Metal hydride formation and hydrogen storage in AlLi










- Slides: 23

Metal hydride formation and hydrogen storage in Al-Li alloys IRI Symposium May 22, 2003 A. Rivera Defects in Materials, IRI, TUDelft Work supported by the Delft Institute for Sustainable Energy (DISE) Contributors at DM: A. van Veen (head), F. Labohm, J. de Roode, W. J. Legerstee, K. T. Westerduin, S. W. H. Eijt, H. Schut. External contributors (Materials Science Faculty, TUDelft): R. Delhez, N. van der Pers

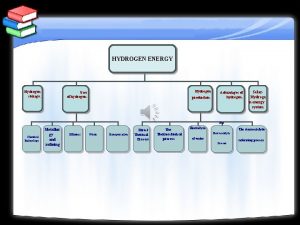
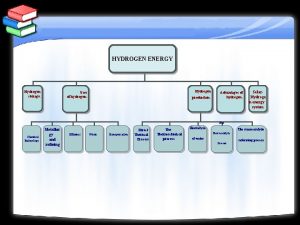
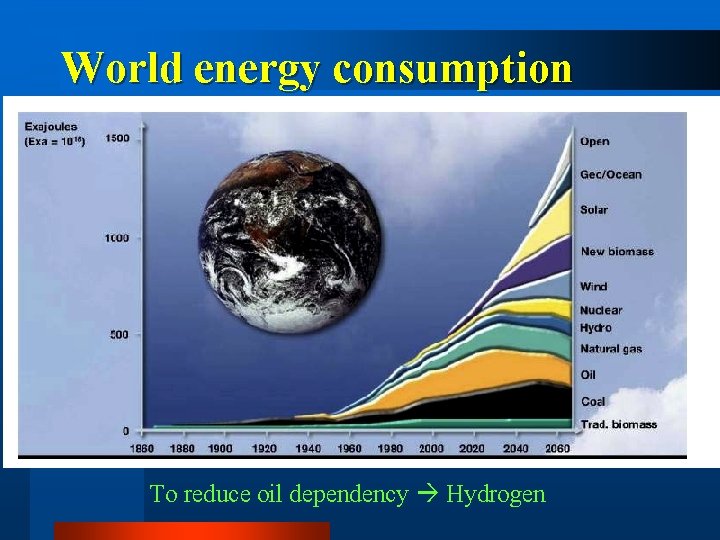
World energy consumption To reduce oil dependency Hydrogen

How to store hydrogen? l A 1000 kg car consumes – 5 -6 kg fuel/100 km l The same car would consume – 2 kg H 2/100 km in combustion mode or – 1 kg H 2 /100 km in fuel cell mode However, at room temperature and atmospheric pressure 1 kg H 2 occupies 11 m 3 l Storage: l – Pressurised vessels – Liquified H 2 – Sorbed at surface or bulk materials

Contents l Material requirements – Examples Non-transition light metal hydrides l Experimental developments l Al-Li materials l Conclusions and further work l

Material requirements l l l l Storage capacity > 5 wt. % Fast reaction kinetics H 2 release: 100 k. Pa at T < 200 ºC Reversibility in the range 0 – 200 ºC Resistance to degradation Cost Safety

Sources of inefficiencies Hysteresis between absorption and desorption l Hydride stability l Limited kinetics l – Poor heat conduction – Small diffusion constant – Surface reactions Necessity for initial hydriding activation l Sensitivity to air, impurities or other gases l Volume expansion l Decrepitation into fine powder l

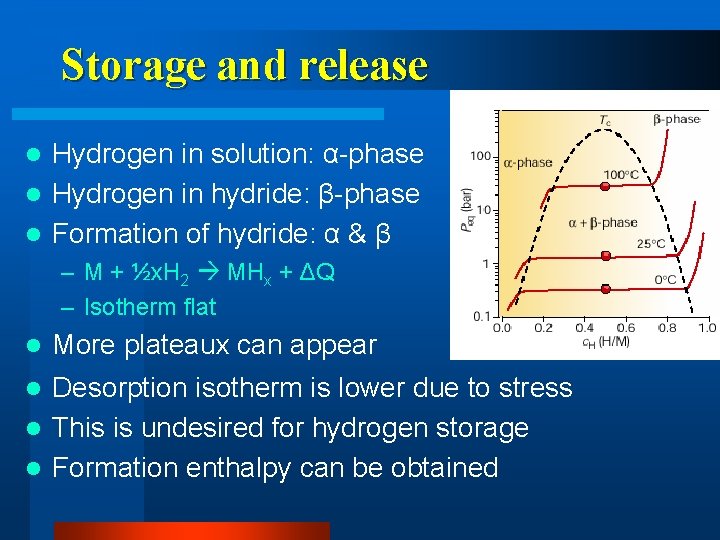
Storage and release Hydrogen in solution: α-phase l Hydrogen in hydride: β-phase l Formation of hydride: α & β l – M + ½x. H 2 MHx + ΔQ – Isotherm flat l More plateaux can appear Desorption isotherm is lower due to stress l This is undesired for hydrogen storage l Formation enthalpy can be obtained l

Kinetics E. g. Mg. H 2 at 600 K l Slow diffusion l 1 μm / s

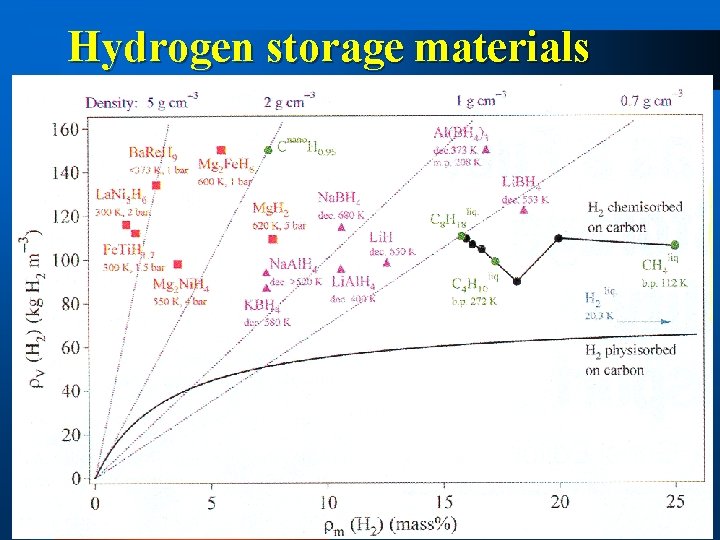
Hydrogen storage materials

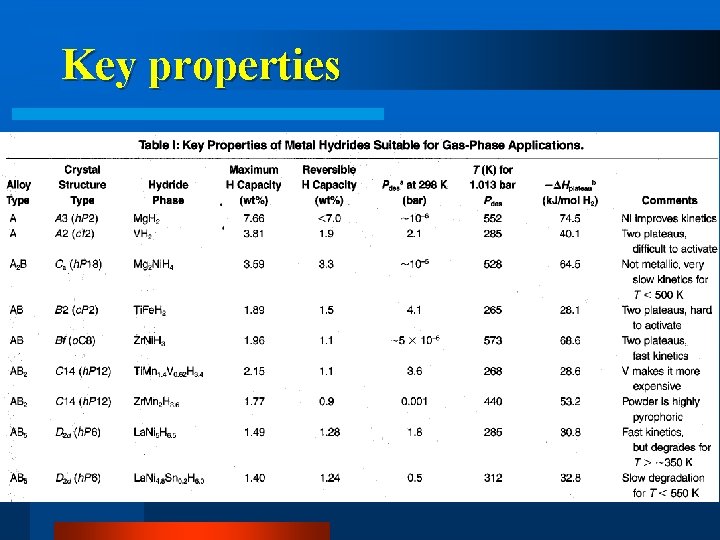
Key properties

Non-transition light metal hydrides Li. Al. H 4, Na. Al. H 4 (in water irreversible full H 2 release) l High capacities (10 and 5 wt. %, respectively) l No reversible due to decomposition l – 3 Li. Al. H 4 Li 3 Al. H 6 + 2 Al + 3 H 2 – Li 3 Al. H 6 + 2 Al 3 Li. H + 3 Al + 1. 5 H 2 – 3 Li. H + 3 Al. Li + 1. 5 H 2 Slow kinetics l Catalysts, as Fe, Ti and Zr l – Make some steps reversible – Improve the kinetics [150 -175 o. C] [180 -220 o. C] [387 -425 o. C]

Our approach Objective: to develop nanostructured light weight alloys for hydrogen storage l Choice: Al-Li compounds l Preparation l – Sputtering of Al-Li alloy or Li. Al. H 4 – Laser ablation of Al-Li alloy or Li. Al. H 4 – Cathodic charge, ion implantation gas or plasma exposure + annealing l Characterisation – Volumetric analyses, Permeation, TDS, XRD, NDP, PBA – Occasionally ERDA, SEM, TEM

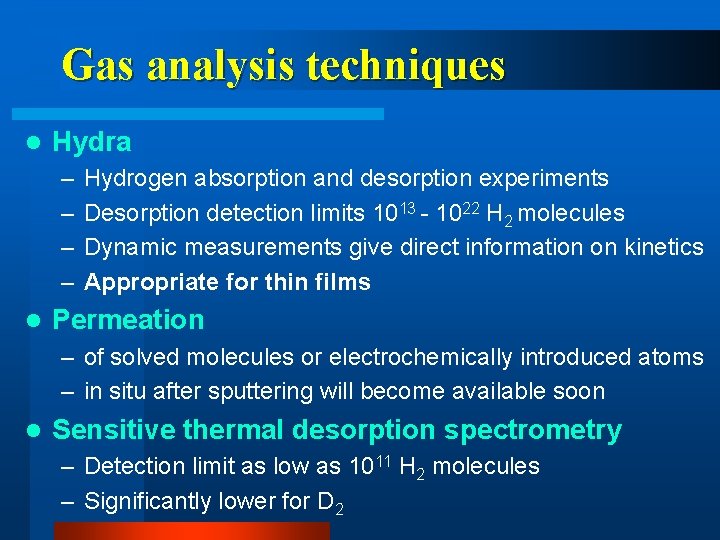
Gas analysis techniques l Hydra – – l Hydrogen absorption and desorption experiments Desorption detection limits 1013 - 1022 H 2 molecules Dynamic measurements give direct information on kinetics Appropriate for thin films Permeation – of solved molecules or electrochemically introduced atoms – in situ after sputtering will become available soon l Sensitive thermal desorption spectrometry – Detection limit as low as 1011 H 2 molecules – Significantly lower for D 2

Hydra

Hydra Mix volume Expansion volume M 1 10 -2 -10 Pa M 2 10 -105 Pa M 0 0. 1 -6 MPa Gas inlet To pumps Pd filter Mass analyser Cell (90 -900 K)

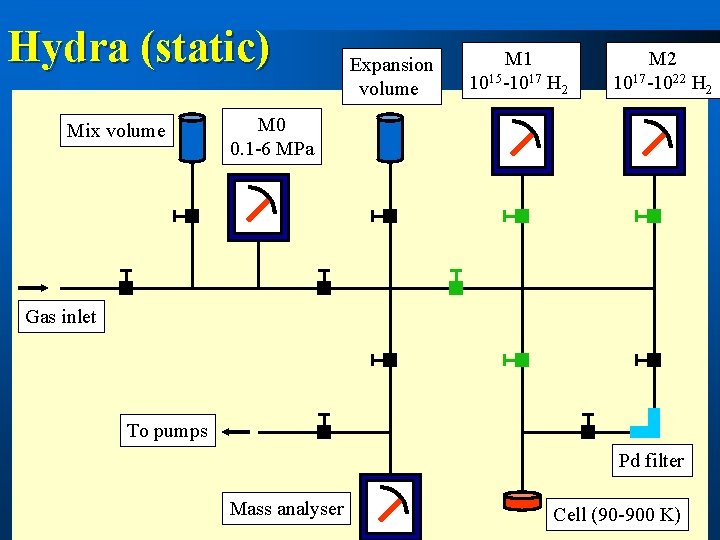
Hydra (static) Mix volume Expansion volume M 1 1015 -1017 H 2 M 2 1017 -1022 H 2 M 0 0. 1 -6 MPa Gas inlet To pumps Pd filter Mass analyser Cell (90 -900 K)

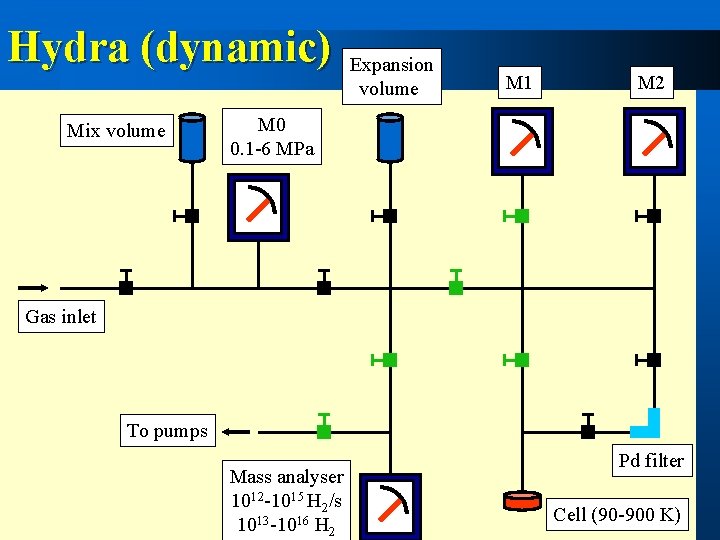
Hydra (dynamic) Mix volume Expansion volume M 1 M 2 M 0 0. 1 -6 MPa Gas inlet To pumps Mass analyser 1012 -1015 H 2/s 1013 -1016 H 2 Pd filter Cell (90 -900 K)

Hydra software

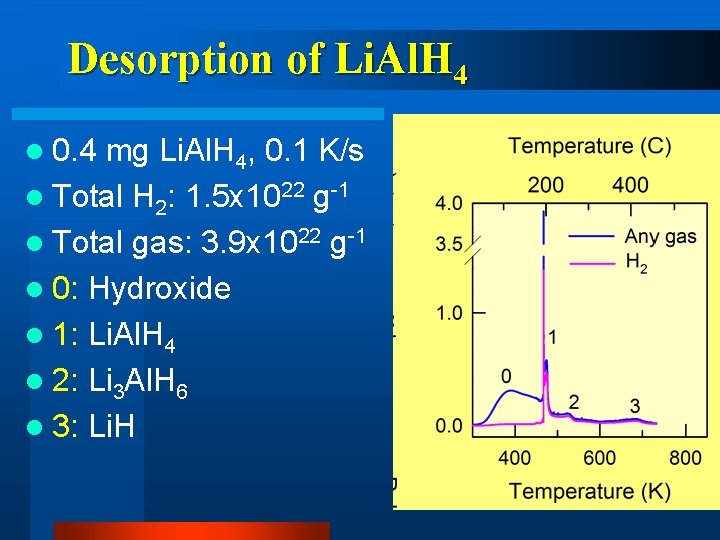
Desorption of Li. Al. H 4 l 0. 4 mg Li. Al. H 4, 0. 1 K/s l Total H 2: 1. 5 x 1022 g-1 l Total gas: 3. 9 x 1022 g-1 l 0: Hydroxide l 1: Li. Al. H 4 l 2: Li 3 Al. H 6 l 3: Li. H

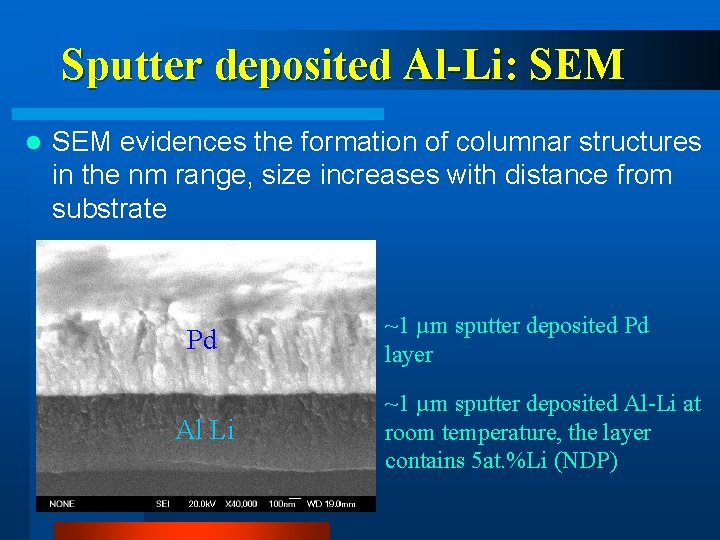
Sputter deposited Al-Li: SEM l SEM evidences the formation of columnar structures in the nm range, size increases with distance from substrate Pd Al Li ~1 µm sputter deposited Pd layer ~1 µm sputter deposited Al-Li at room temperature, the layer contains 5 at. %Li (NDP)

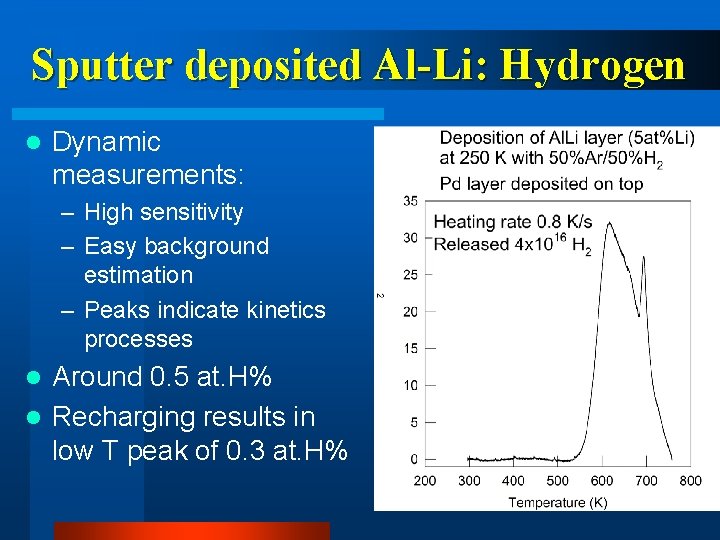
Sputter deposited Al-Li: Hydrogen l Dynamic measurements: – High sensitivity – Easy background estimation – Peaks indicate kinetics processes Around 0. 5 at. H% l Recharging results in low T peak of 0. 3 at. H% l

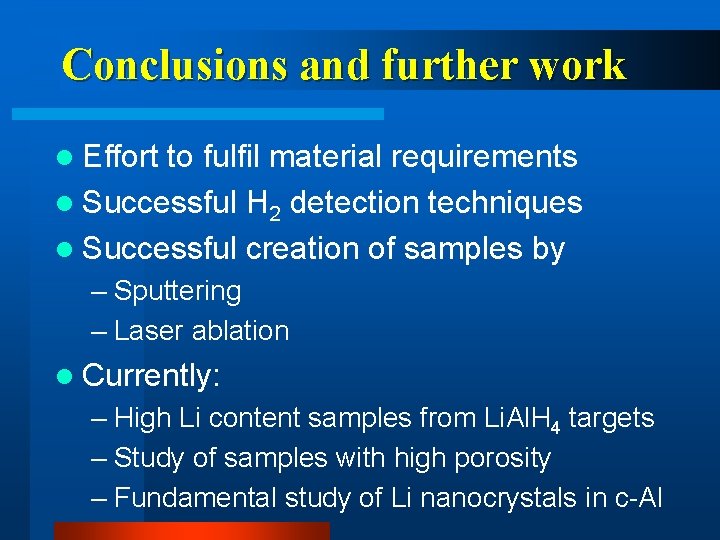
Conclusions and further work l Effort to fulfil material requirements l Successful H 2 detection techniques l Successful creation of samples by – Sputtering – Laser ablation l Currently: – High Li content samples from Li. Al. H 4 targets – Study of samples with high porosity – Fundamental study of Li nanocrystals in c-Al

Further information l Contact – A. Rivera: – A. van Veen: Rivera@iri. tudelft. nl Av. Veen@iri. tudelft. nl
 Hydride vs hydrogen
Hydride vs hydrogen Dzmitry malevich
Dzmitry malevich Beta hydride elimination
Beta hydride elimination Hydride types
Hydride types Beta hydride elimination
Beta hydride elimination Lithium oxide hydrochloric acid
Lithium oxide hydrochloric acid Hydrogen storage
Hydrogen storage Hydrogen storage
Hydrogen storage Hydrogen storage
Hydrogen storage Acidity trends periodic table
Acidity trends periodic table Compare the properties of metals and nonmetals
Compare the properties of metals and nonmetals Uses for non metals
Uses for non metals Metal and non metal elements in periodic table
Metal and non metal elements in periodic table Dr terry blanch
Dr terry blanch Metals vs nonmetals
Metals vs nonmetals Example of metal
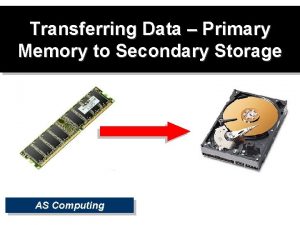
Example of metal Primary storage vs secondary storage
Primary storage vs secondary storage Storage devices of computer
Storage devices of computer Alabanza si hubiera estado allí
Alabanza si hubiera estado allí Recurso literario
Recurso literario Tiina civil
Tiina civil Dimensiones de la vocacion
Dimensiones de la vocacion Demostrativo singular masculino
Demostrativo singular masculino Allí
Allí