RSPT 1060 MODULE C Lesson 6 Solutions Concentrations













































- Slides: 55

RSPT 1060 MODULE C Lesson 6 Solutions, Concentrations, & Medication Delivery

OBJECTIVES At the end of this module, the student will be able to… Define terms associated with solutions, concentrations and medication delivery. Describe the relationship between matter and mixtures. Differentiate between a homogeneous and heterogeneous solution. Give an example of a colloid, suspension and solution found in the human body. Identify the components of a solution. Give an example of each of the following solutions: – – – • • – Gas dissolving in a liquid. Solid dissolving in a liquid. List the things that affect solids or liquids dissolving in a liquid.

OBJECTIVES At the end of this module, the student will be able to… – – – – List the things that affect gases dissolving in a liquid. Differentiate between a dilute, saturated and a supersaturated solution and a precipitate. Differentiate between osmosis and diffusion. Explain osmotic pressure and give examples of how it works Describe the different forms of tonicity Explain the effects of a hypertonic, hypotonic and isotonic solution as it is injected into the blood stream Explain the effects of a hypertonic, hypotonic and isotonic solution as it is inhaled into the tracheobronchial tree. Explain the reason for performing drug calculations in respiratory therapy.

OBJECTIVES – – – – – At the end of this module, the student will be able to… Given a bottle of medication, identify the concentration of the drug Convert a ratio (dilution) solution and a % solution to mg/m. L Perform drug calculations given %weight/volume solutions (%) Perform drug calculations given ratio solutions (1: 100) Perform calculations using the Universal Formula for solving w/v solutions Calculate drug dilution problems Given a medication and a physician order, calculate the dosage or volume required to deliver the ordered amount of medication Differentiate between Young's Rule, Clarks Rule, Fried's Rule and Body Surface Area for calculating pediatric dosages. Given an adult dose of medication, use an infant's age in months, child's age in years, weight or body surface area to determine the correct dosage.

WEB SITES http: //www. school-forchampions. com/science/chemixtures. htm http: //www. psinvention. com/mixtures. htm http: //en. wikipedia. org/wiki/Mixture http: //en. wikipedia. org/wiki/Molal http: //el. hct. ac. ae/HSci/Pharm/Drugs. html

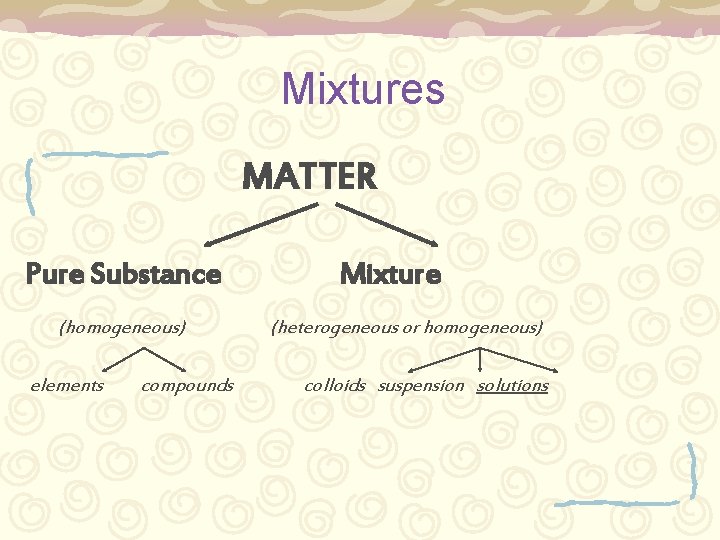
Mixtures MATTER Pure Substance (homogeneous) elements compounds Mixture (heterogeneous or homogeneous) colloids suspension solutions

Heterogeneous mixtures Heterogeneous – colloid & suspension – – – Not uniform Large particles Concentrations vary throughout May settle Can be easily separated by physical means (filtration)

Homogeneous mixtures Homogeneous – solution – – – Usually transparent Small (invisible) particles Will not settle Uniform concentration throughout Can be separated by physical means but not easily. (evaporation)

Mixtures Three types: – Colloids – Suspensions – Solutions

MIXTURES - Colloid Examples: Cellular protoplasm, milk, fat in blood, proteins in blood (albumin) – – – Heterogeneous Large molecules Attract and hold water Usually uniformly dispersed Usually do not settle Suspended in a gel

MIXTURES - Suspension Examples: red blood cells in plasma – – Heterogeneous Large particles that float in the liquid Dispersed by agitation Will settle if agitation stops

MIXTURES - Solution Example: Saline (salt + water), medications, electrolytes in body fluids – Homogeneous – Solute evenly dispersed throughout solvent so concentration is same throughout • Solute – smaller quantity dissolved, can be solid, liquid or gas, “active ingredient”. • Solvent – larger quantity, where solute is dissolved. – “Aqueous” solution has water as the solvent.

Solutions - Gases in liquids Ability of a gas to dissolve in a liquid depends upon : – Henry’s Law – dissolving (into) – Graham’s Law – diffusion (through) – Fick’s Law - overall relationships • • Surface area Thickness Partial pressure Diffusion coefficient

Solutions - Solids & liquids in liquids Ability of a solute to dissolve in a solvent also depends upon: – Physical properties of solute & solvent (density, solubility coefficient) – Pressure of solute – Temperature of solute & solvent – Presence of other solutes

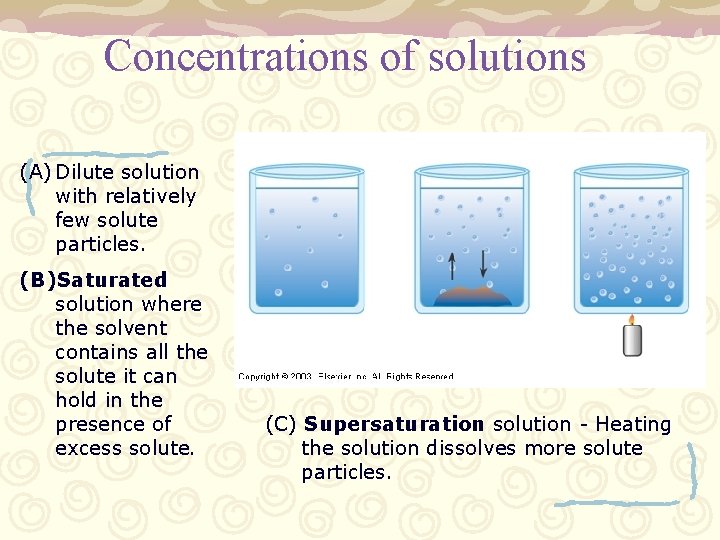
Concentrations of solutions – More or less solute or solvent will change the overall concentration of the solution. • Dilute – small amount of solute in solvent • Saturated – maximum amount of solute in solvent • Precipitate – Excess solute in solvent where some solute settles out at bottom of solvent. – As the concentration changes, the properties of the solution change (freezing point, boiling point…) • Examples: salt on roads, anti-freeze in radiator

Concentrations of solutions (A) Dilute solution with relatively few solute particles. (B)Saturated solution where the solvent contains all the solute it can hold in the presence of excess solute. (C) Supersaturation solution - Heating the solution dissolves more solute particles.

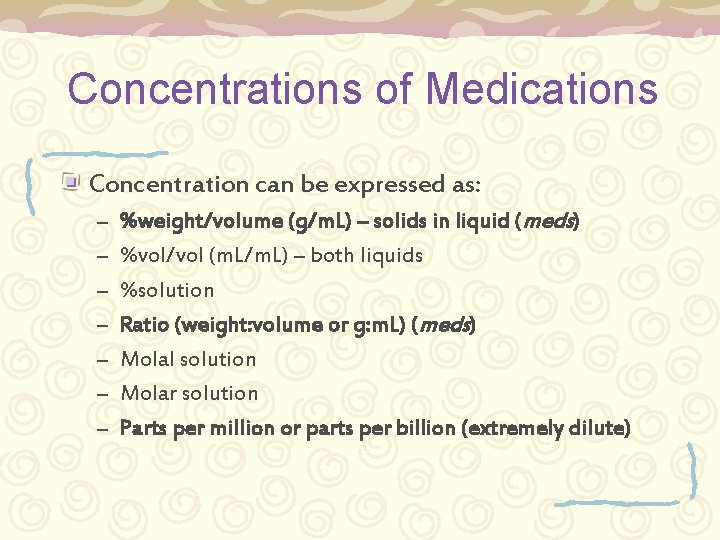
Concentrations of Medications Concentration can be expressed as: – – – – %weight/volume (g/m. L) – solids in liquid (meds) %vol/vol (m. L/m. L) – both liquids %solution Ratio (weight: volume or g: m. L) (meds) Molal solution Molar solution Parts per million or parts per billion (extremely dilute)

Medications (drug solutions) Medications are solutes in solvents. Calculations help quantify amounts of drug (solute) in sterile water or saline (solvent). Calculations also help express different concentrations: – %weight/volume (g/m. L) – solids in liquid (meds) – Ratio (weight: volume or g: m. L) (meds) – Parts per million or parts per billion (extremely dilute)

Respiratory Therapy Medications Preparations: – Multi dose – need to be measured and diluted – Unit dose – already diluted and ready to use Ultimate Goal of calculating is to know how many cc or m. L to administer.

Treatment Demonstration Nebulization of medication – Solute = medication – Solvent = saline or water Order: 2. 5 mg Albuterol in 2. 0 m. L N/S by hand held nebulizer Q 4 hours. – – – Medication Drug dosage Diluent Method of delivery Frequency

How many m. L of drug do we need? Two Ways to Determine: 1. Dosage on hand = Dosage desired Volume on hand Volume desired DH = DD * Called a weight-volume problem VH VD *Need to have drug in mg/m. L format 2. Universal Drug Calculation: #cc x #% x 10 = #mg *Need to know drug %

Weight/Volume Method Used if we have the medication in a vial that tells us how much of the drug we have in solution. Expressed as the amount of solid dissolved in a liquid. “g/m. L”

Weight/Volume Solutions Weight/volume solutions are ALWAYS expressed as a % where the percent represents the number of grams of drug in 100 ml of solvent. – 0. 5% Solution = 0. 5 grams per 100 m. L – 2. 25% Solution = 2. 25 grams per 100 m. L In order for us to use this, we must convert the g/100 m. L to mg/m. L – 0. 5% = 0. 5 grams per 100 m. L OR 500 mg per 100 m. L – 2. 25% = 2. 25 grams per 100 m. L OR 2, 250 mg per 100 m. L


Medication Problems

Respiratory Therapy Medications Preparations: – Multi dose – need to be measured and diluted – Unit dose – already diluted and ready to use Ultimate Goal of calculating is to know how many cc or m. L to administer.

Treatment Demonstration Nebulization of medication – Solute = medication – Solvent = saline or water Order: 2. 5 mg Albuterol in 2. 0 m. L N/S by hand held nebulizer Q 4 hours. – – – Medication Drug dosage Diluent Method of delivery Frequency

Medication Example The physician order states that you are to administer 2. 5 mg of albuterol. You have a 0. 5% albuterol solution. How much medication (in m. L) should you draw up? How many milligrams are in a 0. 5% solution?

Medication Example Continued DH = DD VH VD 500 mg = 2. 5 mg 100 m. L x m. L By cross-multiplying:

Universal Drug Calculation If you know the % of the solution, you can use the Universal Drug Calculation: #cc x #% x 10 = #mg

Medication Example The physician order states that you are to administer 2. 5 mg of albuterol. You have a 0. 5% albuterol solution. How much medication (in m. L) should you draw up? #cc x #% x 10 = #mg

Practice Sibberson’s Practical Math For Respiratory Care: Chapter #6, Sample Problems Second Set, page 72. May skip step in Sample Problem First Set.

Medication Order Isuprel 5 mg of a 1: 100 m. L solution in 2 m. L normal saline by small volume nebulizer Q 4 hours. – – – Medication Drug dosage Diluent Method of delivery Frequency

Ratio Solutions In this scenario, you are given the concentration of the medication not as a percentage, but rather as a ratio. The ratio is expressing the concentration as a weight/volume relationship. One Gram in some amount of m. L of solution

Ratio Solutions Ratio solutions = 1 gram/? ? ? m. L – 1: 100 = 1 gram per 100 m. L – 1: 200 = 1 gram per 200 m. L Convert to mg/m. L – 1: 100 = 1000 mg per 100 m. L – 1: 200 = 1000 mg per 200 m. L


Medication Example The physician orders 5 mg of Isuprel. You have a 1: 100 solution. Determine how much medication (in m. L) to give. What concentration of drug do you have? – 1: 100…What does that mean?

Medication Example DH = DD VH VD 1000 mg = 5 mg 100 m. L x

Universal Drug Calculation Need to convert the ratio to a percentage. 1: 100 = 1/100 =. 01 * 100% = 1%

Universal Drug Calculation The physician orders 5 mg of Isuprel. You have a 1: 100 solution. Determine how much medication to give (#m. L). 1: 100 = 1% solution

Practice Sibberson’s Practical Math For Respiratory Care: Chapter #6, Sample Problems Fourth Set, page 72. May skip step in Sample Problem Third Set.

Pressures in solutions § § Solutes in solvents exert a pressure Two kinds of pressure gradients exist: § Diffusion § § The passive movement from an area of high concentration to one of lower concentration Osmotic § The movement of water from an area of low concentration to an area of high concentration.

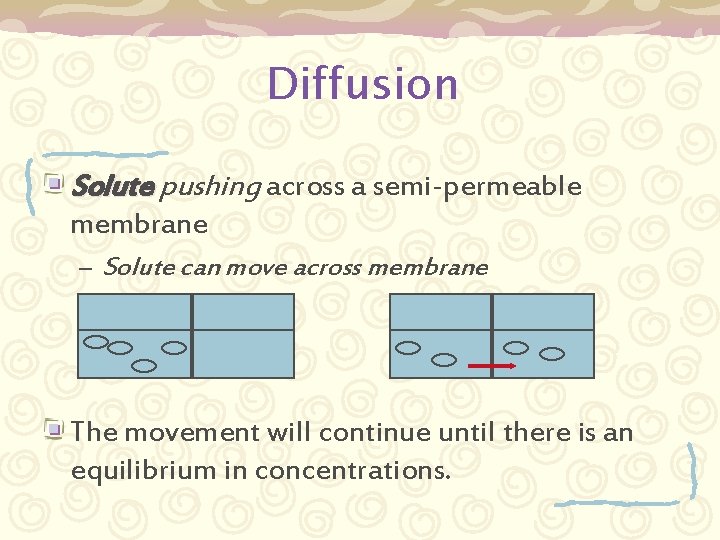
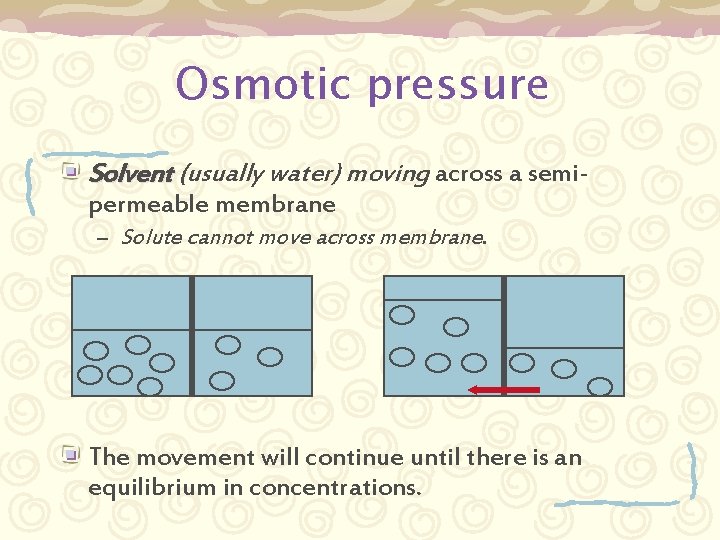
Diffusion Solute pushing across a semi-permeable membrane – Solute can move across membrane The movement will continue until there is an equilibrium in concentrations.

Osmotic pressure Solvent (usually water) moving across a semipermeable membrane – Solute cannot move across membrane. The movement will continue until there is an equilibrium in concentrations.

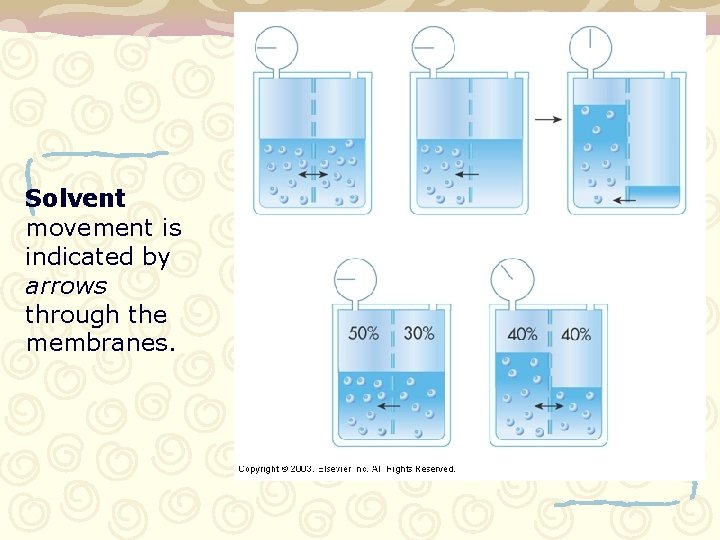
Solvent movement is indicated by arrows through the membranes.

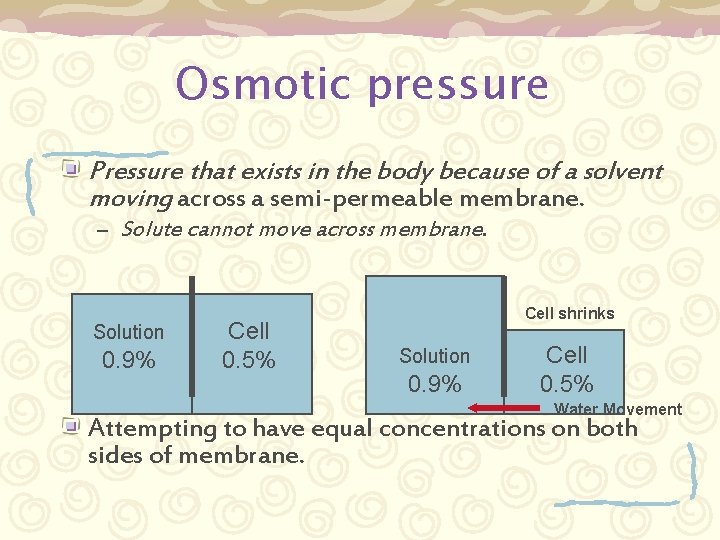
Osmotic pressure Pressure that exists in the body because of a solvent moving across a semi-permeable membrane. – Solute cannot move across membrane. Solution 0. 9% Cell 0. 5% Cell shrinks Solution 0. 9% Cell 0. 5% Water Movement Attempting to have equal concentrations on both sides of membrane.

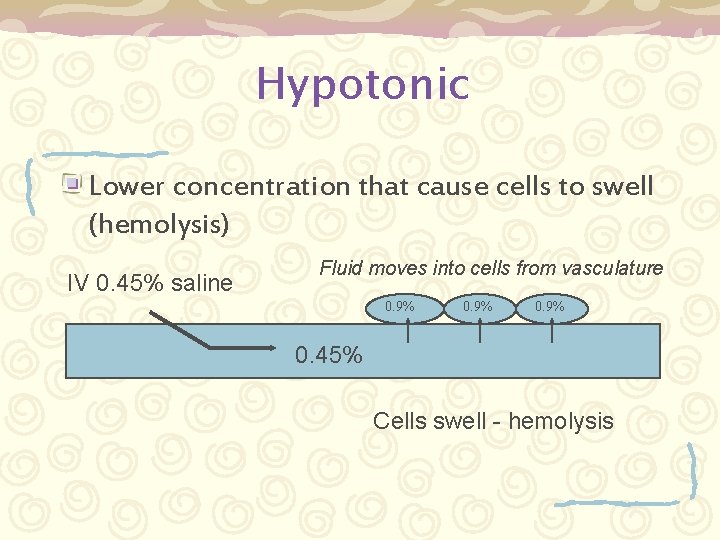
Tonicity Def: The amount of osmotic pressure in a solution. – Isotonic – having the same concentration as that of the body fluids (such as 0. 9% “normal” saline) – Hypertonic – higher concentration that cause cells to shrink (crenation) – Hypotonic – lower concentration that cause cells to swell (hemolysis)

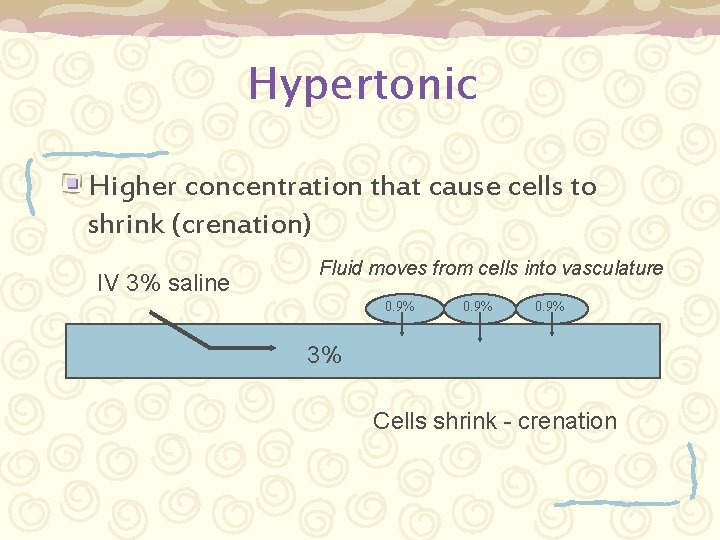
Hypertonic Higher concentration that cause cells to shrink (crenation) IV 3% saline Fluid moves from cells into vasculature 0. 9% 3% Cells shrink - crenation

Hypotonic Lower concentration that cause cells to swell (hemolysis) IV 0. 45% saline Fluid moves into cells from vasculature 0. 9% 0. 45% Cells swell - hemolysis

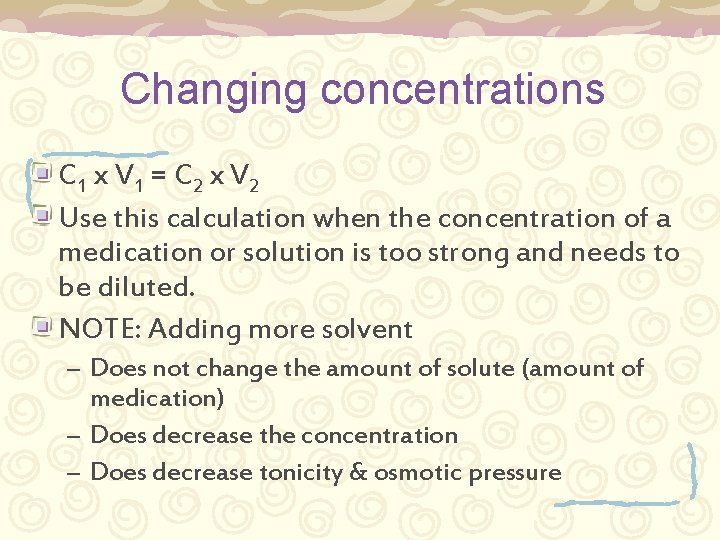
Changing concentrations C 1 x V 1 = C 2 x V 2 Use this calculation when the concentration of a medication or solution is too strong and needs to be diluted. NOTE: Adding more solvent – Does not change the amount of solute (amount of medication) – Does decrease the concentration – Does decrease tonicity & osmotic pressure

Dilution Example If you have 10 cc of 20% Mucomyst and need a 10% solution, what do you need to do? Question: How many cc of saline need to be added to 10 cc of 20% Mucomyst to obtain 10% Mucomyst?

Dilution If you have 20 cc of 0. 9% normal saline and need 0. 3% saline, what do you need to do? Question: How many cc of sterile water need to be added to 20 cc of 0. 9% Saline to obtain 0. 3% Saline?

Questions When you add more solvent (water or saline) to a medication will you be giving more medication (solute)? When you add more solvent (water or saline) to a medication what will happen to the concentration (tonicity)? (increase, decrease or stay the same) When you add more solvent (water or saline) to a medication what will happen to the time it takes to aerosolize? (increase, decrease or stay the same)

Pediatric calculations Body surface area (Dubois Chart) – (Child BSA m 2 / 1. 73) x adult dosage Fried’s Rule – Infants < 1 year – (Infant age in months / 150 months ) x adult dosage Young’s Rule – Child 1 – 12 years – (Child’s age in years/age + 12) x adult dosage Clark’s Rule – (Child’s weight in pounds/150 pounds) x adult dosage

ASSIGNMENTS Read: – Egan Chapter 11 – pages 255 – 258 & 259 – 261 Sibberson’s Practical Math For Respiratory Care: – Chapter #6, Sample Problems & Practice Exercises, pages 67 – 79. Instructor assignments