Copper Hydride Catalyzed Hydroamination of Alkenes and Alkynes














- Slides: 29

Copper Hydride Catalyzed Hydroamination of Alkenes and Alkynes Reporter: Pengfei Yuan Supervisor: Prof. Yong Huang 2016 -06 -22 1

Contents 1. Introduction 2. Study of copper(I) Hydride complexes, hydroxylamine esters and related reactions 3. Copper(I) hydride-catalyzed hydroamination: scope, and mechanistic insight 4. Outlook 5. Acknowledgement 2

Contents 1. Introduction 2. Study of copper(I) Hydride complexes, hydroxylamine esters and related reactions 3. Copper(I) hydride-catalyzed hydroamination: scope, and mechanistic insight 4. Outlook 5. Acknowledgement 3

Copper hydride Source of copper Red solid Air sensitive 4

Hydroamination 5

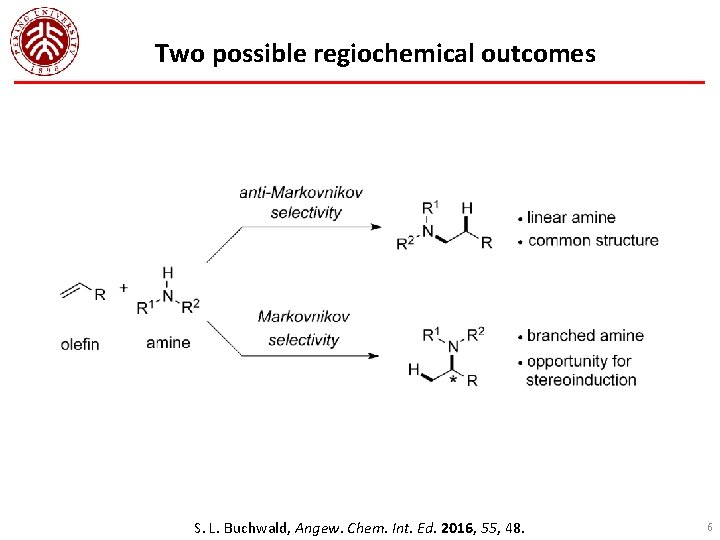
Two possible regiochemical outcomes S. L. Buchwald, Angew. Chem. Int. Ed. 2016, 55, 48. 6

Contents 1. Introduction 2. Study of copper(I) Hydride complexes, hydroxylamine esters and related reactions 3. Copper(I) hydride-catalyzed hydroamination: scope, and mechanistic insight 4. Outlook 5. Acknowledgement 7

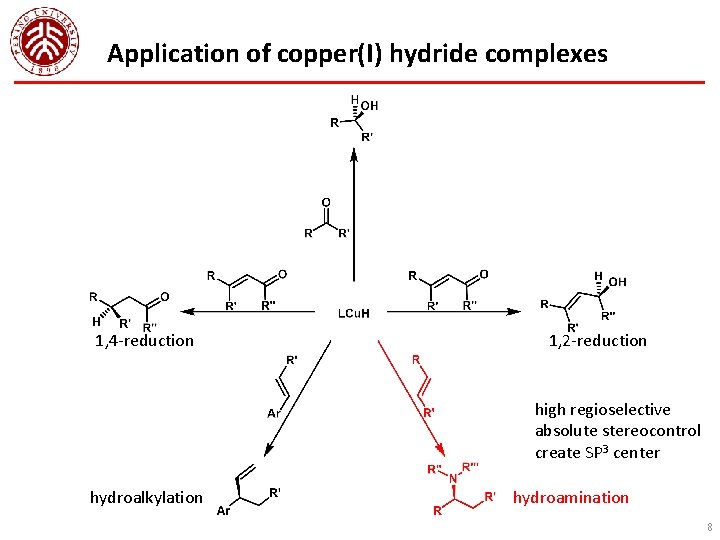
Application of copper(I) hydride complexes 1, 4 -reduction 1, 2 -reduction high regioselective absolute stereocontrol create SP 3 center hydroalkylation hydroamination 8

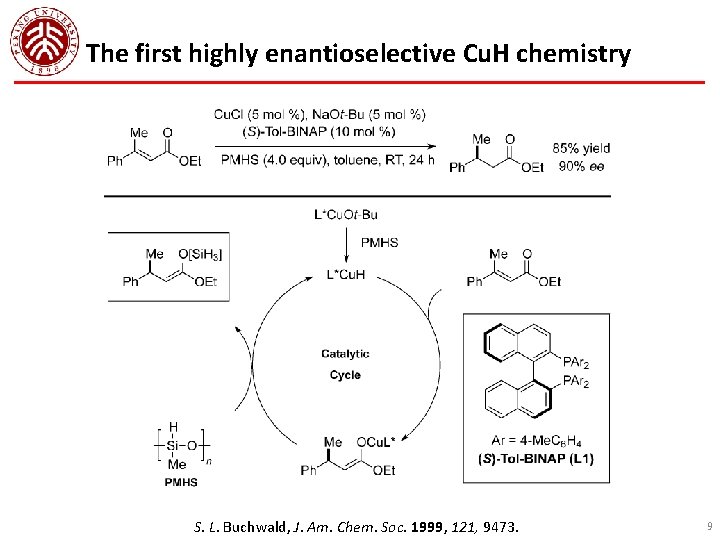
The first highly enantioselective Cu. H chemistry S. L. Buchwald, J. Am. Chem. Soc. 1999, 121, 9473. 9

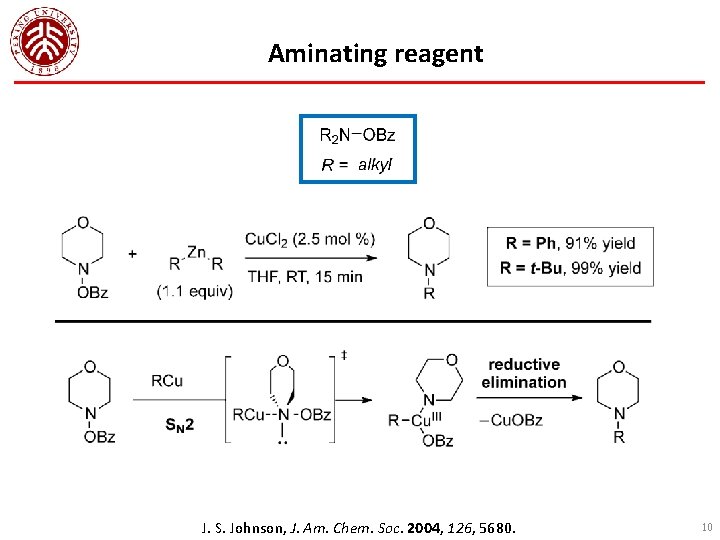
Aminating reagent J. S. Johnson, J. Am. Chem. Soc. 2004, 126, 5680. 10

Contents 1. Introduction 2. Study of copper(I) Hydride complexes, hydroxylamine esters and related reactions 3. Copper(I) hydride-catalyzed hydroamination: scope, and mechanistic insight 4. Outlook 5. Acknowledgement 11

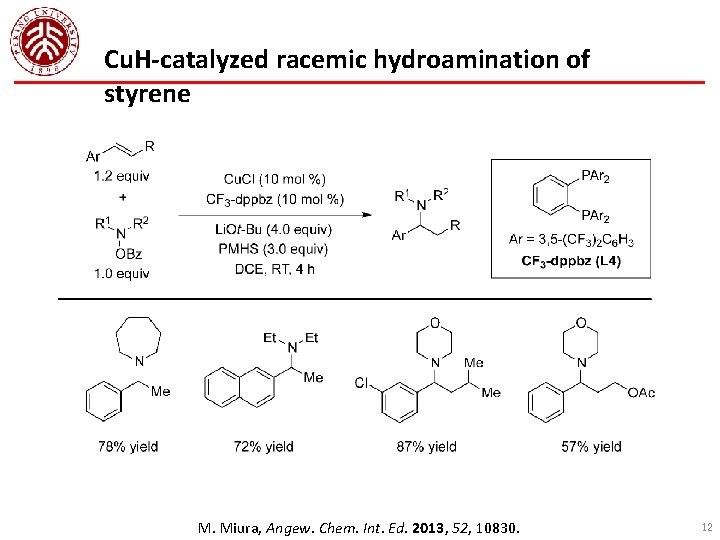
Cu. H-catalyzed racemic hydroamination of styrene M. Miura, Angew. Chem. Int. Ed. 2013, 52, 10830. 12

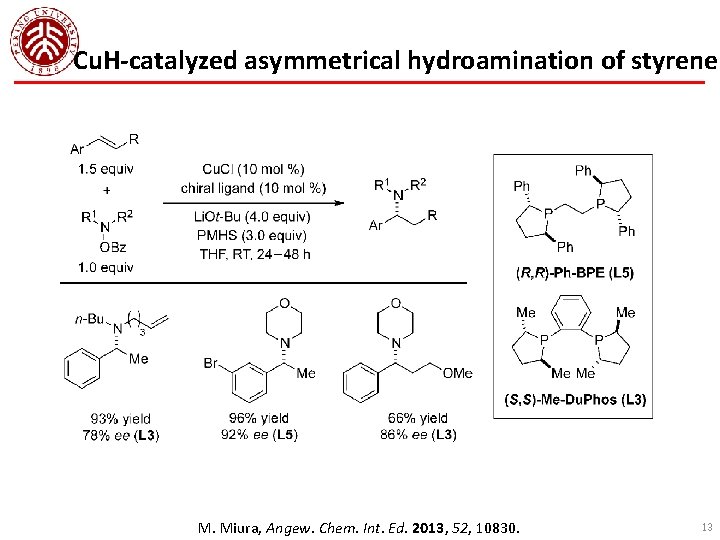
Cu. H-catalyzed asymmetrical hydroamination of styrene M. Miura, Angew. Chem. Int. Ed. 2013, 52, 10830. 13

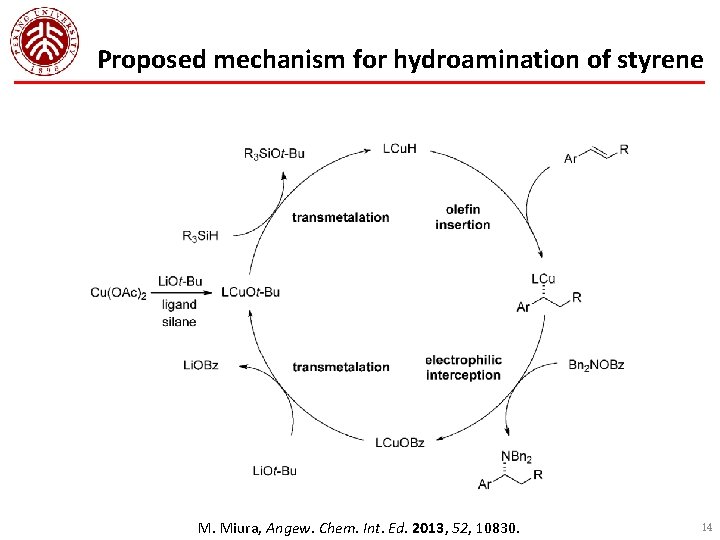
Proposed mechanism for hydroamination of styrene M. Miura, Angew. Chem. Int. Ed. 2013, 52, 10830. 14

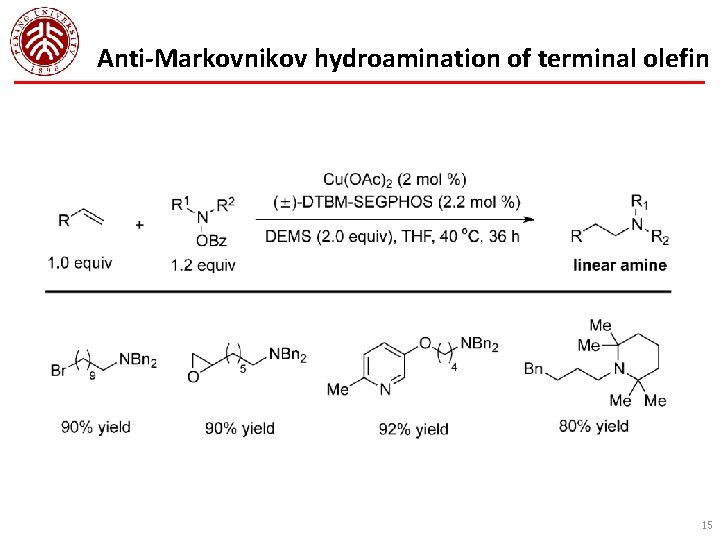
Anti-Markovnikov hydroamination of terminal olefin 15

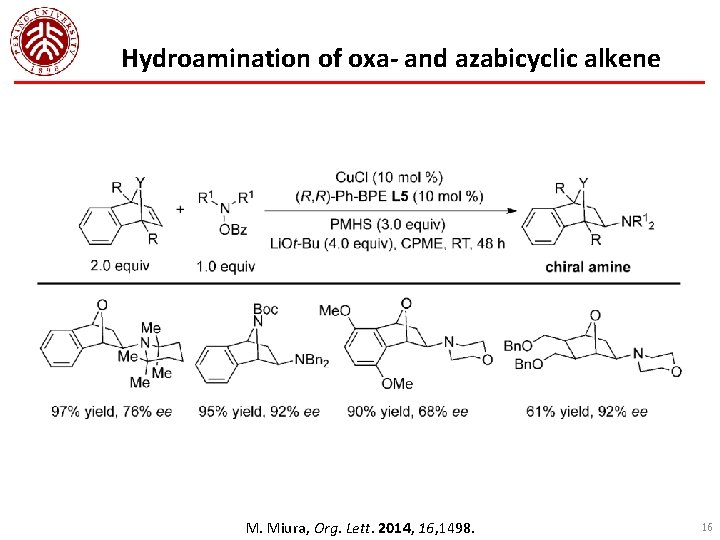
Hydroamination of oxa- and azabicyclic alkene M. Miura, Org. Lett. 2014, 16, 1498. 16

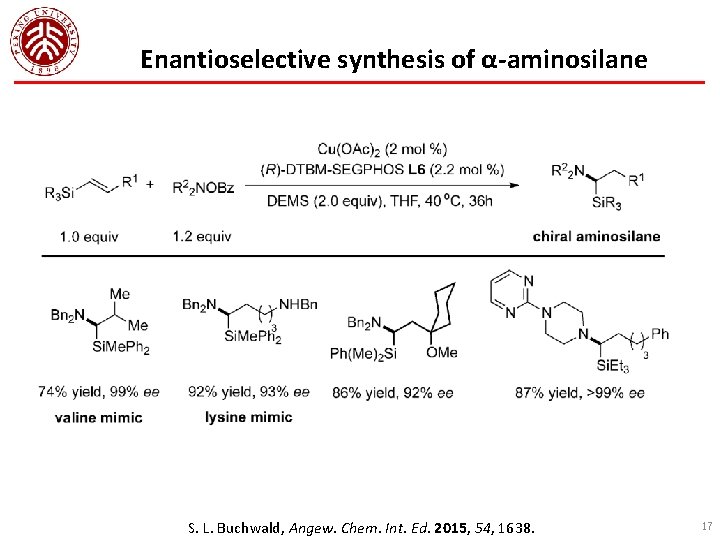
Enantioselective synthesis of α-aminosilane S. L. Buchwald, Angew. Chem. Int. Ed. 2015, 54, 1638. 17

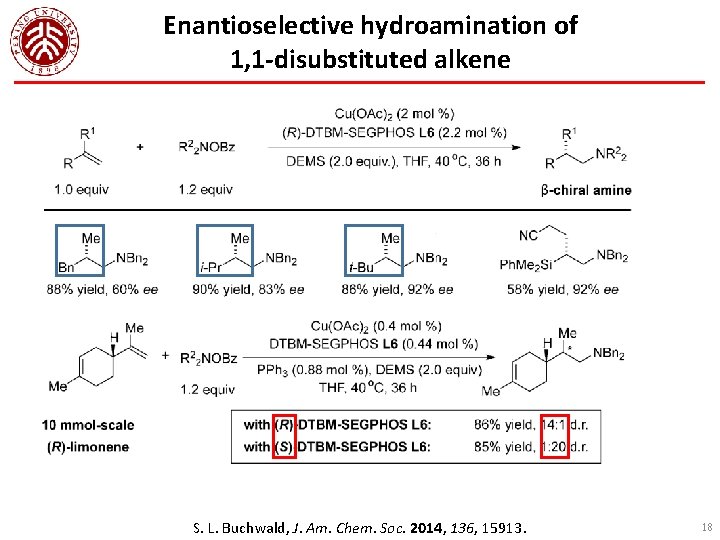
Enantioselective hydroamination of 1, 1 -disubstituted alkene S. L. Buchwald, J. Am. Chem. Soc. 2014, 136, 15913. 18

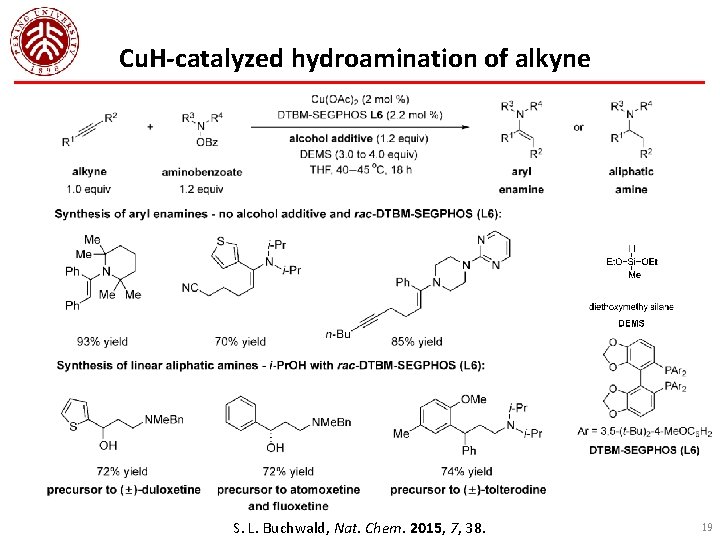
Cu. H-catalyzed hydroamination of alkyne S. L. Buchwald, Nat. Chem. 2015, 7, 38. 19

Proposed mechanism for hydroamination of alkyne S. L. Buchwald, Nat. Chem. 2015, 7, 38. 20

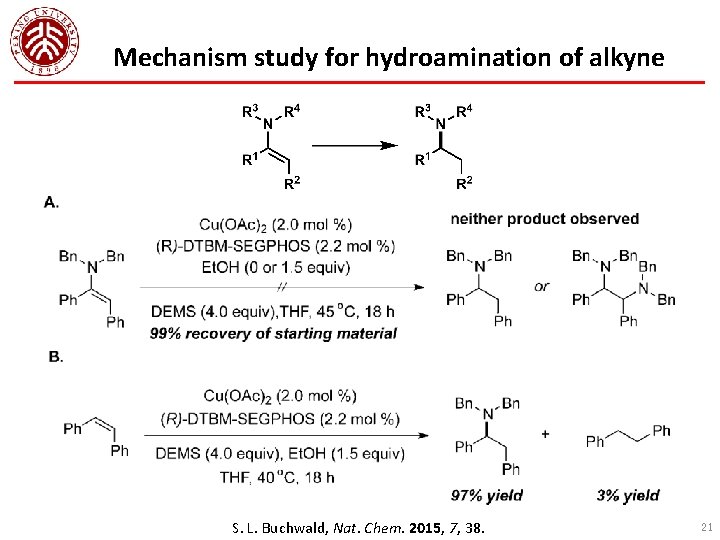
Mechanism study for hydroamination of alkyne S. L. Buchwald, Nat. Chem. 2015, 7, 38. 21

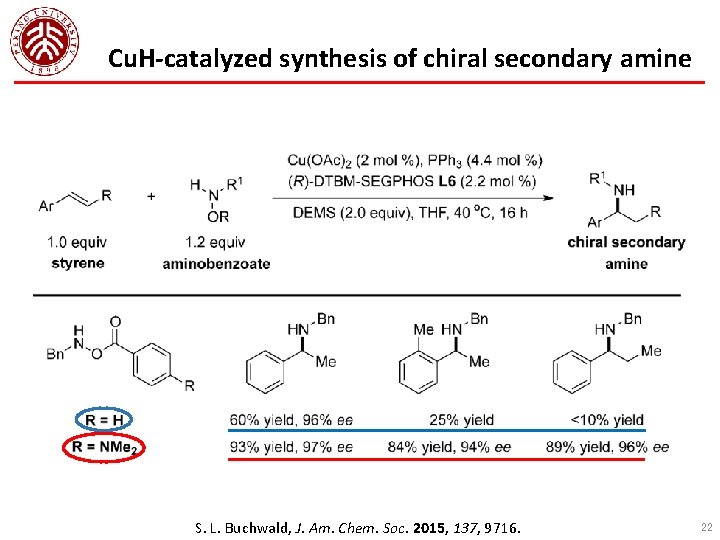
Cu. H-catalyzed synthesis of chiral secondary amine S. L. Buchwald, J. Am. Chem. Soc. 2015, 137, 9716. 22

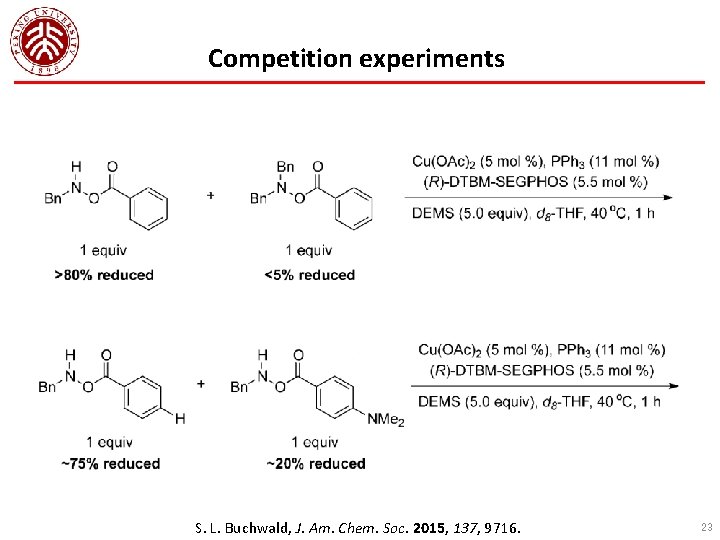
Competition experiments S. L. Buchwald, J. Am. Chem. Soc. 2015, 137, 9716. 23

Hydroamination of unactivated internal olefin S. L. Buchwald, Science, 2015, 349, 62. 24

Calculation S. L. Buchwald, Science, 2015, 349, 62. 25

Contents 1. Introduction 2. Study of copper(I) Hydride complexes, hydroxylamine esters and related reactions 3. Copper(I) hydride-catalyzed hydroamination: scope, and mechanistic insight 4. Outlook 5. Acknowledgement 26

Outlook Ø Amines derived from tri- and tetrasubstituted alkenes; Ø Utilize electrophilic aminating agents to transfer aromatic amines or ammonia or its equivalents. 27

Contents 1. Introduction 2. Study of copper(I) Hydride complexes, hydroxylamine esters and related reactions 3. Copper(I) hydride-catalyzed hydroamination: scope, and mechanistic insight 4. Outlook and conclusions 5. Acknowledgement 28

Acknowledgment Ø Prof. Huang Ø Jiean Chen Ø All members here Thanks for your attention! 29
 Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Alkanes alkenes alkynes
Alkanes alkenes alkynes Buchwald hydroamination
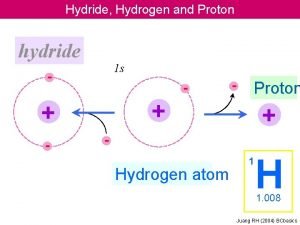
Buchwald hydroamination Hydride vs proton
Hydride vs proton Beta hydride elimination
Beta hydride elimination Hydride types
Hydride types Beta hydride elimination
Beta hydride elimination Metal hydride
Metal hydride Dehydration of 2-methylcyclohexanol
Dehydration of 2-methylcyclohexanol Benzoin condensation mechanism
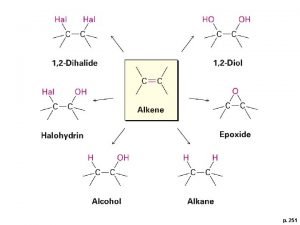
Benzoin condensation mechanism Is acid catalyzed hydration syn or anti
Is acid catalyzed hydration syn or anti Difusion molecular
Difusion molecular Is acid catalyzed hydration syn or anti
Is acid catalyzed hydration syn or anti Is acid catalyzed hydration syn or anti
Is acid catalyzed hydration syn or anti Acid catalyzed hydrolysis of nitrile
Acid catalyzed hydrolysis of nitrile Carboxylic acid base
Carboxylic acid base Acid catalyzed dehydration of cyclohexanol
Acid catalyzed dehydration of cyclohexanol Enolate anion
Enolate anion Enzymes speed up chemical reactions by ____
Enzymes speed up chemical reactions by ____ Muon-catalyzed fusion
Muon-catalyzed fusion Alkenes reactions and synthesis
Alkenes reactions and synthesis Halogenation of alkynes
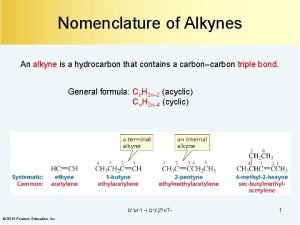
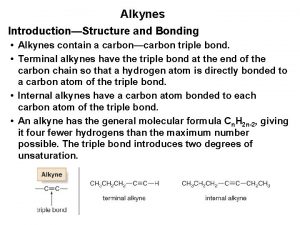
Halogenation of alkynes Naming alkynes
Naming alkynes What is the general formula for alkenes?
What is the general formula for alkenes? Hybridization of alkynes
Hybridization of alkynes What are the first 10 alkynes
What are the first 10 alkynes Preparations of alkynes
Preparations of alkynes Alkynes
Alkynes Alkynes
Alkynes