7. 8 × 10– 3 M, (b) 2. 4 × 10– 3 M Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

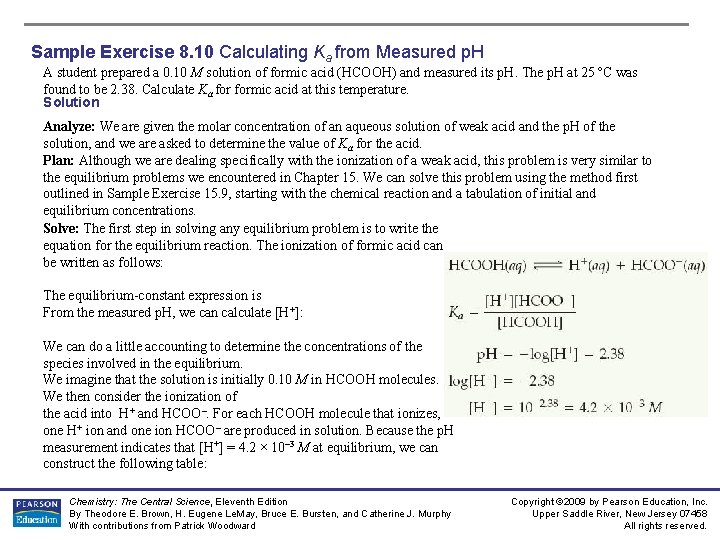
Sample Exercise 8. 10 Calculating Ka from Measured p. H A student prepared a 0. 10 M solution of formic acid (HCOOH) and measured its p. H. The p. H at 25 ºC was found to be 2. 38. Calculate Ka formic acid at this temperature. Solution Analyze: We are given the molar concentration of an aqueous solution of weak acid and the p. H of the solution, and we are asked to determine the value of Ka for the acid. Plan: Although we are dealing specifically with the ionization of a weak acid, this problem is very similar to the equilibrium problems we encountered in Chapter 15. We can solve this problem using the method first outlined in Sample Exercise 15. 9, starting with the chemical reaction and a tabulation of initial and equilibrium concentrations. Solve: The first step in solving any equilibrium problem is to write the equation for the equilibrium reaction. The ionization of formic acid can be written as follows: The equilibrium-constant expression is From the measured p. H, we can calculate [H+]: We can do a little accounting to determine the concentrations of the species involved in the equilibrium. We imagine that the solution is initially 0. 10 M in HCOOH molecules. We then consider the ionization of the acid into H+ and HCOO–. For each HCOOH molecule that ionizes, one H+ ion and one ion HCOO– are produced in solution. Because the p. H measurement indicates that [H+] = 4. 2 × 10– 3 M at equilibrium, we can construct the following table: Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 10 Calculating Ka from Measured p. H Solution (Continued) Notice that we have neglected the very small concentration of H+(aq) that is due to the autoionization of H 2 O. Notice also that the amount of HCOOH that ionizes is very small compared with the initial concentration of the acid. To the number of significant figures we are using, the subtraction yields 0. 10 M: We can now insert the equilibrium centrations into the expression for Ka: Check: The magnitude of our answer is reasonable because Ka for a weak acid is usually between 10– 3 and 10– 10. Practice Exercise Niacin, one of the B vitamins, has the following molecular structure: A 0. 020 M solution of niacin has a p. H of 3. 26. What is the acid-dissociation constant, Ka, for niacin? Answers: 1. 5 × 10– 5 Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

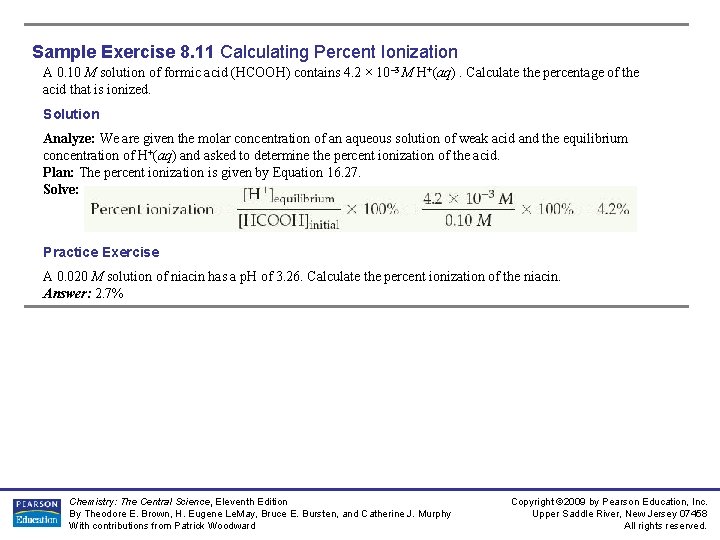
Sample Exercise 8. 11 Calculating Percent Ionization A 0. 10 M solution of formic acid (HCOOH) contains 4. 2 × 10– 3 M H+(aq). Calculate the percentage of the acid that is ionized. Solution Analyze: We are given the molar concentration of an aqueous solution of weak acid and the equilibrium concentration of H+(aq) and asked to determine the percent ionization of the acid. Plan: The percent ionization is given by Equation 16. 27. Solve: Practice Exercise A 0. 020 M solution of niacin has a p. H of 3. 26. Calculate the percent ionization of the niacin. Answer: 2. 7% Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

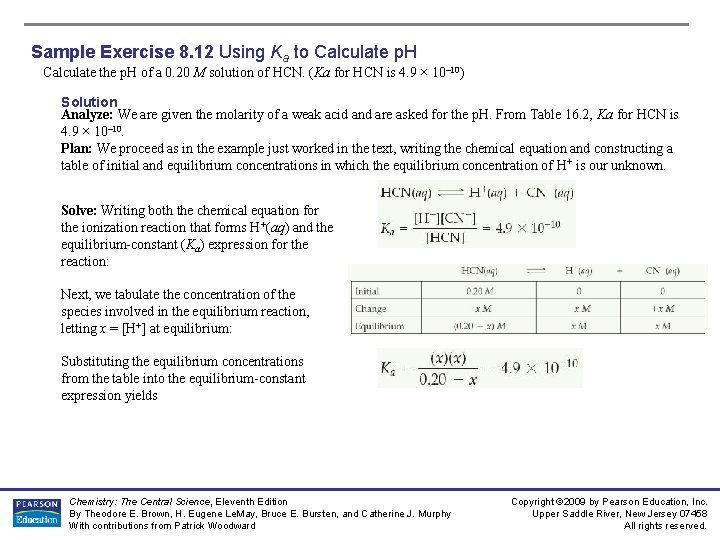
Sample Exercise 8. 12 Using Ka to Calculate p. H Calculate the p. H of a 0. 20 M solution of HCN. (Ka for HCN is 4. 9 × 10– 10) Solution Analyze: We are given the molarity of a weak acid and are asked for the p. H. From Table 16. 2, Ka for HCN is 4. 9 × 10– 10. Plan: We proceed as in the example just worked in the text, writing the chemical equation and constructing a table of initial and equilibrium concentrations in which the equilibrium concentration of H + is our unknown. Solve: Writing both the chemical equation for the ionization reaction that forms H+(aq) and the equilibrium-constant (Ka) expression for the reaction: Next, we tabulate the concentration of the species involved in the equilibrium reaction, letting x = [H+] at equilibrium: Substituting the equilibrium concentrations from the table into the equilibrium-constant expression yields Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

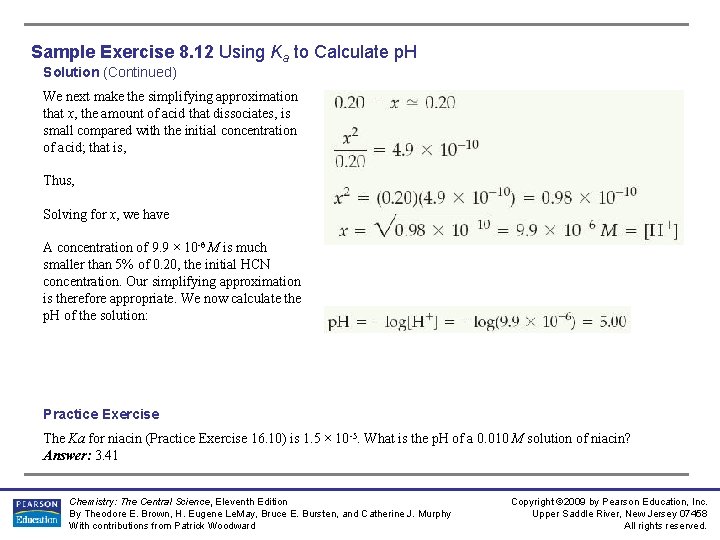
Sample Exercise 8. 12 Using Ka to Calculate p. H Solution (Continued) We next make the simplifying approximation that x, the amount of acid that dissociates, is small compared with the initial concentration of acid; that is, Thus, Solving for x, we have A concentration of 9. 9 × 10 -6 M is much smaller than 5% of 0. 20, the initial HCN concentration. Our simplifying approximation is therefore appropriate. We now calculate the p. H of the solution: Practice Exercise The Ka for niacin (Practice Exercise 16. 10) is 1. 5 × 10 -5. What is the p. H of a 0. 010 M solution of niacin? Answer: 3. 41 Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

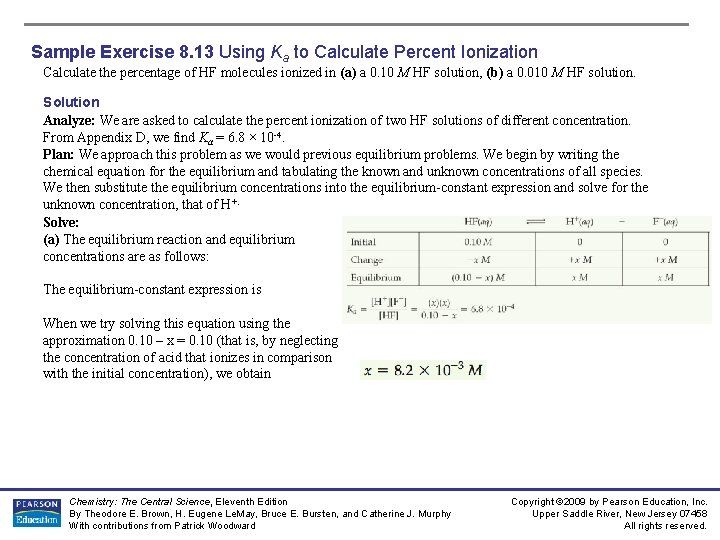
Sample Exercise 8. 13 Using Ka to Calculate Percent Ionization Calculate the percentage of HF molecules ionized in (a) a 0. 10 M HF solution, (b) a 0. 010 M HF solution. Solution Analyze: We are asked to calculate the percent ionization of two HF solutions of different concentration. From Appendix D, we find Ka = 6. 8 × 10 -4. Plan: We approach this problem as we would previous equilibrium problems. We begin by writing the chemical equation for the equilibrium and tabulating the known and unknown concentrations of all species. We then substitute the equilibrium concentrations into the equilibrium-constant expression and solve for the unknown concentration, that of H+. Solve: (a) The equilibrium reaction and equilibrium concentrations are as follows: The equilibrium-constant expression is When we try solving this equation using the approximation 0. 10 – x = 0. 10 (that is, by neglecting the concentration of acid that ionizes in comparison with the initial concentration), we obtain Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

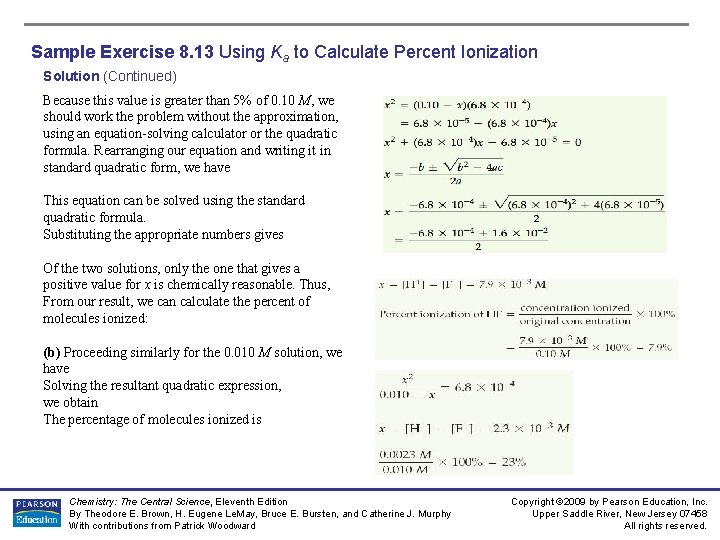
Sample Exercise 8. 13 Using Ka to Calculate Percent Ionization Solution (Continued) Because this value is greater than 5% of 0. 10 M, we should work the problem without the approximation, using an equation-solving calculator or the quadratic formula. Rearranging our equation and writing it in standard quadratic form, we have This equation can be solved using the standard quadratic formula. Substituting the appropriate numbers gives Of the two solutions, only the one that gives a positive value for x is chemically reasonable. Thus, From our result, we can calculate the percent of molecules ionized: (b) Proceeding similarly for the 0. 010 M solution, we have Solving the resultant quadratic expression, we obtain The percentage of molecules ionized is Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 13 Using Ka to Calculate Percent Ionization Solution (Continued) Comment: Notice that if we do not use the quadratic formula to solve the problem properly, we calculate 8. 2% ionization for (a) and 26% ionization for (b). Notice also that in diluting the solution by a factor of 10, the percentage of molecules ionized increases by a factor of 3. This result is in accord with what we see in Figure 16. 9. It is also what we would expect from Le Châtelier’s principle. (Section 15. 7) There are more “particles” or reaction components on the right side of the equation than on the left. Dilution causes the reaction to shift in the direction of the larger number of particles because this counters the effect of the decreasing concentration of particles. Practice Exercise In Practice Exercise 8. 11, we found that the percent ionization of niacin (Ka = 1. 5 × 10 -5) in a 0. 020 M solution is 2. 7%. Calculate the percentage of niacin molecules ionized in a solution that is (a) 0. 010 M, (b) 1. 0 × 10 -3 M. Answers: (a) 3. 9%, (b) 12% Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

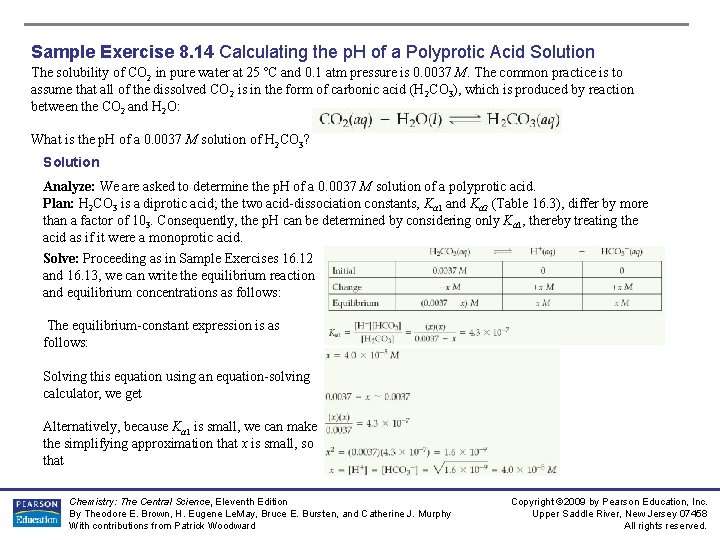
Sample Exercise 8. 14 Calculating the p. H of a Polyprotic Acid Solution The solubility of CO 2 in pure water at 25 ºC and 0. 1 atm pressure is 0. 0037 M. The common practice is to assume that all of the dissolved CO 2 is in the form of carbonic acid (H 2 CO 3), which is produced by reaction between the CO 2 and H 2 O: What is the p. H of a 0. 0037 M solution of H 2 CO 3? Solution Analyze: We are asked to determine the p. H of a 0. 0037 M solution of a polyprotic acid. Plan: H 2 CO 3 is a diprotic acid; the two acid-dissociation constants, Ka 1 and Ka 2 (Table 16. 3), differ by more than a factor of 103. Consequently, the p. H can be determined by considering only Ka 1, thereby treating the acid as if it were a monoprotic acid. Solve: Proceeding as in Sample Exercises 16. 12 and 16. 13, we can write the equilibrium reaction and equilibrium concentrations as follows: The equilibrium-constant expression is as follows: Solving this equation using an equation-solving calculator, we get Alternatively, because Ka 1 is small, we can make the simplifying approximation that x is small, so that Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 14 Calculating the p. H of a Polyprotic Acid Solution (Continued) Thus, Solving for x, we have The small value of x indicates that our simplifying assumption was justified. The p. H is therefore Comment: If we were asked to solve for [CO 32 -], we would need to use Ka 2. Let’s illustrate that calculation. Using the values of [HCO 3–] and [H+] calculated above, and setting [CO 32–] = y, we have the following initial and equilibrium concentration values: Assuming that y is small compared to 4. 0 × 10– 5, we have The value calculated for y is indeed very small compared to 4. 0 × 10 -5, showing that our assumption was justified. It also shows that the ionization of HCO 3– is negligible compared to that of H 2 CO 3, as far as production of H+ is concerned. However, it is the only source of CO 32–, which has a very low concentration in the solution. Our calculations thus tell us that in a solution of carbon dioxide in water, most of the CO 2 is in the form of CO 2 or H 2 CO 3, a small fraction ionizes to form H+ and HCO 3–, and an even smaller fraction ionizes to give CO 32–. Notice also that [CO 32–] is numerically equal to Ka 2. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

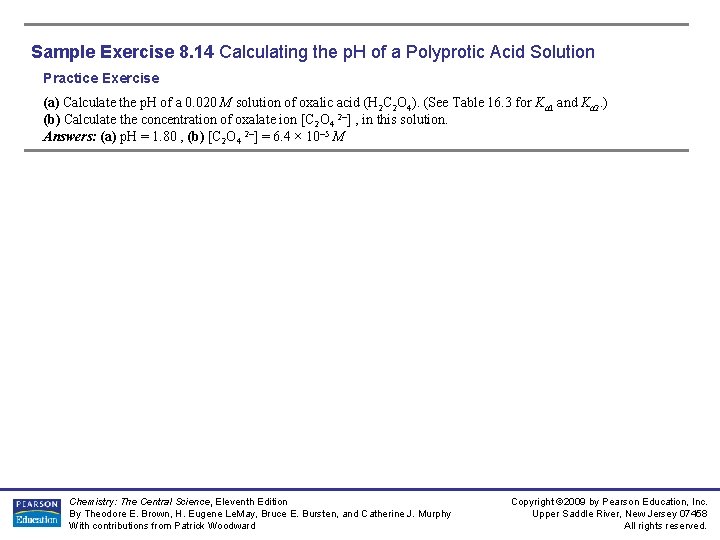
Sample Exercise 8. 14 Calculating the p. H of a Polyprotic Acid Solution Practice Exercise (a) Calculate the p. H of a 0. 020 M solution of oxalic acid (H 2 C 2 O 4). (See Table 16. 3 for Ka 1 and Ka 2. ) (b) Calculate the concentration of oxalate ion [C 2 O 4 2–] , in this solution. Answers: (a) p. H = 1. 80 , (b) [C 2 O 4 2–] = 6. 4 × 10– 5 M Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

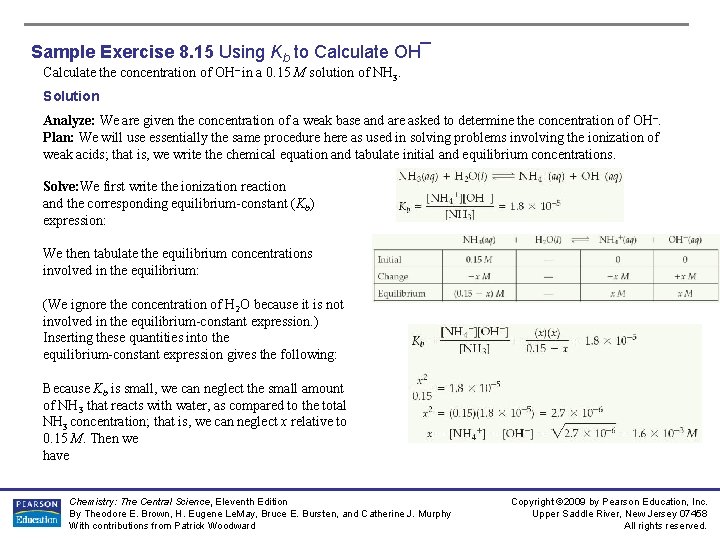
Sample Exercise 8. 15 Using Kb to Calculate OH¯ Calculate the concentration of OH– in a 0. 15 M solution of NH 3. Solution Analyze: We are given the concentration of a weak base and are asked to determine the concentration of OH –. Plan: We will use essentially the same procedure here as used in solving problems involving the ionization of weak acids; that is, we write the chemical equation and tabulate initial and equilibrium concentrations. Solve: We first write the ionization reaction and the corresponding equilibrium-constant (Kb) expression: We then tabulate the equilibrium concentrations involved in the equilibrium: (We ignore the concentration of H 2 O because it is not involved in the equilibrium-constant expression. ) Inserting these quantities into the equilibrium-constant expression gives the following: Because Kb is small, we can neglect the small amount of NH 3 that reacts with water, as compared to the total NH 3 concentration; that is, we can neglect x relative to 0. 15 M. Then we have Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

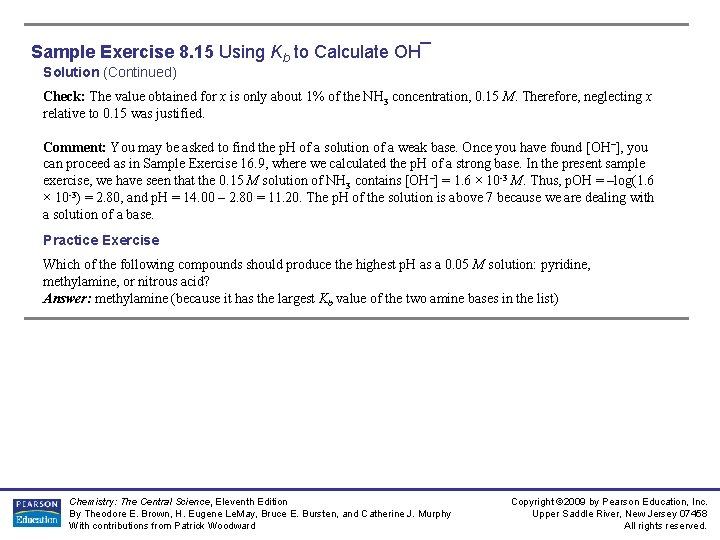
Sample Exercise 8. 15 Using Kb to Calculate OH¯ Solution (Continued) Check: The value obtained for x is only about 1% of the NH 3 concentration, 0. 15 M. Therefore, neglecting x relative to 0. 15 was justified. Comment: You may be asked to find the p. H of a solution of a weak base. Once you have found [OH–], you can proceed as in Sample Exercise 16. 9, where we calculated the p. H of a strong base. In the present sample exercise, we have seen that the 0. 15 M solution of NH 3 contains [OH–] = 1. 6 × 10 -3 M. Thus, p. OH = –log(1. 6 × 10 -3) = 2. 80, and p. H = 14. 00 – 2. 80 = 11. 20. The p. H of the solution is above 7 because we are dealing with a solution of a base. Practice Exercise Which of the following compounds should produce the highest p. H as a 0. 05 M solution: pyridine, methylamine, or nitrous acid? Answer: methylamine (because it has the largest Kb value of the two amine bases in the list) Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

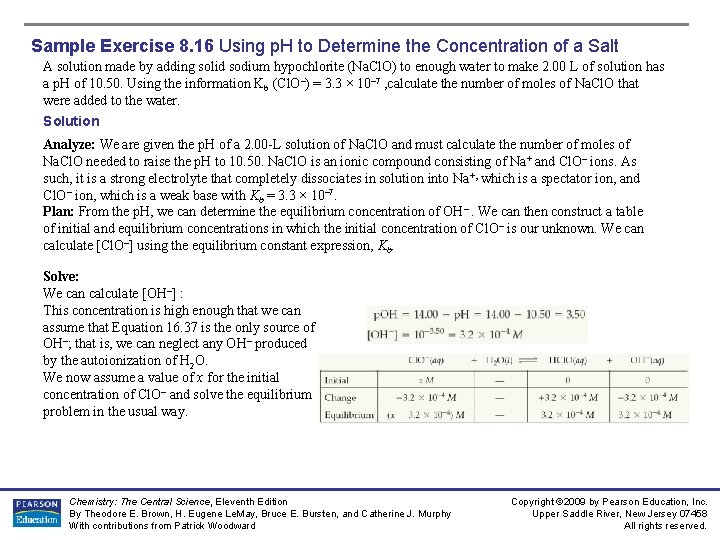
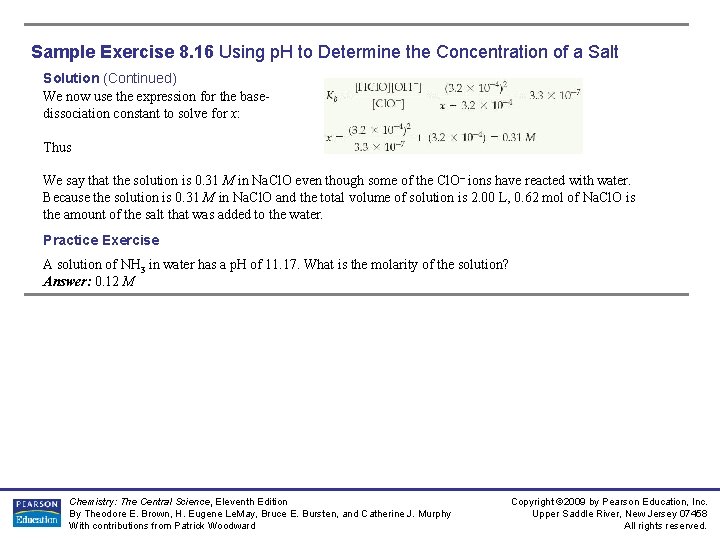
Sample Exercise 8. 16 Using p. H to Determine the Concentration of a Salt A solution made by adding solid sodium hypochlorite (Na. Cl. O) to enough water to make 2. 00 L of solution has a p. H of 10. 50. Using the information Kb (Cl. O–) = 3. 3 × 10– 7 , calculate the number of moles of Na. Cl. O that were added to the water. Solution Analyze: We are given the p. H of a 2. 00 -L solution of Na. Cl. O and must calculate the number of moles of Na. Cl. O needed to raise the p. H to 10. 50. Na. Cl. O is an ionic compound consisting of Na+ and Cl. O– ions. As such, it is a strong electrolyte that completely dissociates in solution into Na +, which is a spectator ion, and Cl. O– ion, which is a weak base with Kb = 3. 3 × 10– 7. Plan: From the p. H, we can determine the equilibrium concentration of OH–. We can then construct a table of initial and equilibrium concentrations in which the initial concentration of Cl. O – is our unknown. We can calculate [Cl. O–] using the equilibrium constant expression, Kb. Solve: We can calculate [OH–] : This concentration is high enough that we can assume that Equation 16. 37 is the only source of OH–; that is, we can neglect any OH– produced by the autoionization of H 2 O. We now assume a value of x for the initial concentration of Cl. O– and solve the equilibrium problem in the usual way. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 16 Using p. H to Determine the Concentration of a Salt Solution (Continued) We now use the expression for the basedissociation constant to solve for x: Thus We say that the solution is 0. 31 M in Na. Cl. O even though some of the Cl. O– ions have reacted with water. Because the solution is 0. 31 M in Na. Cl. O and the total volume of solution is 2. 00 L, 0. 62 mol of Na. Cl. O is the amount of the salt that was added to the water. Practice Exercise A solution of NH 3 in water has a p. H of 11. 17. What is the molarity of the solution? Answer: 0. 12 M Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 17 Calculating Ka or Kb for a Conjugate Acid-Base Pair Calculate (a) the base-dissociation constant, Kb, for the fluoride ion (F–); (b) the acid dissociation constant, Ka, for the ammonium ion (NH 4+). Ka for the acid, HF, is Ka= 6. 8 × 10 -4, Kb for NH 3 is Kb = 1. 8 × 10 -5 Solution Analyze: We are asked to determine dissociation constants for F–, the conjugate base of HF, and NH 4+ , the conjugate acid of NH 3. Plan: Although neither F– nor NH 4+ appears in the tables, we can find the tabulated values for ionization constants for HF and NH 3, and use the relationship between Ka and Kb to calculate the ionization constants for each of the conjugates. Solve: (a) Ka for the weak acid, HF, is Ka = 6. 8 × 10 -4. We can calculate Kb for the conjugate base, F–: (b) Kb for NH 3 is Kb = 1. 8 × 10 -5. , We can calculate Ka for the conjugate acid, NH 4+ : Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 17 Calculating Ka or Kb for a Conjugate Acid-Base Pair Practice Exercise (a) Which of the following anions has the largest base-dissociation constant: NO 2–, PO 43–, or N 3–? (b) The base quinoline has the following structure: Its conjugate acid is listed in handbooks as having a p. Ka of 4. 90. What is the base dissociation constant for quinoline? Answers: (a) PO 43– (Kb = 2. 4 × 10 -2), (b) 7. 9 × 10 -10 Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 18 Determining Whether Salt Solutions Are Acidic, Basic, or Neutral Determine whether aqueous solutions of each of the following salts will be acidic, basic, or neutral: (a) Ba(CH 3 COO)2, (b) NH 4 Cl, (c) CH 3 NH 3 Br, (d) KNO 3, (e) Al(Cl. O 4)3. Solution Analyze: We are given the chemical formulas of five ionic compounds (salts) and asked whether their aqueous solutions will be acidic, basic, or neutral. Plan: We can determine whether a solution of a salt is acidic, basic, or neutral by identifying the ions in solution and by assessing how each ion will affect the p. H. Solve: (a) This solution contains barium ions and acetate ions. The cation, Ba 2+, is an ion of one of the heavy alkaline earth metals and will therefore not affect the p. H (summary point 4). The anion, CH 3 COO–, is the conjugate base of the weak acid CH 3 COOH and will hydrolyze to produce OH– ions, thereby making the solution basic (summary point 2). (b) This solution contains NH 4+ and Cl– ions. NH 4+ is the conjugate acid of a weak base (NH 3) and is therefore acidic (summary point 3). Cl– is the conjugate base of a strong acid (HCl) and therefore has no influence on the p. H of the solution (summary point 1). Because the solution contains an ion that is acidic (NH 4+) and one that has no influence on p. H (Cl–), the solution of NH 4 Cl will be acidic. (c) This solution contains CH 3 NH 3+ and Br– ions. CH 3 NH 3+ is the conjugate acid of a weak base (CH 3 NH 2, an amine) and is therefore acidic (summary point 3). Br – is the conjugate base of a strong acid (HBr) and is therefore p. H-neutral (summary point 1). Because the solution contains one ion that is acidic and one that is neutral, the solution of CH 3 NH 3 Br will be acidic. (d) This solution contains the K+ ion, which is a cation of group 1 A, and the ion NO 3–, which is the conjugate base of the strong acid HNO 3. Neither of the ions will react with water to any appreciable extent (summary points 1 and 4), making the solution neutral. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 18 Determining Whether Salt Solutions Are Acidic, Basic, or Neutral Solution (Continued) (e) This solution contains Al 3+ and Cl. O 4– ions. Cations, such as Al 3+, that are not in groups 1 A or 2 A are acidic (summary point 5). The Cl. O 4– ion is the conjugate base of a strong acid (HCl. O 4) and therefore does not affect p. H (summary point 1). Thus, the solution of Al(Cl. O 4)3 will be acidic. Practice Exercise In each of the following, indicate which salt in each of the following pairs will form the more acidic (or less basic) 0. 010 M solution: (a) Na. NO 3, or Fe(NO 3)3; (b) KBr, or KBr. O; (c) CH 3 NH 3 Cl, or Ba. Cl 2, (d) NH 4 NO 2, or NH 4 NO 3. Answers: (a) Fe(NO 3)3, (b) KBr, (c) CH 3 NH 3 Cl, (d) NH 4 NO 3 Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 19 Predicting Whether the Solution of an Amphiprotic Anion is Acidic or Basic Predict whether the salt Na 2 HPO 4 will form an acidic solution or a basic solution on dissolving in water. Ka (HPO 42 -/PO 43 -) = 4. 2 × 10 -13 , Ka (H 2 PO 4 -/HPO 42 -) = 6. 2 × 10 -8 Solution Analyze: We are asked to predict whether a solution of Na 2 HPO 4 will be acidic or basic. This substance is an ionic compound composed of Na+ and HPO 42– ions. Plan: We need to evaluate each ion, predicting whether each is acidic or basic. Because Na + is a cation of group 1 A, we know that it has no influence on p. H. It is merely a spectator ion in acid–base chemistry. Thus, our analysis of whether the solution is acidic or basic must focus on the behavior of the HPO 42– ion. We need to consider the fact that HPO 42– can act as either an acid or a base. The reaction with the larger equilibrium constant will determine whether the solution is acidic or basic Solve: The value of Ka for Equation 16. 45, , is 4. 2 × 10 -13. We must calculate the value of Kb for Equation 16. 46 from the value of Ka for its conjugate acid, H 2 PO 4–. We make use of the relationship shown in Equation : We want to know Kb for the base HPO 42–, knowing the value of Ka for the conjugate acid HPO 42–: Because Ka for H 2 PO 4– is 6. 2 × 10 -8 , we calculate Kb for H 2 PO 42– to be 1. 6 × 10 -7. This is more than 105 times larger than Ka for H 2 PO 42–; thus, the reaction shown in Equation 16. 46 predominates over that in Equation 16. 45, and the solution will be basic. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 19 Predicting Whether the Solution of an Amphiprotic Anion is Acidic or Basic Practice Exercise Predict whether the dipotassium salt of citric acid (K 2 HC 6 H 5 O 7) will form an acidic or basic solution in water (see Table 16. 3 for data). Answer: acidic Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 20 Calculating the p. H When a Common Ion is Involved What is the p. H of a solution made by adding 0. 30 mol of acetic acid and 0. 30 mol of sodium acetate to enough water to make 1. 0 L of solution? Ka (CH 3 COOH/CH 3 COO– ) = 1. 8 10 -5 Solution Analyze: We are asked to determine the p. H of a solution of a weak electrolyte (CH 3 COOH) and a strong electrolyte (CH 3 COONa) that share a common ion, CH 3 COO–. 1. Consider which solutes are strong electrolytes and which are weak electrolytes, and identify the major species in solution. 2. Identify the important equilibrium that is the source of H+ and therefore determines p. H. 3. Tabulate the concentrations of ions involved in the equilibrium. 4. Use the equilibrium-constant expression to calculate [H+] and then p. H. Solve: First, because CH 3 COOH is a weak electrolyte and CH 3 COONa is a strong electrolyte, the major species in the solution are CH 3 COOH (a weak acid), Na+ (which is neither acidic nor basic and is therefore a spectator in the acid–base chemistry), and CH 3 COO– (which is the conjugate base of CH 3 COOH). Plan: In any problem in which we must determine the p. H of a solution containing a mixture of solutes, it is helpful to proceed by a series of logical steps: Second, [H+] and, therefore, the p. H are controlled by the dissociation equilibrium of CH 3 COOH: (We have written the equilibrium Using H+(aq) rather than H 3 O+(aq) but both representations of the hydrated hydrogen ion are equally valid. ) Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

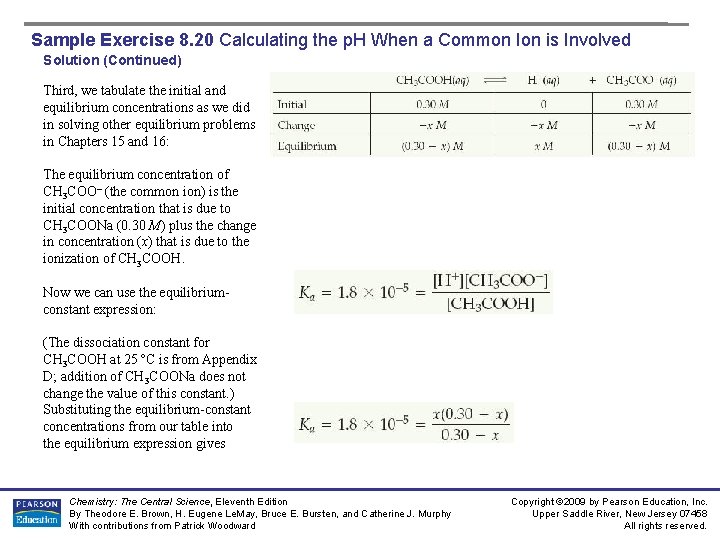
Sample Exercise 8. 20 Calculating the p. H When a Common Ion is Involved Solution (Continued) Third, we tabulate the initial and equilibrium concentrations as we did in solving other equilibrium problems in Chapters 15 and 16: The equilibrium concentration of CH 3 COO– (the common ion) is the initial concentration that is due to CH 3 COONa (0. 30 M) plus the change in concentration (x) that is due to the ionization of CH 3 COOH. Now we can use the equilibriumconstant expression: (The dissociation constant for CH 3 COOH at 25 ºC is from Appendix D; addition of CH 3 COONa does not change the value of this constant. ) Substituting the equilibrium-constant concentrations from our table into the equilibrium expression gives Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 20 Calculating the p. H When a Common Ion is Involved Solution (Continued) Because Ka is small, we assume that x is small compared to the original concentrations of CH 3 COOH and CH 3 COO– (0. 30 M each). Thus, we can ignore the very small x relative to 0. 30 M, giving The resulting value of x is indeed small relative to 0. 30, justifying the approximation made in simplifying the problem. Finally, we calculate the p. H from the equilibrium concentration of H+(aq): Comment: In Section 16. 6 we calculated that a 0. 30 M solution of CH 3 COOH has a p. H of 2. 64, corresponding to H+] = 2. 3 × 10 -3 M. Thus, the addition of CH 3 COONa has substantially decreased , [H+] as we would expect from Le Châtelier’s principle. Practice Exercise Calculate the p. H of a solution containing 0. 085 M nitrous acid (HNO 2; Ka = 4. 5 × 10 -4) and 0. 10 M potassium nitrite (KNO 2). Answer: 3. 42 Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

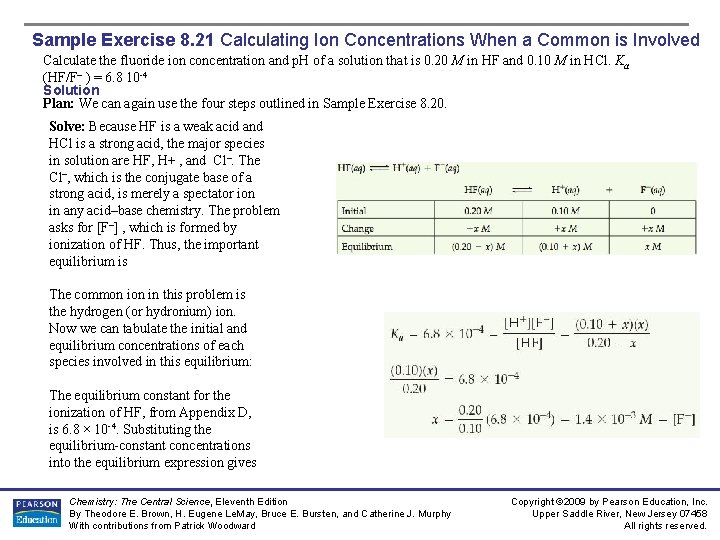
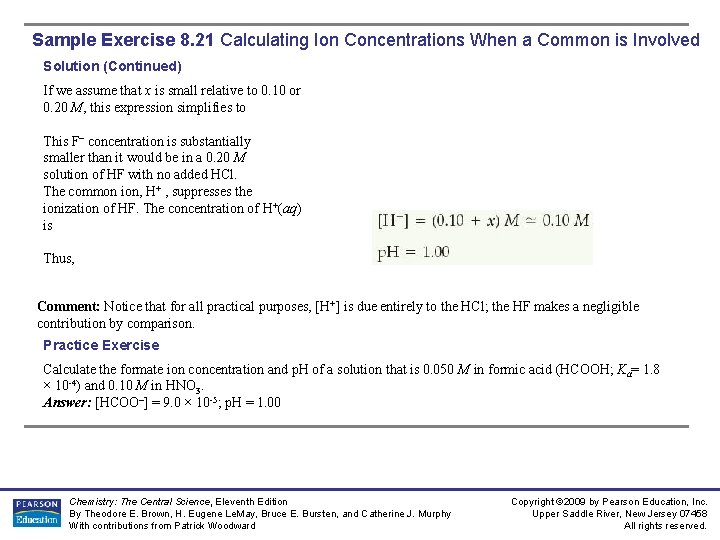
Sample Exercise 8. 21 Calculating Ion Concentrations When a Common is Involved Calculate the fluoride ion concentration and p. H of a solution that is 0. 20 M in HF and 0. 10 M in HCl. Ka (HF/F– ) = 6. 8 10 -4 Solution Plan: We can again use the four steps outlined in Sample Exercise 8. 20. Solve: Because HF is a weak acid and HCl is a strong acid, the major species in solution are HF, H+ , and Cl–. The Cl–, which is the conjugate base of a strong acid, is merely a spectator ion in any acid–base chemistry. The problem asks for [F–] , which is formed by ionization of HF. Thus, the important equilibrium is The common in this problem is the hydrogen (or hydronium) ion. Now we can tabulate the initial and equilibrium concentrations of each species involved in this equilibrium: The equilibrium constant for the ionization of HF, from Appendix D, is 6. 8 × 10 -4. Substituting the equilibrium-constant concentrations into the equilibrium expression gives Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 21 Calculating Ion Concentrations When a Common is Involved Solution (Continued) If we assume that x is small relative to 0. 10 or 0. 20 M, this expression simplifies to This F– concentration is substantially smaller than it would be in a 0. 20 M solution of HF with no added HCl. The common ion, H+ , suppresses the ionization of HF. The concentration of H+(aq) is Thus, Comment: Notice that for all practical purposes, [H+] is due entirely to the HCl; the HF makes a negligible contribution by comparison. Practice Exercise Calculate the formate ion concentration and p. H of a solution that is 0. 050 M in formic acid (HCOOH; Ka= 1. 8 × 10 -4) and 0. 10 M in HNO 3. Answer: [HCOO–] = 9. 0 × 10 -5; p. H = 1. 00 Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

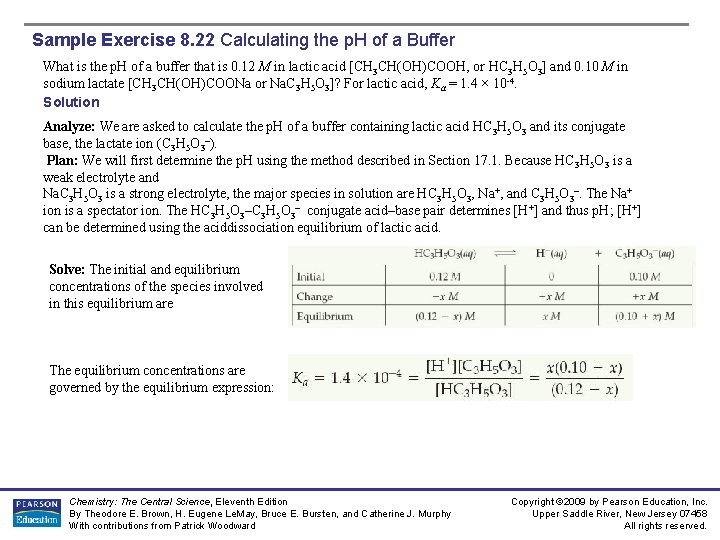
Sample Exercise 8. 22 Calculating the p. H of a Buffer What is the p. H of a buffer that is 0. 12 M in lactic acid [CH 3 CH(OH)COOH, or HC 3 H 5 O 3] and 0. 10 M in sodium lactate [CH 3 CH(OH)COONa or Na. C 3 H 5 O 3]? For lactic acid, Ka = 1. 4 × 10 -4. Solution Analyze: We are asked to calculate the p. H of a buffer containing lactic acid HC 3 H 5 O 3 and its conjugate base, the lactate ion (C 3 H 5 O 3–). Plan: We will first determine the p. H using the method described in Section 17. 1. Because HC 3 H 5 O 3 is a weak electrolyte and Na. C 3 H 5 O 3 is a strong electrolyte, the major species in solution are HC 3 H 5 O 3, Na+, and C 3 H 5 O 3–. The Na+ ion is a spectator ion. The HC 3 H 5 O 3– conjugate acid–base pair determines [H+] and thus p. H; [H+] can be determined using the aciddissociation equilibrium of lactic acid. Solve: The initial and equilibrium concentrations of the species involved in this equilibrium are The equilibrium concentrations are governed by the equilibrium expression: Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

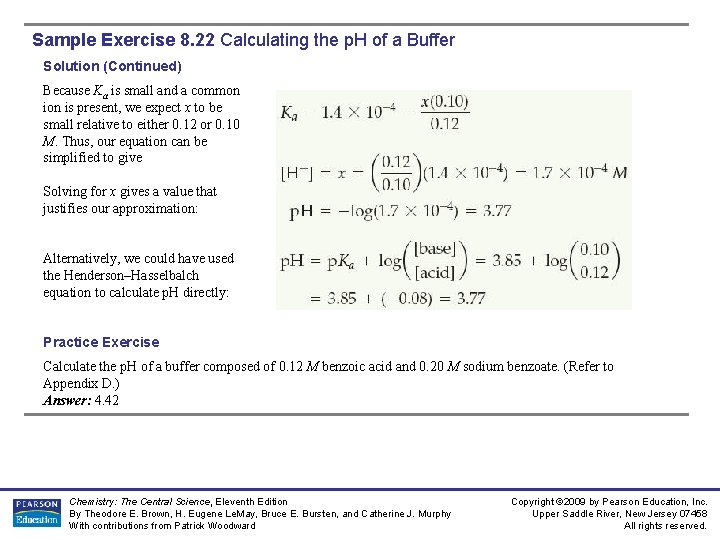
Sample Exercise 8. 22 Calculating the p. H of a Buffer Solution (Continued) Because Ka is small and a common is present, we expect x to be small relative to either 0. 12 or 0. 10 M. Thus, our equation can be simplified to give Solving for x gives a value that justifies our approximation: Alternatively, we could have used the Henderson–Hasselbalch equation to calculate p. H directly: Practice Exercise Calculate the p. H of a buffer composed of 0. 12 M benzoic acid and 0. 20 M sodium benzoate. (Refer to Appendix D. ) Answer: 4. 42 Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

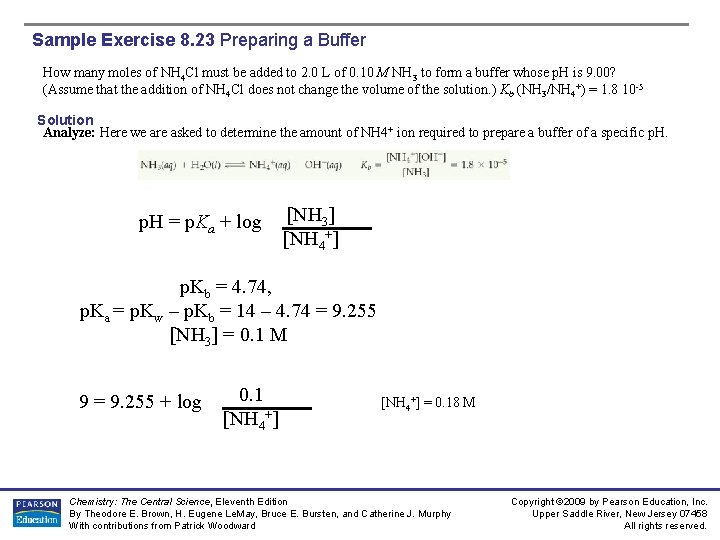
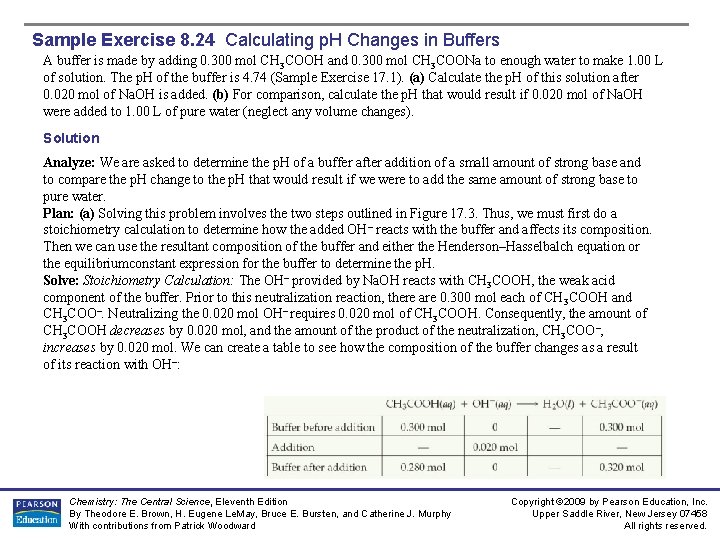
Sample Exercise 8. 23 Preparing a Buffer How many moles of NH 4 Cl must be added to 2. 0 L of 0. 10 M NH 3 to form a buffer whose p. H is 9. 00? (Assume that the addition of NH 4 Cl does not change the volume of the solution. ) Kb (NH 3/NH 4+) = 1. 8 10 -5 Solution Analyze: Here we are asked to determine the amount of NH 4+ ion required to prepare a buffer of a specific p. H = p. Ka + log [NH 3] [NH 4+] p. Kb = 4. 74, p. Ka = p. Kw – p. Kb = 14 – 4. 74 = 9. 255 [NH 3] = 0. 1 M 9 = 9. 255 + log 0. 1 [NH 4+] = 0. 18 M Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.
![Sample Exercise 8 23 Preparing a Buffer Solution Continued NH 4 must equal 0 Sample Exercise 8. 23 Preparing a Buffer Solution (Continued) [NH 4+] must equal 0.](https://slidetodoc.com/presentation_image_h2/36223c3fd92aaa348d1bb831e941e8ba/image-39.jpg)
Sample Exercise 8. 23 Preparing a Buffer Solution (Continued) [NH 4+] must equal 0. 18 M. The number of moles of NH 4 Cl needed to produce this concentration is given by the product of the volume of the solution and its molarity: Comment: Because NH 4+ and NH 3 are a conjugate acid–base pair, we could use the Henderson– Hasselbalch equation (Equation 17. 9) to solve this problem. To do so requires first using Equation 16. 41 to calculate p. Ka for NH 4+ from the value of p. Kb for NH 3. We suggest you try this approach to convince yourself that you can use the Henderson–Hasselbalch equation for buffers for which you are given Kb for the conjugate base rather than Ka for the conjugate acid. Practice Exercise Calculate the concentration of sodium benzoate that must be present in a 0. 20 M solution of benzoic acid (C 6 H 5 COOH) to produce a p. H of 4. 00. Answer: 0. 13 M Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

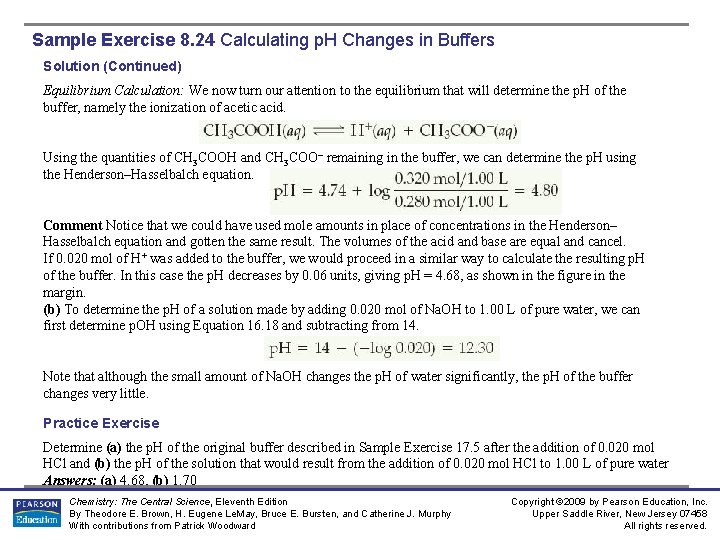
Sample Exercise 8. 24 Calculating p. H Changes in Buffers A buffer is made by adding 0. 300 mol CH 3 COOH and 0. 300 mol CH 3 COONa to enough water to make 1. 00 L of solution. The p. H of the buffer is 4. 74 (Sample Exercise 17. 1). (a) Calculate the p. H of this solution after 0. 020 mol of Na. OH is added. (b) For comparison, calculate the p. H that would result if 0. 020 mol of Na. OH were added to 1. 00 L of pure water (neglect any volume changes). Solution Analyze: We are asked to determine the p. H of a buffer after addition of a small amount of strong base and to compare the p. H change to the p. H that would result if we were to add the same amount of strong base to pure water. Plan: (a) Solving this problem involves the two steps outlined in Figure 17. 3. Thus, we must first do a stoichiometry calculation to determine how the added OH– reacts with the buffer and affects its composition. Then we can use the resultant composition of the buffer and either the Henderson–Hasselbalch equation or the equilibriumconstant expression for the buffer to determine the p. H. Solve: Stoichiometry Calculation: The OH– provided by Na. OH reacts with CH 3 COOH, the weak acid component of the buffer. Prior to this neutralization reaction, there are 0. 300 mol each of CH 3 COOH and CH 3 COO–. Neutralizing the 0. 020 mol OH– requires 0. 020 mol of CH 3 COOH. Consequently, the amount of CH 3 COOH decreases by 0. 020 mol, and the amount of the product of the neutralization, CH 3 COO–, increases by 0. 020 mol. We can create a table to see how the composition of the buffer changes as a result of its reaction with OH–: Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 24 Calculating p. H Changes in Buffers Solution (Continued) Equilibrium Calculation: We now turn our attention to the equilibrium that will determine the p. H of the buffer, namely the ionization of acetic acid. Using the quantities of CH 3 COOH and CH 3 COO– remaining in the buffer, we can determine the p. H using the Henderson–Hasselbalch equation. Comment Notice that we could have used mole amounts in place of concentrations in the Henderson– Hasselbalch equation and gotten the same result. The volumes of the acid and base are equal and cancel. If 0. 020 mol of H+ was added to the buffer, we would proceed in a similar way to calculate the resulting p. H of the buffer. In this case the p. H decreases by 0. 06 units, giving p. H = 4. 68, as shown in the figure in the margin. (b) To determine the p. H of a solution made by adding 0. 020 mol of Na. OH to 1. 00 L of pure water, we can first determine p. OH using Equation 16. 18 and subtracting from 14. Note that although the small amount of Na. OH changes the p. H of water significantly, the p. H of the buffer changes very little. Practice Exercise Determine (a) the p. H of the original buffer described in Sample Exercise 17. 5 after the addition of 0. 020 mol HCl and (b) the p. H of the solution that would result from the addition of 0. 020 mol HCl to 1. 00 L of pure water Answers: (a) 4. 68, (b) 1. 70 Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

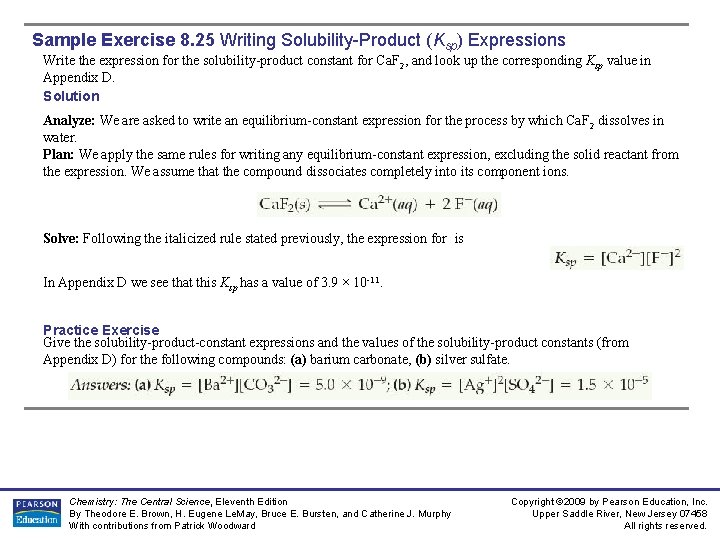
Sample Exercise 8. 25 Writing Solubility-Product (Ksp) Expressions Write the expression for the solubility-product constant for Ca. F 2, and look up the corresponding Ksp value in Appendix D. Solution Analyze: We are asked to write an equilibrium-constant expression for the process by which Ca. F 2 dissolves in water. Plan: We apply the same rules for writing any equilibrium-constant expression, excluding the solid reactant from the expression. We assume that the compound dissociates completely into its component ions. Solve: Following the italicized rule stated previously, the expression for is In Appendix D we see that this Ksp has a value of 3. 9 × 10 -11. Practice Exercise Give the solubility-product-constant expressions and the values of the solubility-product constants (from Appendix D) for the following compounds: (a) barium carbonate, (b) silver sulfate. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

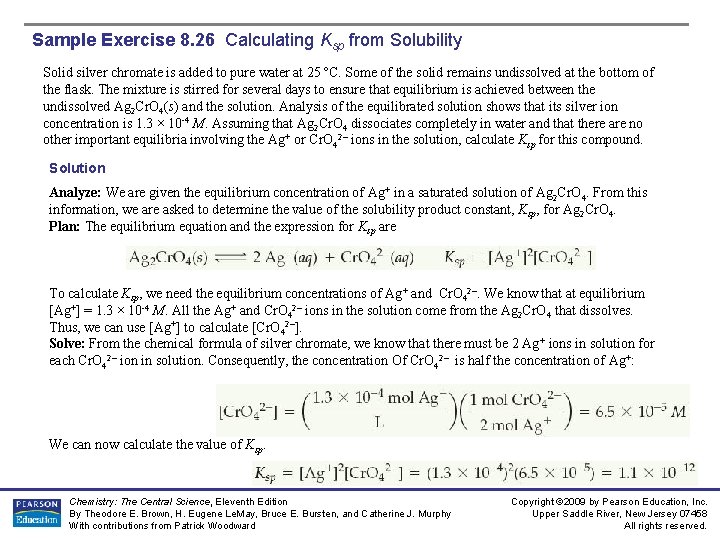
Sample Exercise 8. 26 Calculating Ksp from Solubility Solid silver chromate is added to pure water at 25 ºC. Some of the solid remains undissolved at the bottom of the flask. The mixture is stirred for several days to ensure that equilibrium is achieved between the undissolved Ag 2 Cr. O 4(s) and the solution. Analysis of the equilibrated solution shows that its silver ion concentration is 1. 3 × 10 -4 M. Assuming that Ag 2 Cr. O 4 dissociates completely in water and that there are no other important equilibria involving the Ag+ or Cr. O 42– ions in the solution, calculate Ksp for this compound. Solution Analyze: We are given the equilibrium concentration of Ag+ in a saturated solution of Ag 2 Cr. O 4. From this information, we are asked to determine the value of the solubility product constant, Ksp, for Ag 2 Cr. O 4. Plan: The equilibrium equation and the expression for Ksp are To calculate Ksp, we need the equilibrium concentrations of Ag+ and Cr. O 42–. We know that at equilibrium [Ag+] = 1. 3 × 10 -4 M. All the Ag+ and Cr. O 42– ions in the solution come from the Ag 2 Cr. O 4 that dissolves. Thus, we can use [Ag+] to calculate [Cr. O 42–]. Solve: From the chemical formula of silver chromate, we know that there must be 2 Ag+ ions in solution for each Cr. O 42– ion in solution. Consequently, the concentration Of Cr. O 42– is half the concentration of Ag+: We can now calculate the value of Ksp. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 26 Calculating Ksp from Solubility Solution (Continued) Check: We obtain a small value, as expected for a slightly soluble salt. Furthermore, the calculated value agrees well with the one given in Appendix D, 1. 2 × 10 -12. Practice Exercise A saturated solution of Mg(OH)2 in contact with undissolved solid is prepared at 25 ºC. The p. H of the solution is found to be 10. 17. Assuming that Mg(OH)2 dissociates completely in water and that there are no other simultaneous equilibria involving the Mg 2+ or OH– ions in the solution, calculate Ksp for this compound. Answer: 1. 6 × 10 -12 Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

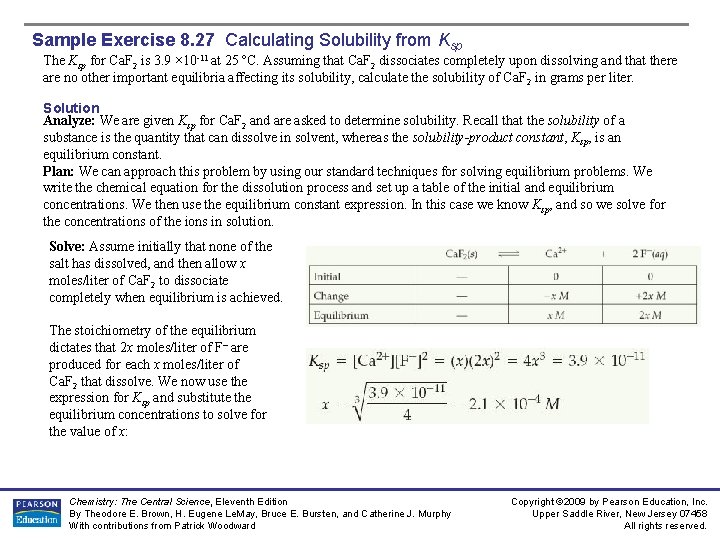
Sample Exercise 8. 27 Calculating Solubility from Ksp The Ksp for Ca. F 2 is 3. 9 × 10 -11 at 25 ºC. Assuming that Ca. F 2 dissociates completely upon dissolving and that there are no other important equilibria affecting its solubility, calculate the solubility of Ca. F 2 in grams per liter. Solution Analyze: We are given Ksp for Ca. F 2 and are asked to determine solubility. Recall that the solubility of a substance is the quantity that can dissolve in solvent, whereas the solubility-product constant, Ksp, is an equilibrium constant. Plan: We can approach this problem by using our standard techniques for solving equilibrium problems. We write the chemical equation for the dissolution process and set up a table of the initial and equilibrium concentrations. We then use the equilibrium constant expression. In this case we know Ksp, and so we solve for the concentrations of the ions in solution. Solve: Assume initially that none of the salt has dissolved, and then allow x moles/liter of Ca. F 2 to dissociate completely when equilibrium is achieved. The stoichiometry of the equilibrium dictates that 2 x moles/liter of F– are produced for each x moles/liter of Ca. F 2 that dissolve. We now use the expression for Ksp and substitute the equilibrium concentrations to solve for the value of x: Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 27 Calculating Solubility from Ksp Solution (Remember that to calculate the cube root of a number, you can use the yx function on your calculator, with x =. ) Thus, the molar solubility of Ca. F 2 is 2. 1 × 10 -4 mol/L. The mass of Ca. F 2 that dissolves in water to form a liter of solution is Check: We expect a small number for the solubility of a slightly soluble salt. If we reverse the calculation, we should be able to recalculate the solubility product: Ksp = (2. 1 × 10 -4)(4. 2 × 10 -4)2 = 3. 7 × 10 -11 , close to the starting value for Ksp, 3. 9 × 10 -11, Comment: Because F- is the anion of a weak acid, you might expect that the hydrolysis of the ion would affect the solubility of Ca. F 2. The basicity of F– is so small (Kb = 1. 5 × 10 -11), however, that the hydrolysis occurs to only a slight extent and does not significantly influence the solubility. The reported solubility is 0. 017 g/L at 25 ºC, in good agreement with our calculation Practice Exercise The Ksp for La. F 3 is 2 × 10 -19. What is the solubility of La. F 3 in water in moles per liter? Answer: 9 × 10 -6 mol/L Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

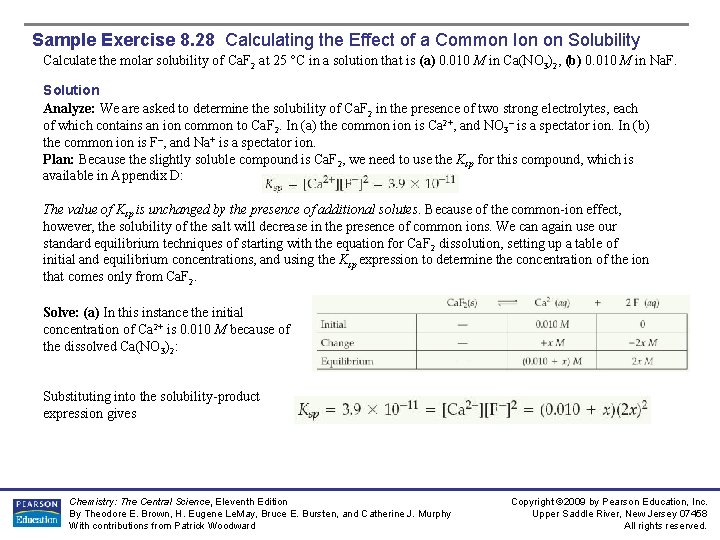
Sample Exercise 8. 28 Calculating the Effect of a Common Ion on Solubility Calculate the molar solubility of Ca. F 2 at 25 °C in a solution that is (a) 0. 010 M in Ca(NO 3)2, (b) 0. 010 M in Na. F. Solution Analyze: We are asked to determine the solubility of Ca. F 2 in the presence of two strong electrolytes, each of which contains an ion common to Ca. F 2. In (a) the common is Ca 2+, and NO 3– is a spectator ion. In (b) the common is F–, and Na+ is a spectator ion. Plan: Because the slightly soluble compound is Ca. F 2, we need to use the Ksp for this compound, which is available in Appendix D: The value of Ksp is unchanged by the presence of additional solutes. Because of the common-ion effect, however, the solubility of the salt will decrease in the presence of common ions. We can again use our standard equilibrium techniques of starting with the equation for Ca. F 2 dissolution, setting up a table of initial and equilibrium concentrations, and using the Ksp expression to determine the concentration of the ion that comes only from Ca. F 2. Solve: (a) In this instance the initial concentration of Ca 2+ is 0. 010 M because of the dissolved Ca(NO 3)2: Substituting into the solubility-product expression gives Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

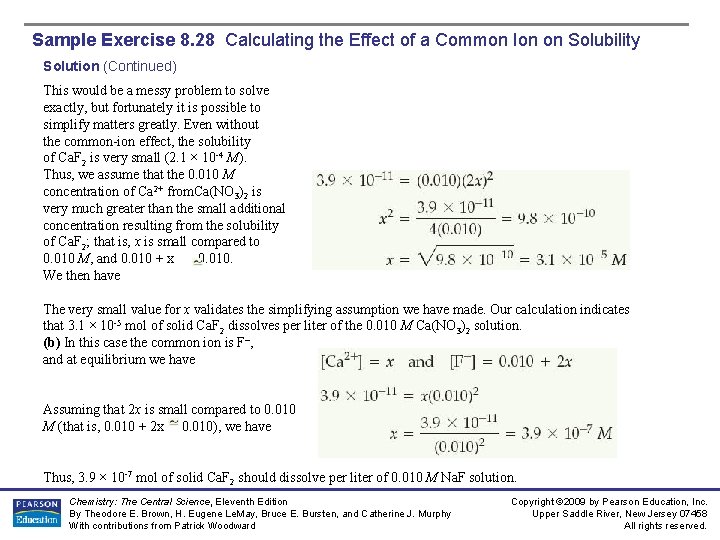
Sample Exercise 8. 28 Calculating the Effect of a Common Ion on Solubility Solution (Continued) This would be a messy problem to solve exactly, but fortunately it is possible to simplify matters greatly. Even without the common-ion effect, the solubility of Ca. F 2 is very small (2. 1 × 10 -4 M). Thus, we assume that the 0. 010 M concentration of Ca 2+ from. Ca(NO 3)2 is very much greater than the small additional concentration resulting from the solubility of Ca. F 2; that is, x is small compared to 0. 010 M, and 0. 010 + x 0. 010. We then have The very small value for x validates the simplifying assumption we have made. Our calculation indicates that 3. 1 × 10 -5 mol of solid Ca. F 2 dissolves per liter of the 0. 010 M Ca(NO 3)2 solution. (b) In this case the common is F–, and at equilibrium we have Assuming that 2 x is small compared to 0. 010 M (that is, 0. 010 + 2 x 0. 010), we have Thus, 3. 9 × 10 -7 mol of solid Ca. F 2 should dissolve per liter of 0. 010 M Na. F solution. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 28 Calculating the Effect of a Common Ion on Solubility Solution (Continued) Comment: The molar solubility of Ca. F 2 in pure water is 2. 1 × 10 -4 M (Sample Exercise 17. 11). By comparison, our calculations above show that the solubility of Ca. F 2 in the presence of 0. 010 M Ca 2+ is 3. 1 × 10 -5 M, and in the presence of 0. 010 M F– ion it is 3. 9 × 10 -7 M. Thus, the addition of either Ca 2+ or F– to a solution of Ca. F 2 decreases the solubility. However, the effect of F- on the solubility is more pronounced than that of Ca 2+ because [F–] appears to the second power in the Ksp expression for Ca. F 2, whereas Ca 2+ appears to the first power. Practice Exercise The value for Ksp for manganese(II) hydroxide, Mn(OH)2, is 1. 6 × 10 -13. Calculate the molar solubility of Mn(OH)2 in a solution that contains 0. 020 M Na. OH. Answer: 4. 0 × 10 -10 M Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

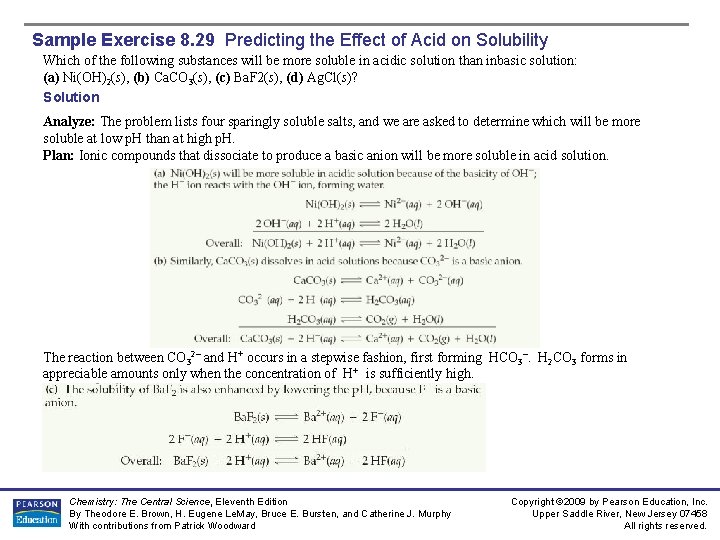
Sample Exercise 8. 29 Predicting the Effect of Acid on Solubility Which of the following substances will be more soluble in acidic solution than inbasic solution: (a) Ni(OH)2(s), (b) Ca. CO 3(s), (c) Ba. F 2(s), (d) Ag. Cl(s)? Solution Analyze: The problem lists four sparingly soluble salts, and we are asked to determine which will be more soluble at low p. H than at high p. H. Plan: Ionic compounds that dissociate to produce a basic anion will be more soluble in acid solution. The reaction between CO 32– and H+ occurs in a stepwise fashion, first forming HCO 3–. H 2 CO 3 forms in appreciable amounts only when the concentration of H+ is sufficiently high. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 29 Predicting the Effect of Acid on Solubility Solution (d) The solubility of Ag. Cl is unaffected by changes in p. H because Cl– is the anion of a strong acid and therefore has negligible basicity. Practice Exercise Write the net ionic equation for the reaction of the following copper(II) compounds with acid: (a) Cu. S, (b) Cu(N 3)2. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

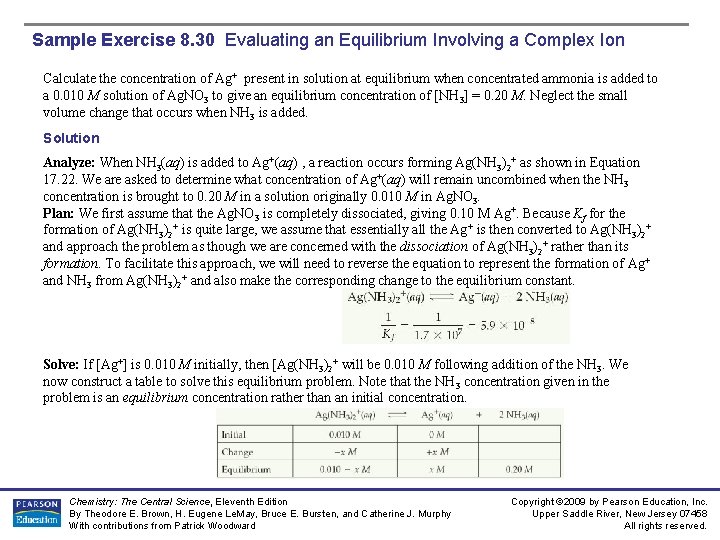
Sample Exercise 8. 30 Evaluating an Equilibrium Involving a Complex Ion Calculate the concentration of Ag+ present in solution at equilibrium when concentrated ammonia is added to a 0. 010 M solution of Ag. NO 3 to give an equilibrium concentration of [NH 3] = 0. 20 M. Neglect the small volume change that occurs when NH 3 is added. Solution Analyze: When NH 3(aq) is added to Ag+(aq) , a reaction occurs forming Ag(NH 3)2+ as shown in Equation 17. 22. We are asked to determine what concentration of Ag+(aq) will remain uncombined when the NH 3 concentration is brought to 0. 20 M in a solution originally 0. 010 M in Ag. NO 3. Plan: We first assume that the Ag. NO 3 is completely dissociated, giving 0. 10 M Ag+. Because Kf for the formation of Ag(NH 3)2+ is quite large, we assume that essentially all the Ag+ is then converted to Ag(NH 3)2+ and approach the problem as though we are concerned with the dissociation of Ag(NH 3)2+ rather than its formation. To facilitate this approach, we will need to reverse the equation to represent the formation of Ag + and NH 3 from Ag(NH 3)2+ and also make the corresponding change to the equilibrium constant. Solve: If [Ag+] is 0. 010 M initially, then [Ag(NH 3)2+ will be 0. 010 M following addition of the NH 3. We now construct a table to solve this equilibrium problem. Note that the NH 3 concentration given in the problem is an equilibrium concentration rather than an initial concentration. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

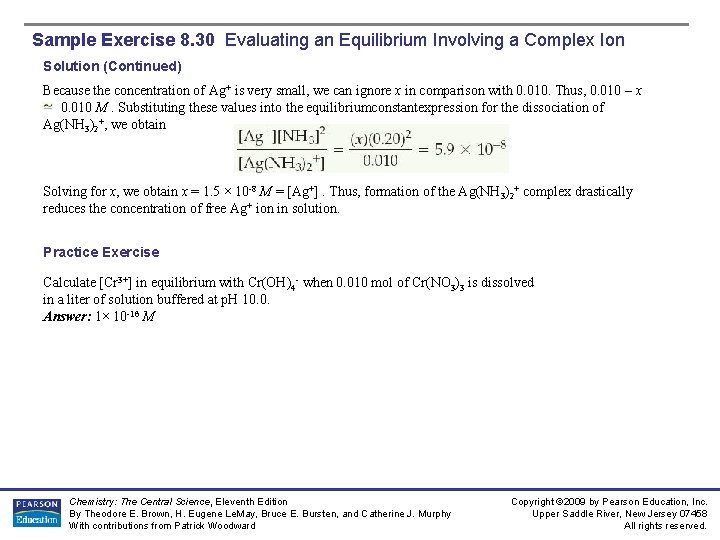
Sample Exercise 8. 30 Evaluating an Equilibrium Involving a Complex Ion Solution (Continued) Because the concentration of Ag+ is very small, we can ignore x in comparison with 0. 010. Thus, 0. 010 – x 0. 010 M. Substituting these values into the equilibriumconstantexpression for the dissociation of Ag(NH 3)2+, we obtain Solving for x, we obtain x = 1. 5 × 10 -8 M = [Ag+]. Thus, formation of the Ag(NH 3)2+ complex drastically reduces the concentration of free Ag+ ion in solution. Practice Exercise Calculate [Cr 3+] in equilibrium with Cr(OH)4 - when 0. 010 mol of Cr(NO 3)3 is dissolved in a liter of solution buffered at p. H 10. 0. Answer: 1× 10 -16 M Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 31 Predicting Whether a Precipitate Will Form Will a precipitate of Pb. SO 4 form when 0. 10 L of 8. 0 × 10 -3 M Pb(NO 3)2 is added to 0. 40 L of 5. 0 × 10 -3 M Na 2 SO 4? Pb. SO 4 has a Ksp of 6. 3 × 10 -7 Solution Analyze: The problem asks us to determine whether a precipitate will form when two salt solutions are combined. Plan: We should determine the concentrations of all ions immediately upon mixing of the solutions and compare the value of the reaction quotient, Q, to the solubility-product constant, Ksp, for any potentially insoluble product. The possible metathesis products are Pb. SO 4 and Na. NO 3. Sodium salts are quite soluble; Pb. SO 4 has a Ksp of 6. 3 × 10 -7 (Appendix D), however, and will precipitate if the Pb 2+ and SO 42– concentrations are high enough for Q to exceed Ksp for the salt. Solve: When the two solutions are mixed, the total volume becomes 0. 10 L + 0. 40 L = 0. 50 L. The number of moles of Pb 2+ in 0. 10 L of 8. 0 × 10 -3 M Pb(NO 3)2 is The concentration of Pb 2+ in the 0. 50 -L mixture is therefore The number of moles of SO 42– in 0. 40 L of 5. 0 × 10 -3 MNa 2 SO 4 is Therefore, [SO 42–] in the 0. 50 -L mixture is We then have Because Q > Ksp , Pb. SO 4 will precipitate. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 31 Predicting Whether a Precipitate Will Form Practice Exercise Will a precipitate form when 0. 050 L of 2. 0 × 10 -2 M Na. F is mixed with 0. 010 L of 1. 0 × 10 -2 M Ca(NO 3)2? Answer: Yes, Ca. F 2 precipitates because Q = 4. 6 × 10 -8 is larger than Ksp = 3. 9 × 10 -11 Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 32 Calculating Ion Concentrations for Precipitation A solution contains 1. 0 × 10 -2 M Ag+ and 2. 0 × 10 -2 M Pb 2+. When Cl– is added to the solution, both Ag. Cl (Ksp = 1. 8× 10 -10) and Pb. Cl 2 (Ksp = 1. 7× 10 -5) precipitate from the solution. What concentration of Cl– is necessary to begin the precipitation of each salt? Which salt precipitates first? Solution Analyze: We are asked to determine the concentration of Cl– necessary to begin the precipitation from a solution containing Ag+ and Pb 2+, and to predict which metal chloride will begin to precipitate first. Plan: We are given Ksp values for the two possible precipitates. Using these and the metal ion concentrations, we can calculate what concentration of Cl– ion would be necessary to begin precipitation of each. The salt requiring the lower Cl– ion concentration will precipitate first. Solve: For Ag. Cl we have Because [Ag+] = 1. 0 × 10 -2 M, the greatest concentration of Cl– that can be present without causing precipitation of Ag. Cl can be calculated from the Ksp expression Any Cl– in excess of this very small concentration will cause Ag. Cl to precipitate from solution. Proceeding similarly for Pb. Cl 2, we have Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

Sample Exercise 8. 32 Calculating Ion Concentrations for Precipitation Solution (Continued) Thus, a concentration of Cl– in excess of 2. 9 × 10 -2 M will cause Pb. Cl 2 to precipitate. Comparing the concentrations of Cl– required to precipitate each salt, we see that as Cl– is added to the solution, Ag. Cl will precipitate first because it requires a much smaller concentration of Cl –. Thus, Ag+ can be separated from by slowly adding Cl– so [Cl–] is between 1. 8 × 10 -8 M and 2. 9 × 10 -2 M. Practice Exercise Asolution consists of 0. 050 M Mg 2+ and 0. 020 M Cu 2+. Which ion will precipitate first as OH– is added to the solution? What concentration of OH– is necessary to begin the precipitation of each cation? [Ksp = 1. 8 × 10 -11 for Mg(OH)2, and Ksp = 4. 8 × 10 -20 for Cu(OH)2. ] Answer: Cu(OH)2 precipitates first. Cu(OH)2 begins to precipitate when [OH–] exceeds 1. 5 × 10 -9 M; Mg(OH)2 begins to precipitate when [OH–] exceeds 1. 9 × 10 -5 M. Chemistry: The Central Science, Eleventh Edition By Theodore E. Brown, H. Eugene Le. May, Bruce E. Bursten, and Catherine J. Murphy With contributions from Patrick Woodward Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.