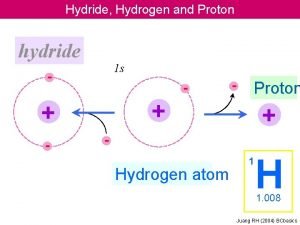
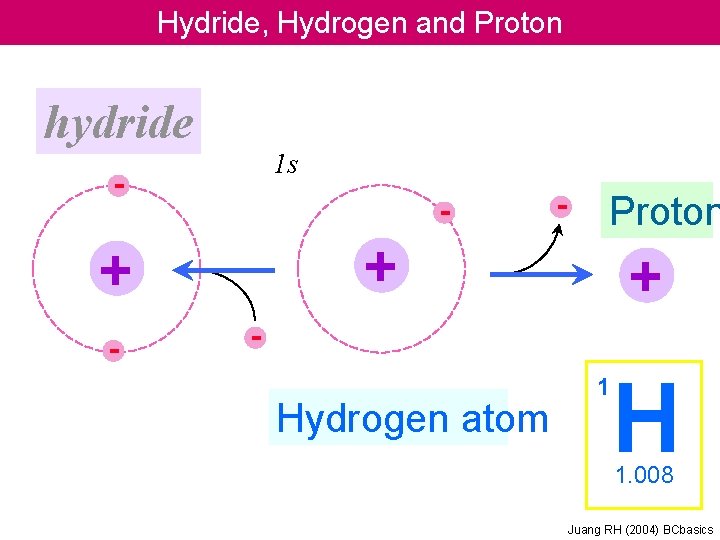
Hydride Hydrogen and Proton hydride 1 s Proton


- Slides: 8

Hydride, Hydrogen and Proton hydride 1 s - - Proton + + - - + - Hydrogen atom 1 H 1. 008 Juang RH (2004) BCbasics

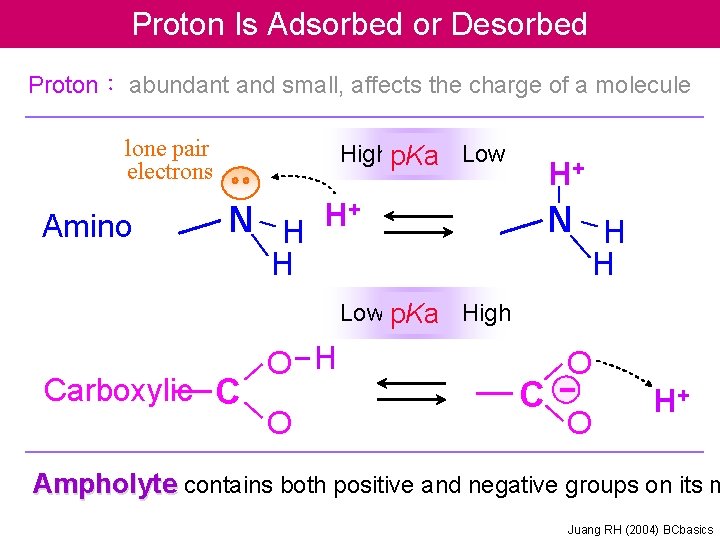
Proton Is Adsorbed or Desorbed Proton: abundant and small, affects the charge of a molecule lone pair electrons Amino Highp. Ka Low N H H+ H+ N H H H Low p. Ka High Carboxylic C O H O C O O H+ Ampholyte contains both positive and negative groups on its m Juang RH (2004) BCbasics

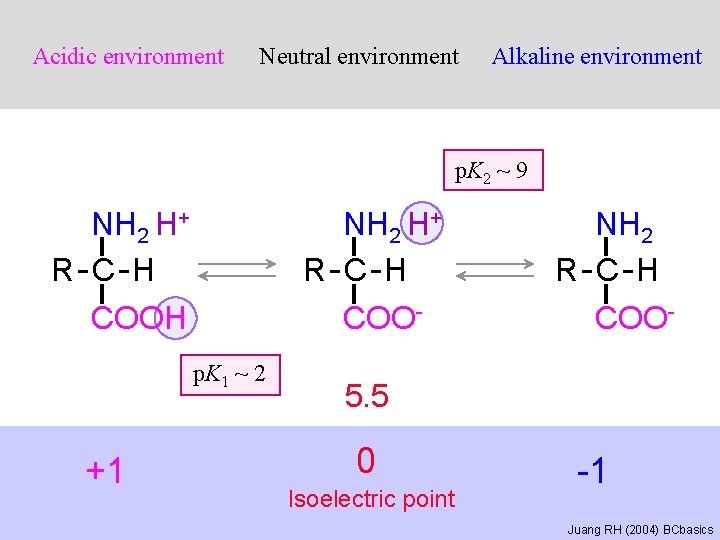
Acidic environment Neutral environment Alkaline environment p. K 2 ~ 9 NH 2 H+ R-C-H COOH NH 2 H+ R-C-H COOp. K 1 ~ 2 +1 NH 2 R-C-H COO- 5. 5 0 Isoelectric point -1 Juang RH (2004) BCbasics

Amino Acids Have Buffering Effect p. H 12 ★ p. K 2 Isoelectric point = p. I 9 6 NH 2 H+ H -C -R COO- 3 ★ p. K 1 + p. K 2 2 p. K 1 0 [OH] → Juang RH (2004) BCbasics

Environment p. H vs Protein Charge Buffer p. H 10 9 8 7 Isoelectric point, p. I + 6 5 4 3 0 - Net Charge of a Protein Juang RH (2004) BCbasics

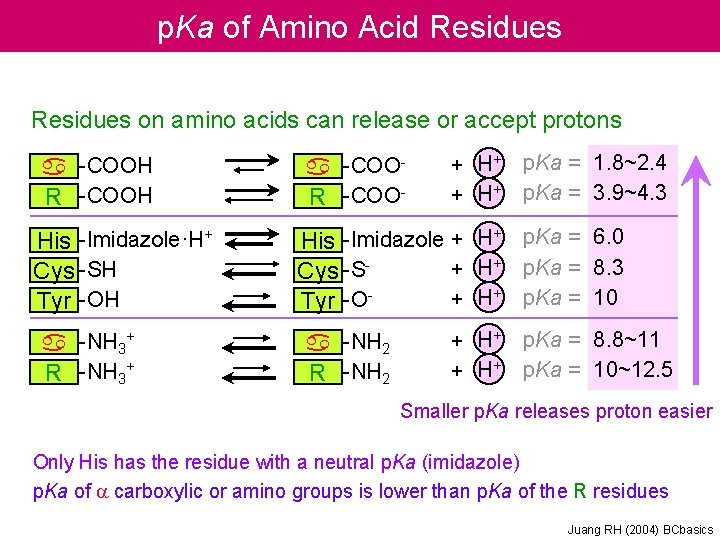
p. Ka of Amino Acid Residues on amino acids can release or accept protons a -COOH R -COOH His -Imidazole·H+ Cys -SH Tyr -OH a -NH 3+ R -NH 3+ a -COOR -COO- + H+ p. Ka = 1. 8~2. 4 p. Ka = 3. 9~4. 3 His -Imidazole + H+ p. Ka = 6. 0 + H+ p. Ka = 8. 3 Cys -S+ H+ p. Ka = 10 Tyr -Oa -NH 2 R -NH 2 + H+ p. Ka = 8. 8~11 p. Ka = 10~12. 5 Smaller p. Ka releases proton easier Only His has the residue with a neutral p. Ka (imidazole) p. Ka of a carboxylic or amino groups is lower than p. Ka of the R residues Juang RH (2004) BCbasics

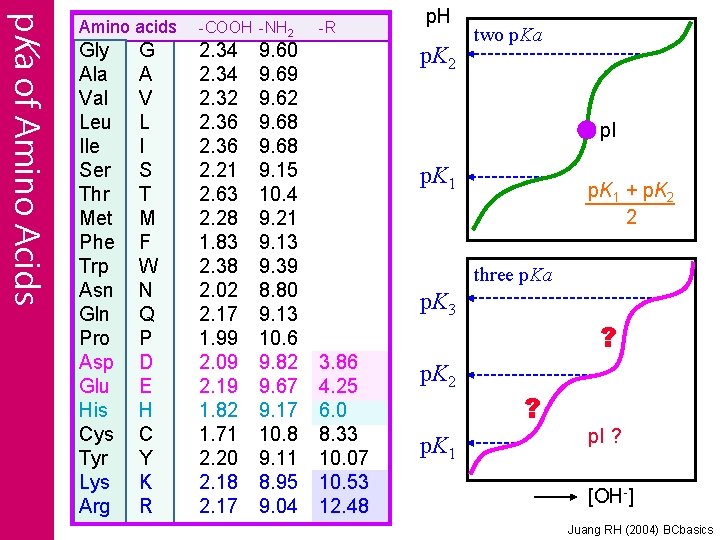
p. Ka of Amino Acids Amino acids Gly Ala Val Leu Ile Ser Thr Met Phe Trp Asn Gln Pro Asp Glu His Cys Tyr Lys Arg G A V L I S T M F W N Q P D E H C Y K R -COOH -NH 2 2. 34 2. 32 2. 36 2. 21 2. 63 2. 28 1. 83 2. 38 2. 02 2. 17 1. 99 2. 09 2. 19 1. 82 1. 71 2. 20 2. 18 2. 17 9. 60 9. 69 9. 62 9. 68 9. 15 10. 4 9. 21 9. 13 9. 39 8. 80 9. 13 10. 6 9. 82 9. 67 9. 17 10. 8 9. 11 8. 95 9. 04 -R p. H p. K 2 two p. Ka p. I p. K 1 + p. K 2 2 three p. Ka p. K 3 3. 86 4. 25 6. 0 8. 33 10. 07 10. 53 12. 48 p. K 2 p. K 1 ? ? p. I ? [OH-] Juang RH (2004) BCbasics

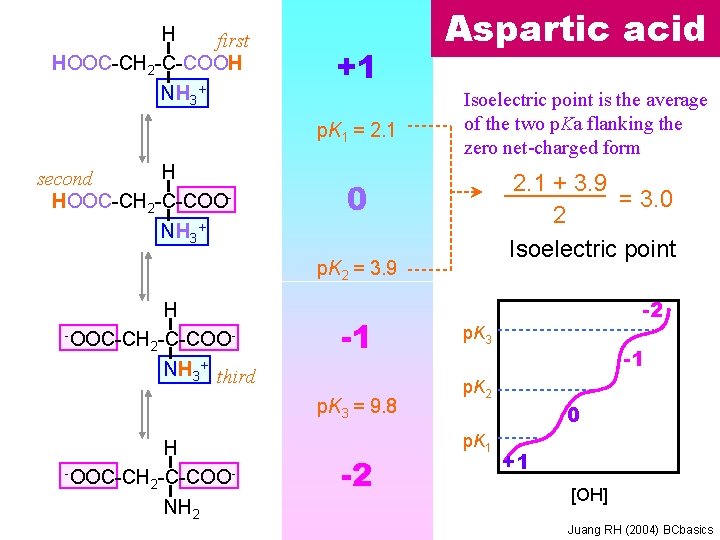
H first HOOC-CH 2 -C-COOH NH 3+ +1 p. K 1 = 2. 1 H second HOOC-CH 2 -C-COO- Aspartic acid Isoelectric point is the average of the two p. Ka flanking the zero net-charged form 2. 1 + 3. 9 = 3. 0 2 Isoelectric point 0 NH 3+ p. K 2 = 3. 9 H -OOC-CH -C-COO 2 -1 NH 3+ third p. K 3 = 9. 8 H -OOC-CH -C-COO 2 NH 2 -2 -2 p. K 3 -1 p. K 2 p. K 1 0 +1 [OH] Juang RH (2004) BCbasics