THE HYDRONIUM ION The proton does not actually



- Slides: 25

THE HYDRONIUM ION • The proton does not actually exist in aqueous solution as a bare H+ ion. • The proton exists as the hydronium ion (H 3 O+). • Consider the acid-base reaction: HCO 3 - + H 2 O H 3 O+ + CO 32 Here water acts as a base, producing the hydronium ion as its conjugate acid. For simplicity, we often just write this reaction as: HCO 3 - H+ + CO 32 -

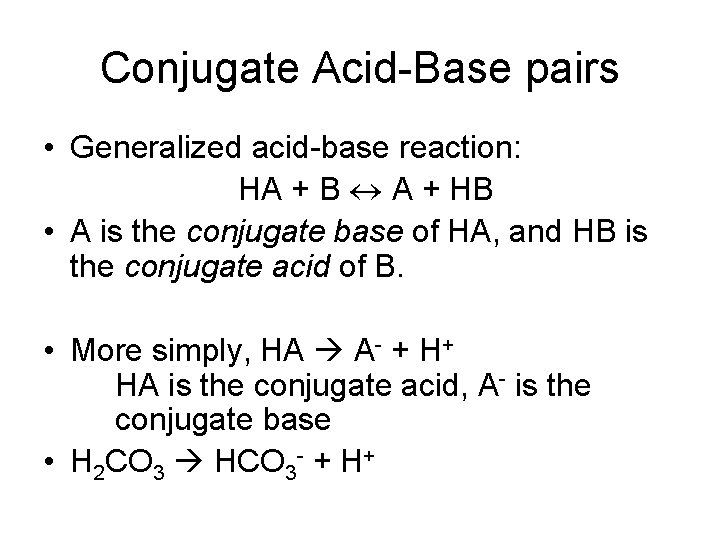
Conjugate Acid-Base pairs • Generalized acid-base reaction: HA + B A + HB • A is the conjugate base of HA, and HB is the conjugate acid of B. • More simply, HA A- + H+ HA is the conjugate acid, A- is the conjugate base • H 2 CO 3 HCO 3 - + H+

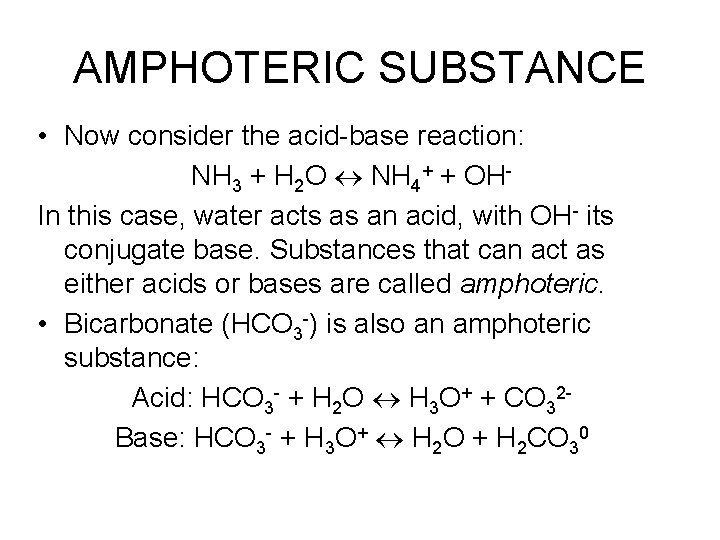
AMPHOTERIC SUBSTANCE • Now consider the acid-base reaction: NH 3 + H 2 O NH 4+ + OHIn this case, water acts as an acid, with OH- its conjugate base. Substances that can act as either acids or bases are called amphoteric. • Bicarbonate (HCO 3 -) is also an amphoteric substance: Acid: HCO 3 - + H 2 O H 3 O+ + CO 32 Base: HCO 3 - + H 3 O+ H 2 O + H 2 CO 30

Strong Acids/ Bases • Strong Acids more readily release H+ into water, they more fully dissociate – H 2 SO 4 2 H+ + SO 42 - • Strong Bases more readily release OHinto water, they more fully dissociate – Na. OH Na+ + OH- Strength DOES NOT EQUAL Concentration!

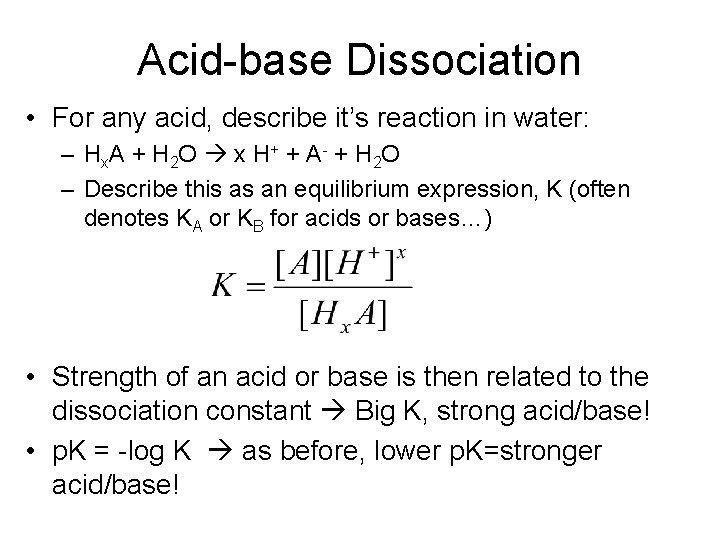
Acid-base Dissociation • For any acid, describe it’s reaction in water: – Hx. A + H 2 O x H+ + A- + H 2 O – Describe this as an equilibrium expression, K (often denotes KA or KB for acids or bases…) • Strength of an acid or base is then related to the dissociation constant Big K, strong acid/base! • p. K = -log K as before, lower p. K=stronger acid/base!

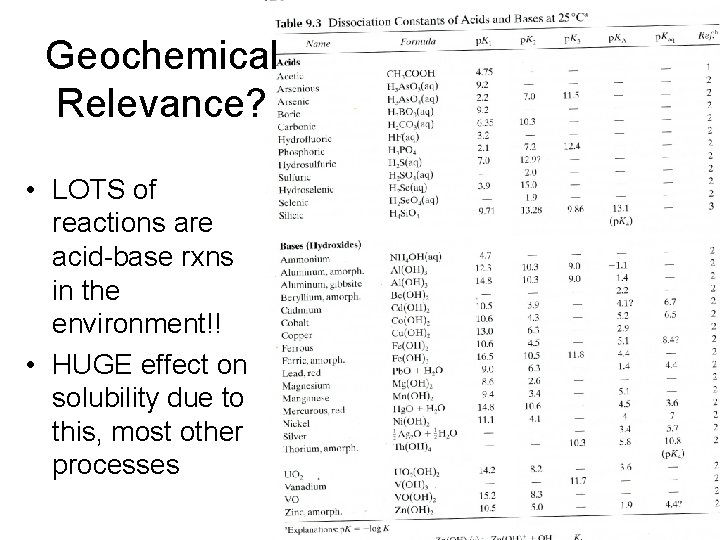
Geochemical Relevance? • LOTS of reactions are acid-base rxns in the environment!! • HUGE effect on solubility due to this, most other processes

Organic acids in natural waters • Humic/nonhumic – designations for organic fractions, – Humics= refractory, acidic, dark, aromatic, large – generally meaning an unspecified mix of organics – Nonhumics – Carbohydrates, proteins, peptides, amino acids, etc. • Aquatic humics include humic and fulvic acids (p. Ka>3. 6) and humin which is more insoluble • Soil fulvic acids also strongly complex metals and can be an important control on metal mobility

p. H • Commonly represented as a range between 0 and 14, and most natural waters are between p. H 4 and 9 • Remember that p. H = - log [H+] – Can p. H be negative? – Of course! p. H -3 [H+]=103 = 1000 molal? – But what’s g. H+? ? Turns out to be quite small 0. 002 or so… – How would you determine this? ?

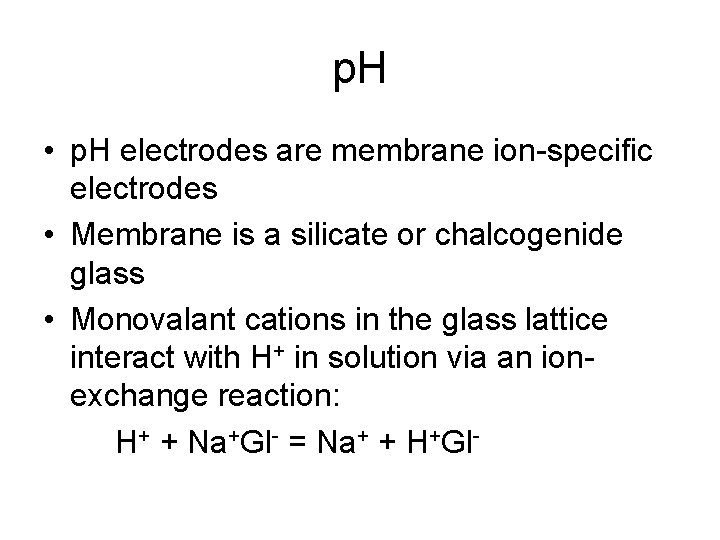
p. H • p. H electrodes are membrane ion-specific electrodes • Membrane is a silicate or chalcogenide glass • Monovalant cations in the glass lattice interact with H+ in solution via an ionexchange reaction: H+ + Na+Gl- = Na+ + H+Gl-

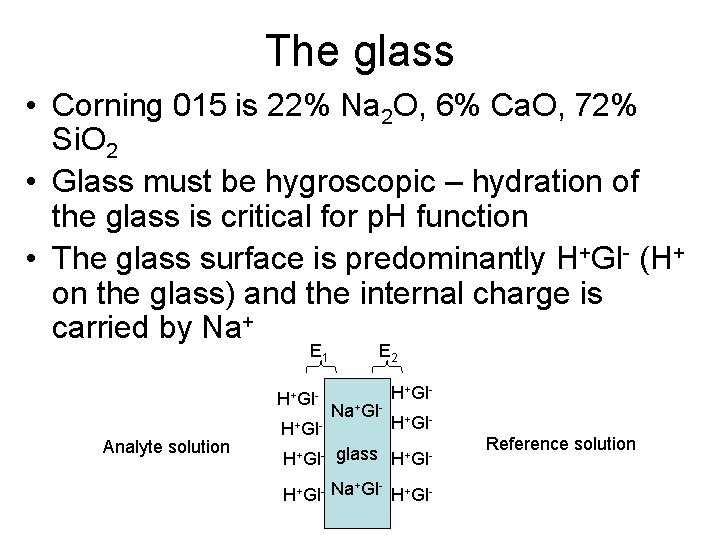
The glass • Corning 015 is 22% Na 2 O, 6% Ca. O, 72% Si. O 2 • Glass must be hygroscopic – hydration of the glass is critical for p. H function • The glass surface is predominantly H+Gl- (H+ on the glass) and the internal charge is carried by Na+ E 1 H+Gl. Analyte solution H+Gl- E 2 Na+Gl- H+Gl- glass H+Gl- + H+Gl- Na Gl H+Gl- Reference solution

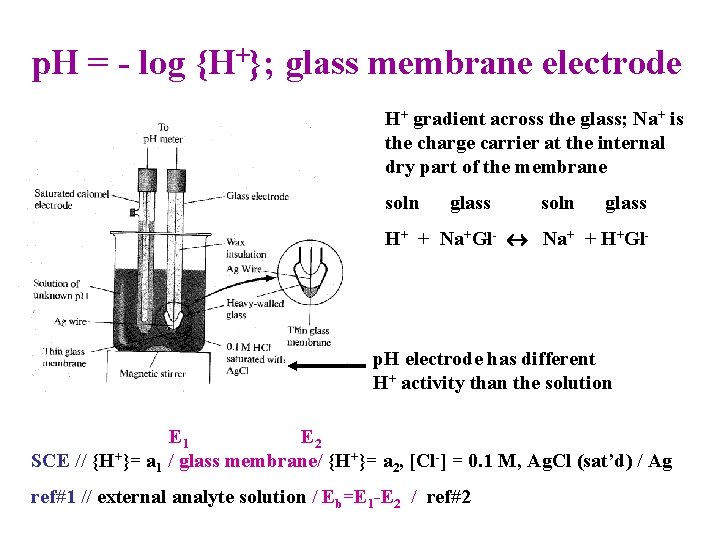
p. H = - log {H+}; glass membrane electrode H+ gradient across the glass; Na+ is the charge carrier at the internal dry part of the membrane soln glass H+ + Na+Gl- Na+ + H+Gl- p. H electrode has different H+ activity than the solution E 1 E 2 SCE // {H+}= a 1 / glass membrane/ {H+}= a 2, [Cl-] = 0. 1 M, Ag. Cl (sat’d) / Ag ref#1 // external analyte solution / Eb=E 1 -E 2 / ref#2

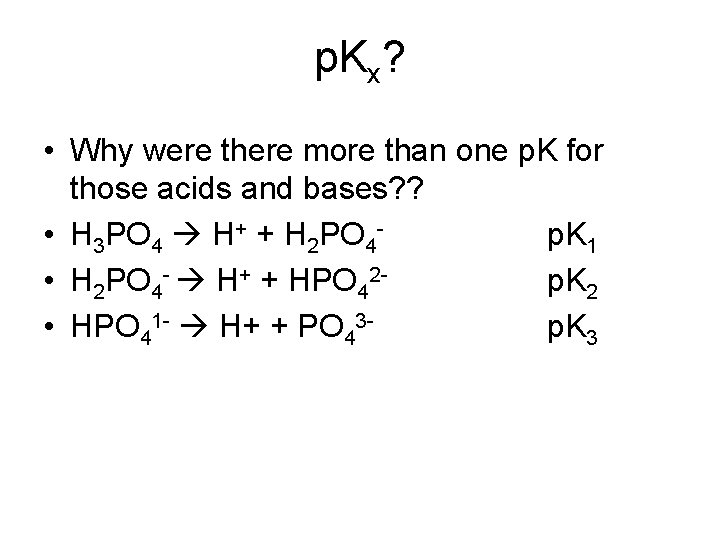
p. H = - log {H+} K = reference and junction potentials Values of NIST primary-standard p. H solutions from 0 to 60 o. C

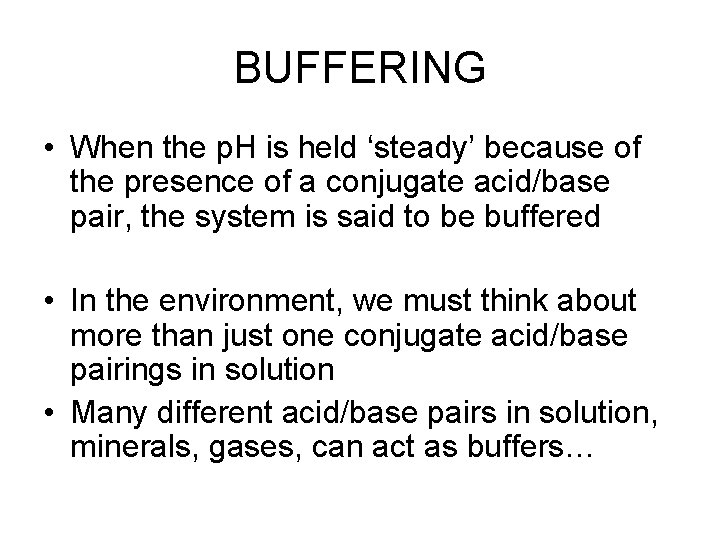
p. Kx? • Why were there more than one p. K for those acids and bases? ? • H 3 PO 4 H+ + H 2 PO 4 p. K 1 • H 2 PO 4 - H+ + HPO 42 p. K 2 • HPO 41 - H+ + PO 43 p. K 3

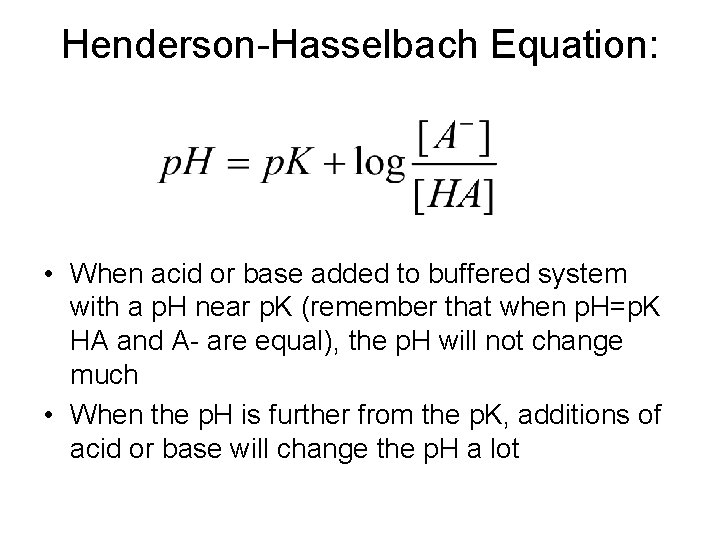
BUFFERING • When the p. H is held ‘steady’ because of the presence of a conjugate acid/base pair, the system is said to be buffered • In the environment, we must think about more than just one conjugate acid/base pairings in solution • Many different acid/base pairs in solution, minerals, gases, can act as buffers…

Henderson-Hasselbach Equation: • When acid or base added to buffered system with a p. H near p. K (remember that when p. H=p. K HA and A- are equal), the p. H will not change much • When the p. H is further from the p. K, additions of acid or base will change the p. H a lot

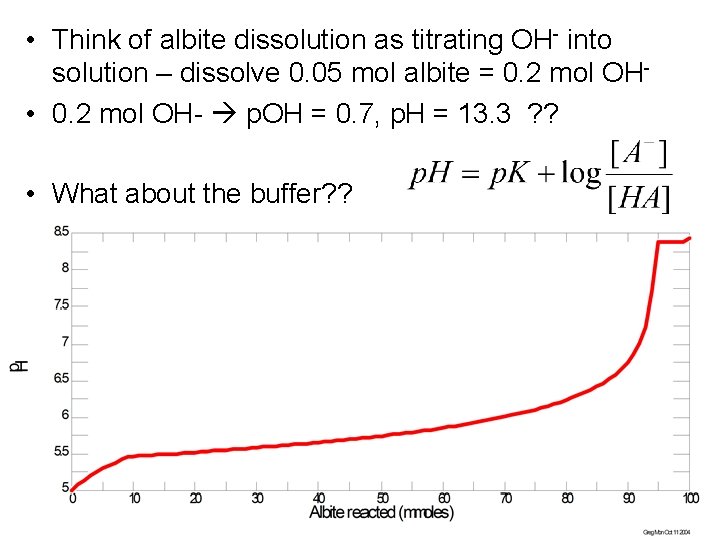
Buffering example • Let’s convince ourselves of what buffering can do… • Take a base-generating reaction: – Albite + 2 H 2 O = 4 OH- + Na+ + Al 3+ + 3 Si. O 2(aq) – What happens to the p. H of a solution containing 100 m. M HCO 3 - which starts at p. H 5? ? – p. K 1 for H 2 CO 3 = 6. 35

• Think of albite dissolution as titrating OH- into solution – dissolve 0. 05 mol albite = 0. 2 mol OH • 0. 2 mol OH- p. OH = 0. 7, p. H = 13. 3 ? ? • What about the buffer? ? – Write the p. H changes via the Henderson-Hasselbach equation • 0. 1 mol H 2 CO 3(aq), as the p. H increases, some of this starts turning into HCO 3 • After 12. 5 mmoles albite react (50 mmoles OH-): – p. H=6. 35+log (HCO 3 -/H 2 CO 3) = 6. 35+log(50/50) • After 20 mmoles albite react (80 mmoles OH-): – p. H=6. 35+log(80/20) = 6. 35 + 0. 6 = 6. 95

Bjerrum Plots • 2 D plots of species activity (y axis) and p. H (x axis) • Useful to look at how conjugate acid-base pairs for many different species behave as p. H changes • At p. H=p. K the activity of the conjugate acid and base are equal

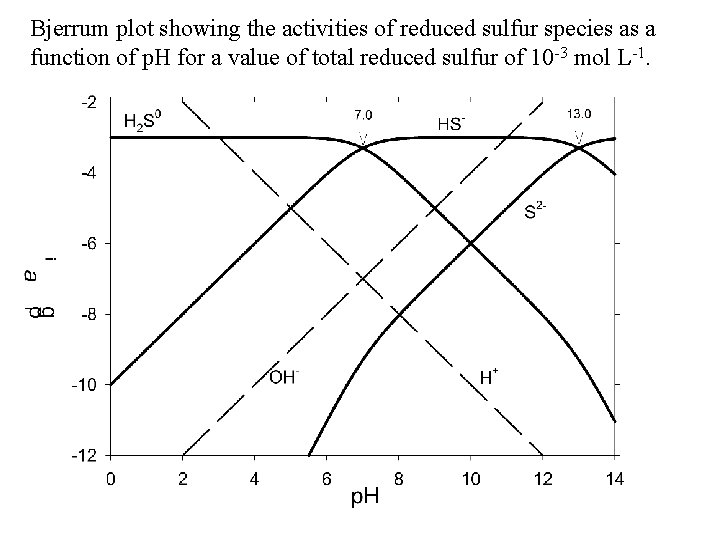
Bjerrum plot showing the activities of reduced sulfur species as a function of p. H for a value of total reduced sulfur of 10 -3 mol L-1.

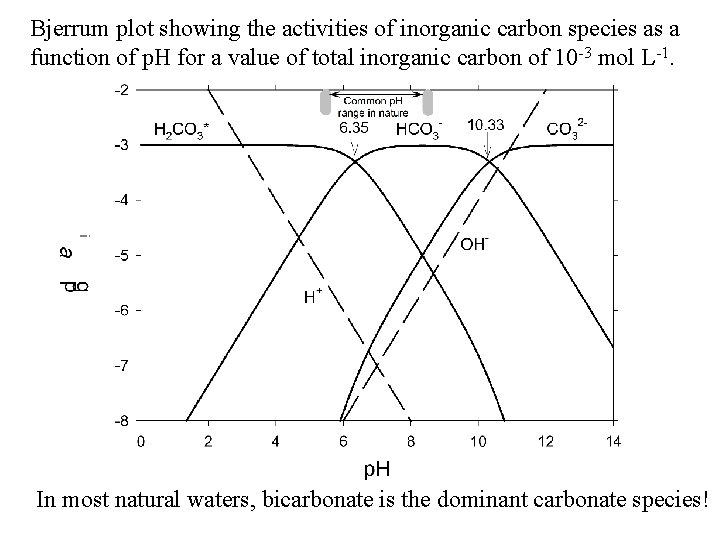
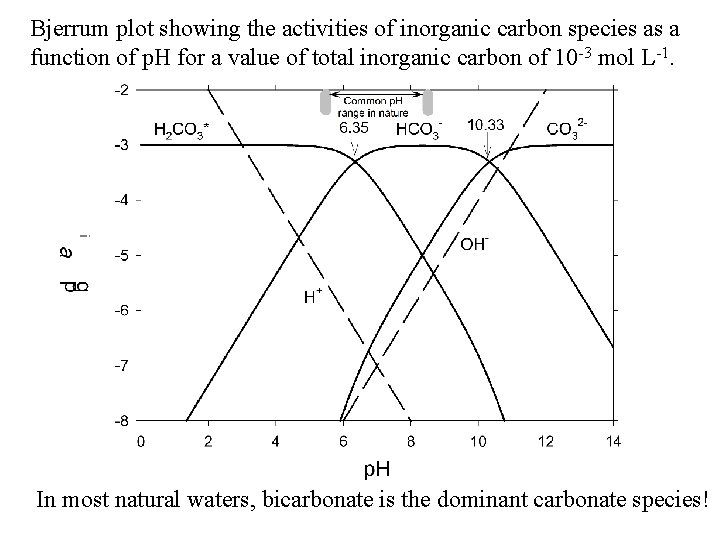
Bjerrum plot showing the activities of inorganic carbon species as a function of p. H for a value of total inorganic carbon of 10 -3 mol L-1. In most natural waters, bicarbonate is the dominant carbonate species!

Titrations • When we add acid or base to a solution containing an ion which can by protonated/deprotonated (i. e. it can accept a H+ or OH-), how does that affect the p. H?

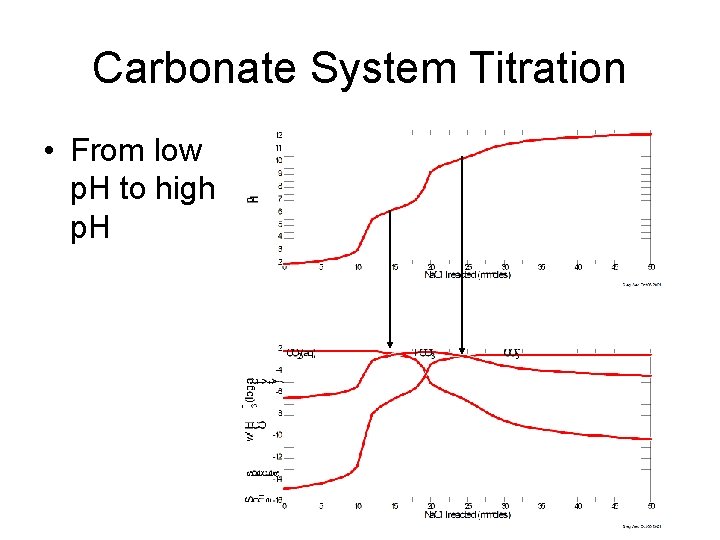
Carbonate System Titration • From low p. H to high p. H

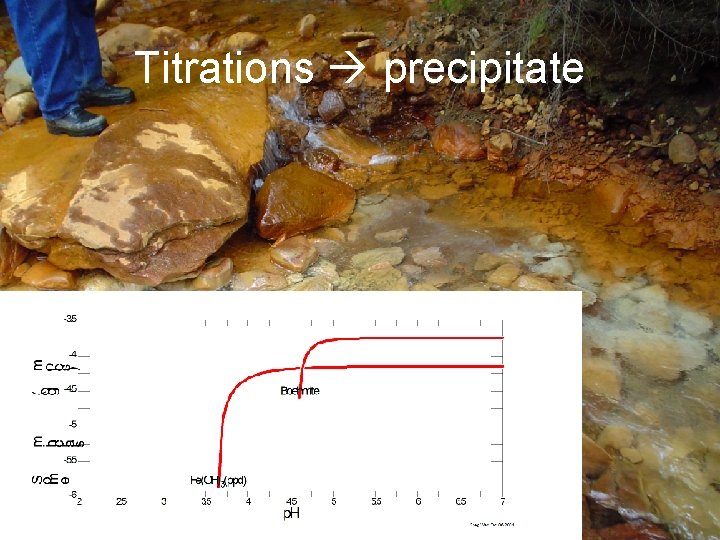
Titrations precipitate

BJERRUM PLOT - CARBONATE • closed systems with a specified total carbonate concentration. They plot the log of the concentrations of various species in the system as a function of p. H. • The species in the CO 2 -H 2 O system: H 2 CO 3*, HCO 3 -, CO 32 -, H+, and OH-. • At each p. K value, conjugate acid-base pairs have equal concentrations. • At p. H < p. K 1, H 2 CO 3* is predominant, and accounts for nearly 100% of total carbonate. • At p. K 1 < p. H < p. K 2, HCO 3 - is predominant, and accounts for nearly 100% of total carbonate. • At p. H > p. K 2, CO 32 - is predominant.

Bjerrum plot showing the activities of inorganic carbon species as a function of p. H for a value of total inorganic carbon of 10 -3 mol L-1. In most natural waters, bicarbonate is the dominant carbonate species!