16 2 Concentrations of Solutions Chapter 16 Solutions


















- Slides: 55

16. 2 Concentrations of Solutions > Chapter 16 Solutions 16. 1 Properties of Solutions 16. 2 Concentrations of Solutions 16. 3 Colligative Properties of Solutions 16. 4 Calculations Involving Colligative Properties 1 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > CHEMISTRY & YOU How can you describe the concentration of a solution? The federal government and state governments set standards limiting the amount of contaminants allowed in drinking water. 2 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Molarity How do you calculate the molarity of a solution? 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Molarity The concentration of a solution is a measure of the amount of solute that is dissolved in a given quantity of solvent. • A solution that contains a relatively small amount of solute is a dilute solution. • A concentrated solution contains a large amount of solute. 4 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Molarity In chemistry, the most important unit of concentration is molarity. • Molarity (M) is the number of moles of solute dissolved in one liter of solution. • Molarity is also known as molar concentration. 5 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

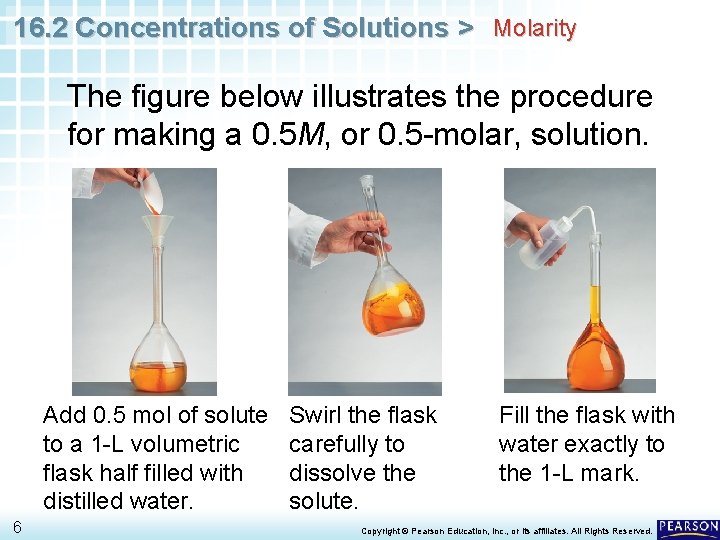
16. 2 Concentrations of Solutions > Molarity The figure below illustrates the procedure for making a 0. 5 M, or 0. 5 -molar, solution. Add 0. 5 mol of solute to a 1 -L volumetric flask half filled with distilled water. 6 Swirl the flask carefully to dissolve the solute. Fill the flask with water exactly to the 1 -L mark. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Molarity To calculate the molarity of a solution, divide the number of moles of solute by the volume of the solution in liters. moles of solute Molarity (M) = liters of solution 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Sample Problem 16. 2 Calculating Molarity Intravenous (IV) saline solutions are often administered to patients in the hospital. One saline solution contains 0. 90 g Na. Cl in exactly 100 m. L of solution. What is the molarity of the solution? 8 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

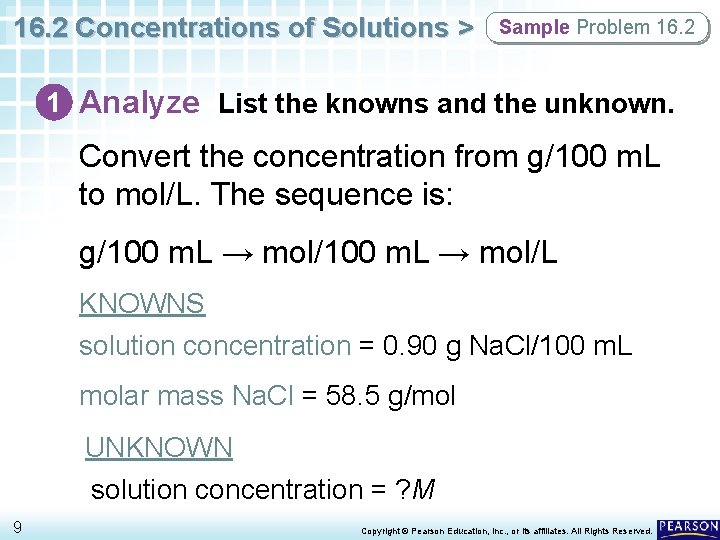
16. 2 Concentrations of Solutions > Sample Problem 16. 2 1 Analyze List the knowns and the unknown. Convert the concentration from g/100 m. L to mol/L. The sequence is: g/100 m. L → mol/L KNOWNS solution concentration = 0. 90 g Na. Cl/100 m. L molar mass Na. Cl = 58. 5 g/mol UNKNOWN solution concentration = ? M 9 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

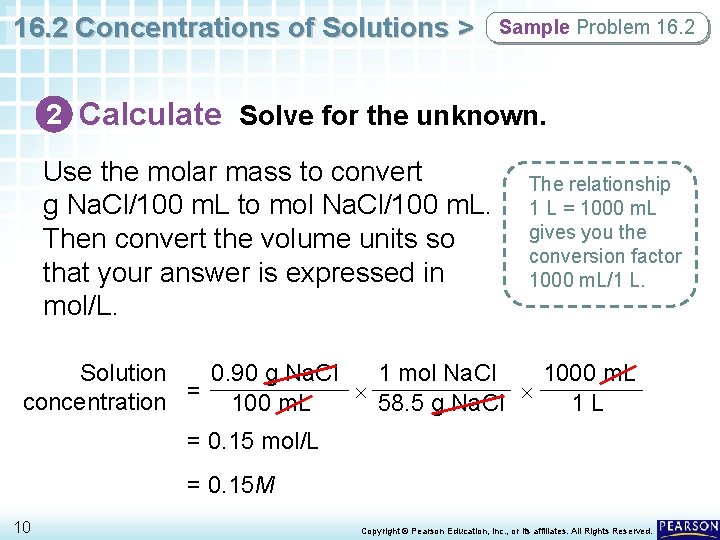
16. 2 Concentrations of Solutions > Sample Problem 16. 2 2 Calculate Solve for the unknown. Use the molar mass to convert g Na. Cl/100 m. L to mol Na. Cl/100 m. L. Then convert the volume units so that your answer is expressed in mol/L. The relationship 1 L = 1000 m. L gives you the conversion factor 1000 m. L/1 L. 0. 90 g Na. Cl 1 mol Na. Cl 1000 m. L Solution 58. 5 g Na. Cl concentration = 100 m. L 1 L = 0. 15 mol/L = 0. 15 M 10 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Sample Problem 16. 2 3 Evaluate Does the result make sense? • The answer should be less than 1 M because a concentration of 0. 90 g/100 m. L is the same as 9. 0 g/1000 m. L (9. 0 g/1 L), and 9. 0 g is less than 1 mol Na. Cl. • The answer is correctly expressed to two significant figures. 11 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Sample Problem 16. 3 Calculating the Moles of Solute in a Solution Household laundry bleach is a dilute aqueous solution of sodium hypochlorite (Na. Cl. O). How many moles of solute are present in 1. 5 L of 0. 70 M Na. Cl. O? 12 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Sample Problem 16. 3 1 Analyze List the knowns and the unknown. The conversion is: volume of solution → moles of solute. Molarity has the units mol/L, so you can use it as a conversion factor between moles of solute and volume of solution. KNOWNS volume of solution = 1. 5 L solution concentration = 0. 70 M Na. Cl. O UNKNOWN moles solute = ? mol 13 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

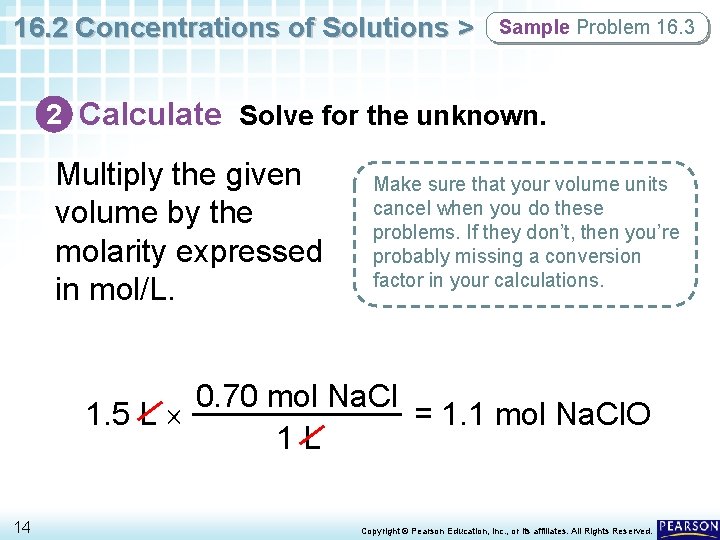
16. 2 Concentrations of Solutions > Sample Problem 16. 3 2 Calculate Solve for the unknown. Multiply the given volume by the molarity expressed in mol/L. Make sure that your volume units cancel when you do these problems. If they don’t, then you’re probably missing a conversion factor in your calculations. 0. 70 mol Na. Cl 1. 5 L = 1. 1 mol Na. Cl. O 1 L 14 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Sample Problem 16. 3 3 Evaluate Does the result make sense? • The answer should be greater than 1 mol but less than 1. 5 mol, because the solution concentration is less than 0. 75 mol/L and the volume is less than 2 L. • The answer is correctly expressed to two significant figures. 15 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > How much water is required to make a 1. 00 M aqueous solution of Na. Cl, if 58. 4 g of Na. Cl are dissolved? A. 1. 00 liter of water B. enough water to make 1. 00 liter of solution C. 1. 00 kg of water D. 100 m. L of water 16 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > How much water is required to make a 1. 00 M aqueous solution of Na. Cl, if 58. 4 g of Na. Cl are dissolved? A. 1. 00 liter of water B. enough water to make 1. 00 liter of solution C. 1. 00 kg of water D. 100 m. L of water 17 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Making Dilutions What effect does dilution have on the amount of solute? 18 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Making Dilutions Both of these solutions contain the same amount of solute. • You can tell by the color of solution (a) that it is more concentrated than solution (b). • Solution (a) has the greater molarity. • The more dilute solution (b) was made from solution (a) by adding more solvent. 19 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

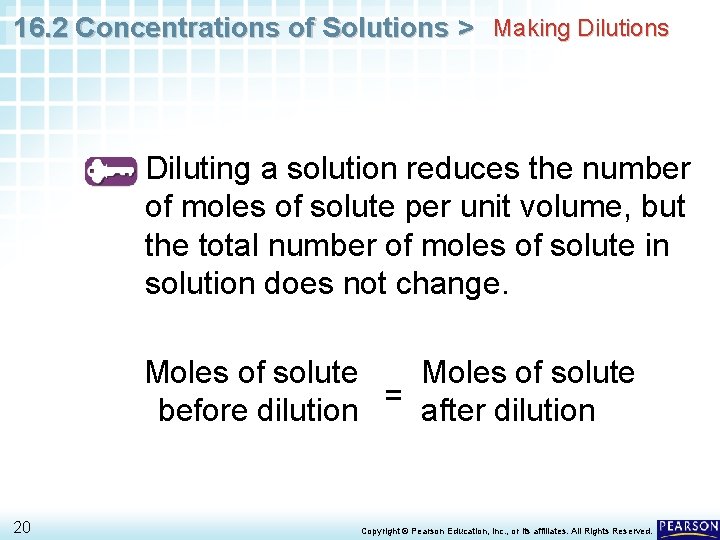
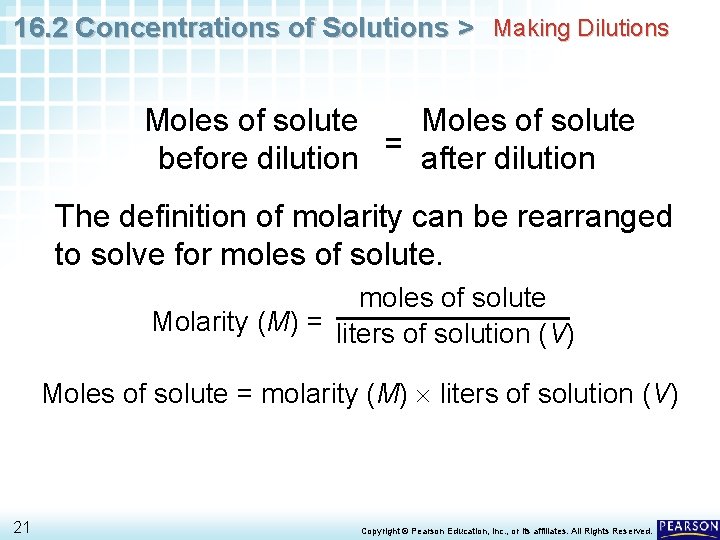
16. 2 Concentrations of Solutions > Making Dilutions Diluting a solution reduces the number of moles of solute per unit volume, but the total number of moles of solute in solution does not change. Moles of solute = before dilution after dilution 20 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Making Dilutions Moles of solute = before dilution after dilution The definition of molarity can be rearranged to solve for moles of solute Molarity (M) = liters of solution (V) Moles of solute = molarity (M) liters of solution (V) 21 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

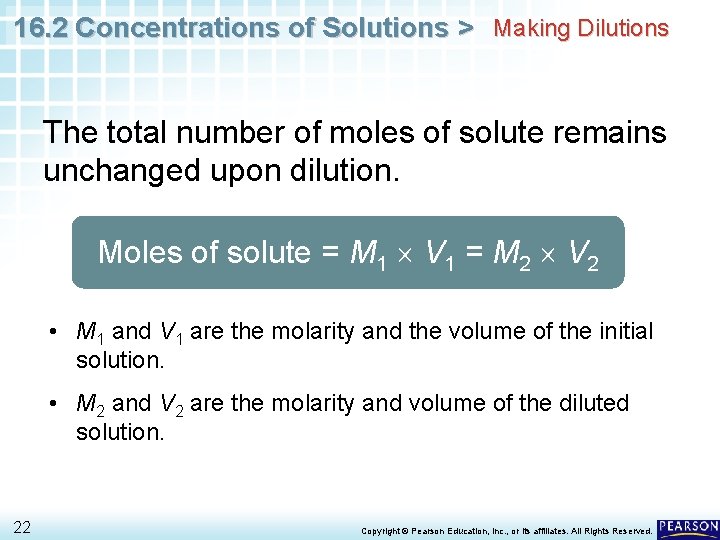
16. 2 Concentrations of Solutions > Making Dilutions The total number of moles of solute remains unchanged upon dilution. Moles of solute = M 1 V 1 = M 2 V 2 • M 1 and V 1 are the molarity and the volume of the initial solution. • M 2 and V 2 are the molarity and volume of the diluted solution. 22 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Making Dilutions The student is preparing 100 m. L of 0. 40 M Mg. SO 4 from a stock solution of 2. 0 M Mg. SO 4. She measures 20 m. L of the stock solution with a 20 -m. L pipet. 23 She transfers the 20 m. L to a 100 -m. L volumetric flask. She carefully adds water to the mark to make 100 m. L of solution. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

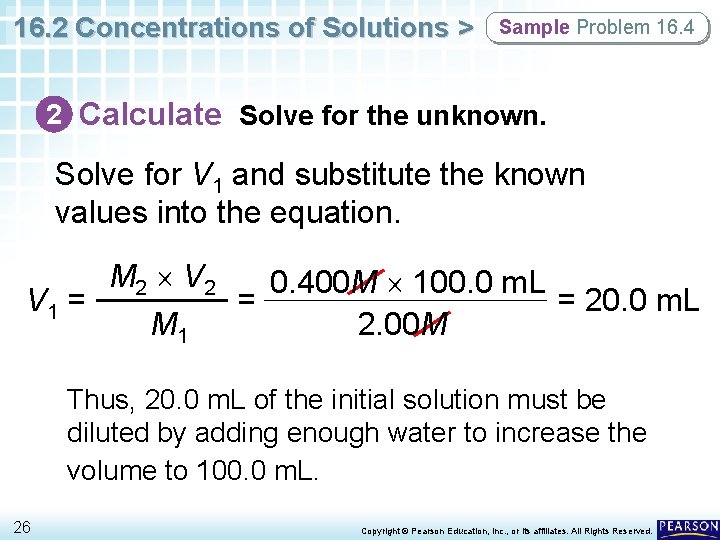
16. 2 Concentrations of Solutions > Sample Problem 16. 4 Preparing a Dilute Solution How many milliliters of aqueous 2. 00 M Mg. SO 4 solution must be diluted with water to prepare 100. 0 m. L of aqueous 0. 400 M Mg. SO 4? 24 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

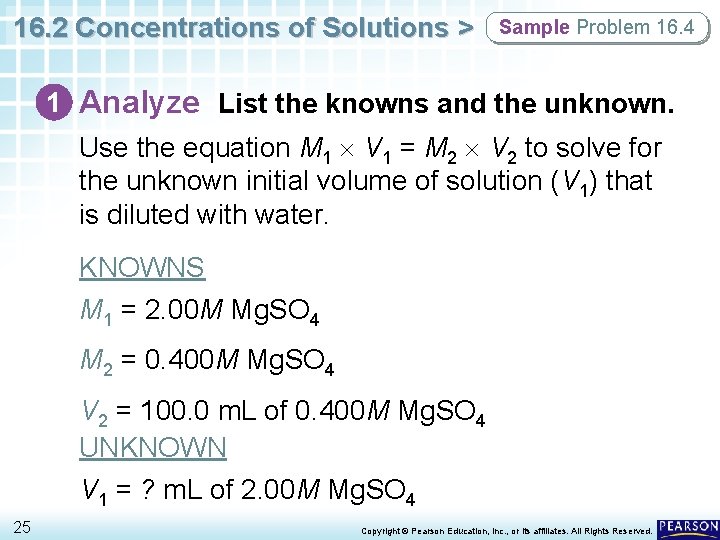
16. 2 Concentrations of Solutions > Sample Problem 16. 4 1 Analyze List the knowns and the unknown. Use the equation M 1 V 1 = M 2 V 2 to solve for the unknown initial volume of solution (V 1) that is diluted with water. KNOWNS M 1 = 2. 00 M Mg. SO 4 M 2 = 0. 400 M Mg. SO 4 V 2 = 100. 0 m. L of 0. 400 M Mg. SO 4 UNKNOWN V 1 = ? m. L of 2. 00 M Mg. SO 4 25 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Sample Problem 16. 4 2 Calculate Solve for the unknown. Solve for V 1 and substitute the known values into the equation. M 2 V 2 0. 400 M 100. 0 m. L V 1 = = = 20. 0 m. L M 1 2. 00 M Thus, 20. 0 m. L of the initial solution must be diluted by adding enough water to increase the volume to 100. 0 m. L. 26 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Sample Problem 16. 4 3 Evaluate Does the result make sense? • The initial concentration is five times larger than the dilute concentration. • Because the number of moles of solute does not change, the initial volume of solution should be one-fifth the final volume of the diluted solution. 27 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

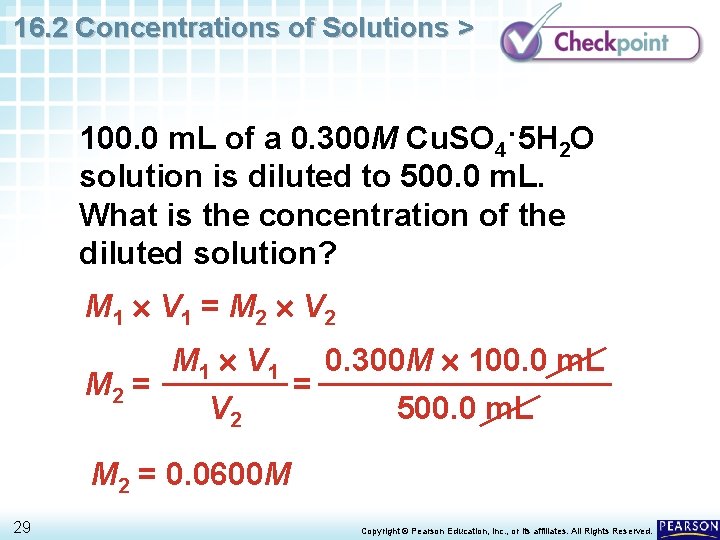
16. 2 Concentrations of Solutions > 100. 0 m. L of a 0. 300 M Cu. SO 4·5 H 2 O solution is diluted to 500. 0 m. L. What is the concentration of the diluted solution? 28 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > 100. 0 m. L of a 0. 300 M Cu. SO 4·5 H 2 O solution is diluted to 500. 0 m. L. What is the concentration of the diluted solution? M 1 V 1 = M 2 V 2 M 1 V 1 0. 300 M 100. 0 m. L M 2 = = V 2 500. 0 m. L M 2 = 0. 0600 M 29 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

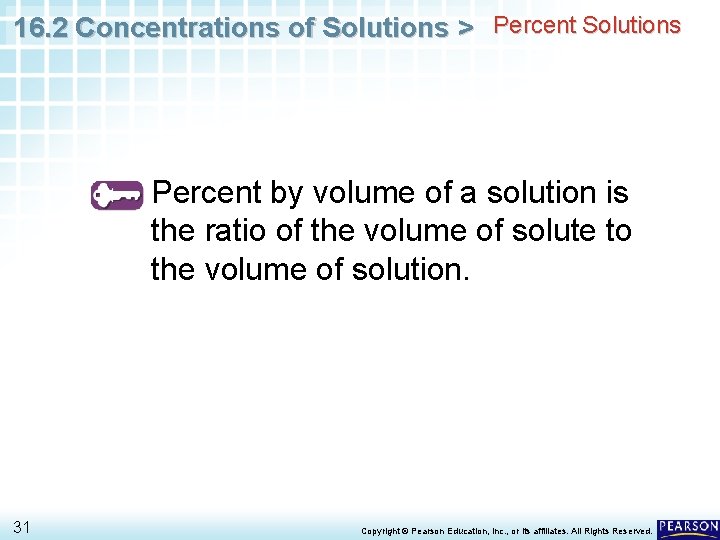
16. 2 Concentrations of Solutions > Percent Solutions How do percent by volume and percent by mass differ? 30 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

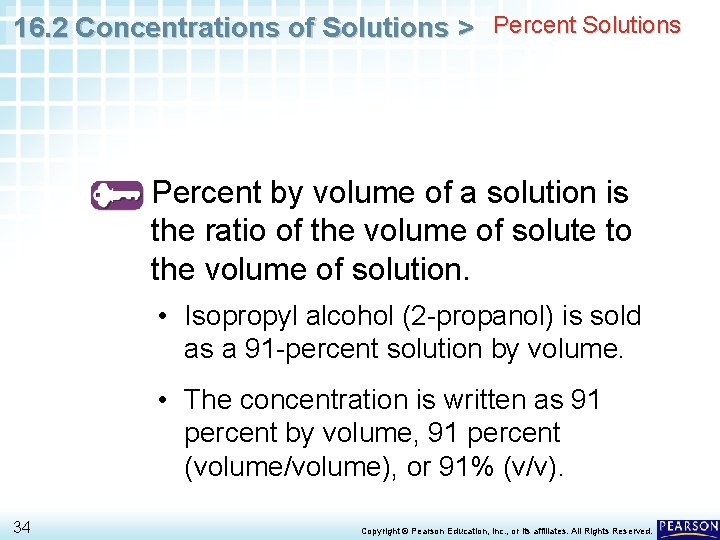
16. 2 Concentrations of Solutions > Percent Solutions Percent by volume of a solution is the ratio of the volume of solute to the volume of solution. 31 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

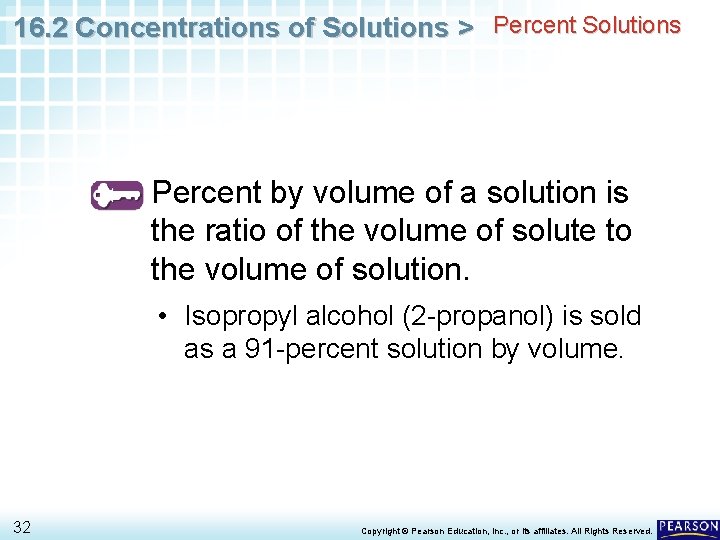
16. 2 Concentrations of Solutions > Percent Solutions Percent by volume of a solution is the ratio of the volume of solute to the volume of solution. • Isopropyl alcohol (2 -propanol) is sold as a 91 -percent solution by volume. 32 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Percent Solutions Percent by volume of a solution is the ratio of the volume of solute to the volume of solution. • Isopropyl alcohol (2 -propanol) is sold as a 91 -percent solution by volume. • You could prepare such a solution by diluting 91 m. L of pure isopropyl alcohol with enough water to make 100 m. L of solution. 33 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Percent Solutions Percent by volume of a solution is the ratio of the volume of solute to the volume of solution. • Isopropyl alcohol (2 -propanol) is sold as a 91 -percent solution by volume. • The concentration is written as 91 percent by volume, 91 percent (volume/volume), or 91% (v/v). 34 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

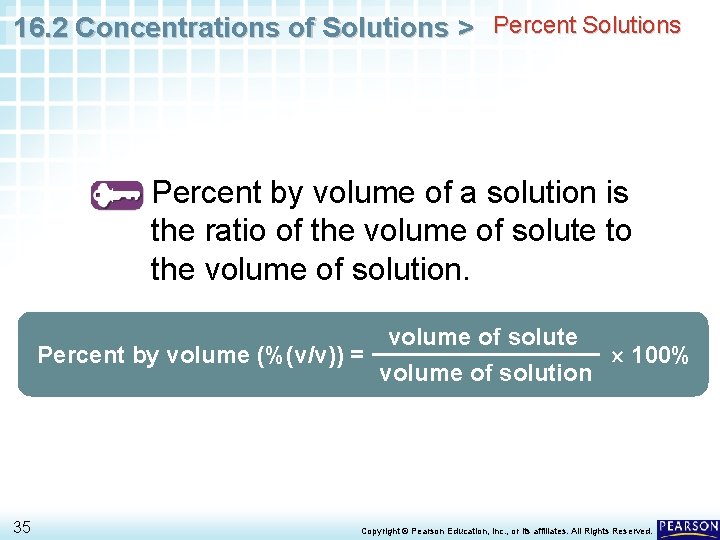
16. 2 Concentrations of Solutions > Percent Solutions Percent by volume of a solution is the ratio of the volume of solute to the volume of solution. volume of solute Percent by volume (%(v/v)) = 100% volume of solution 35 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

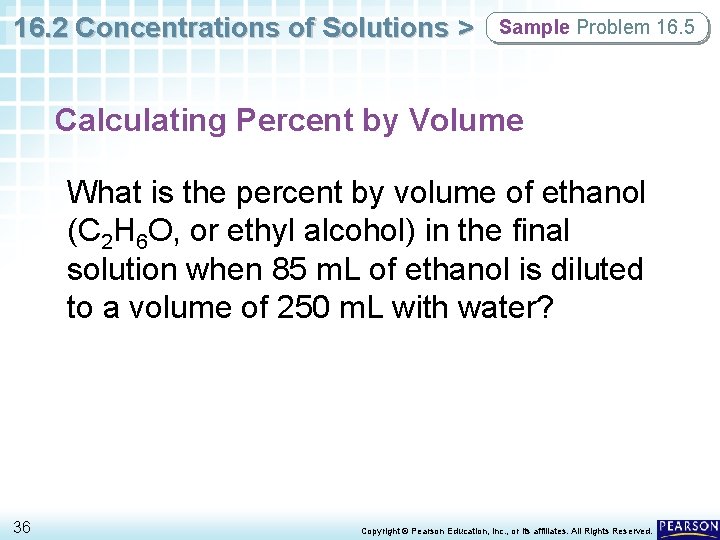
16. 2 Concentrations of Solutions > Sample Problem 16. 5 Calculating Percent by Volume What is the percent by volume of ethanol (C 2 H 6 O, or ethyl alcohol) in the final solution when 85 m. L of ethanol is diluted to a volume of 250 m. L with water? 36 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

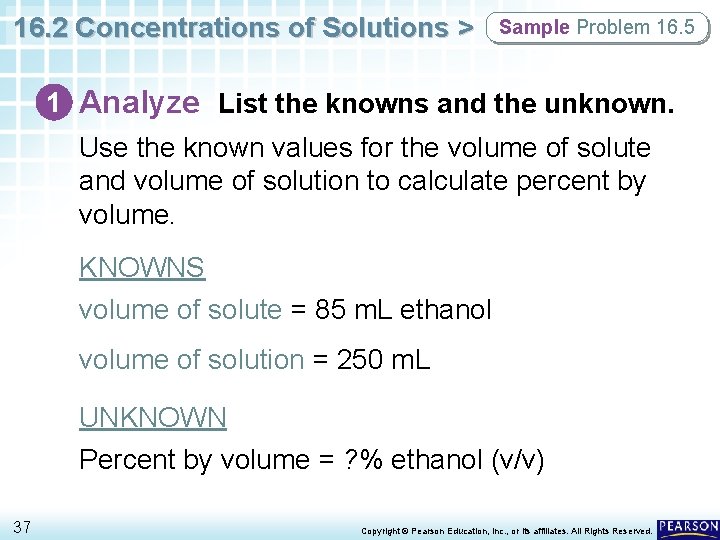
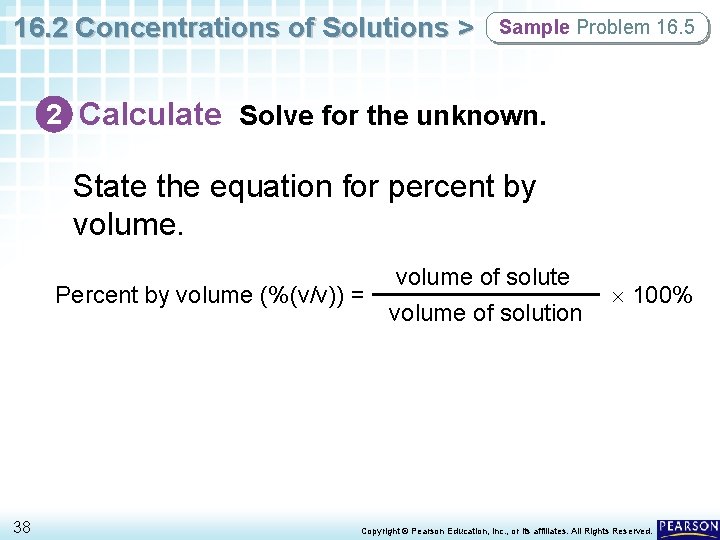
16. 2 Concentrations of Solutions > Sample Problem 16. 5 1 Analyze List the knowns and the unknown. Use the known values for the volume of solute and volume of solution to calculate percent by volume. KNOWNS volume of solute = 85 m. L ethanol volume of solution = 250 m. L UNKNOWN Percent by volume = ? % ethanol (v/v) 37 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Sample Problem 16. 5 2 Calculate Solve for the unknown. State the equation for percent by volume. Percent by volume (%(v/v)) = 38 volume of solute volume of solution 100% Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

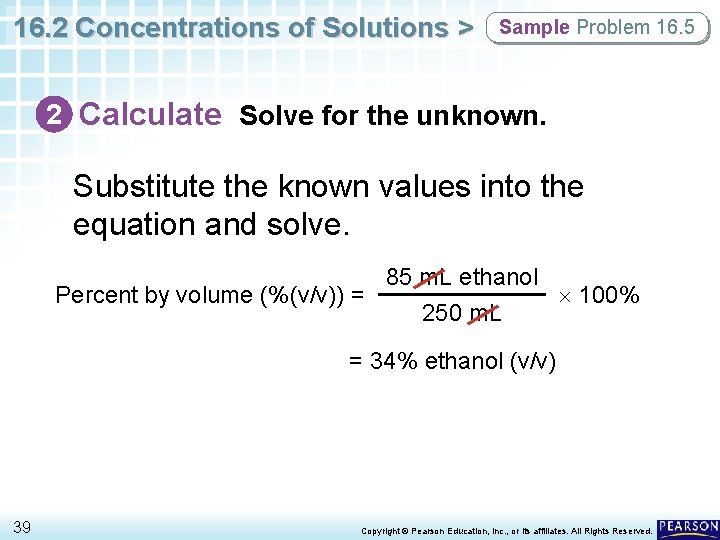
16. 2 Concentrations of Solutions > Sample Problem 16. 5 2 Calculate Solve for the unknown. Substitute the known values into the equation and solve. 85 m. L ethanol Percent by volume (%(v/v)) = 100% 250 m. L = 34% ethanol (v/v) 39 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Sample Problem 16. 5 3 Evaluate Does the result make sense? • The volume of the solute is about one-third the volume of the solution, so the answer is reasonable. • The answer is correctly expressed to two significant figures. 40 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

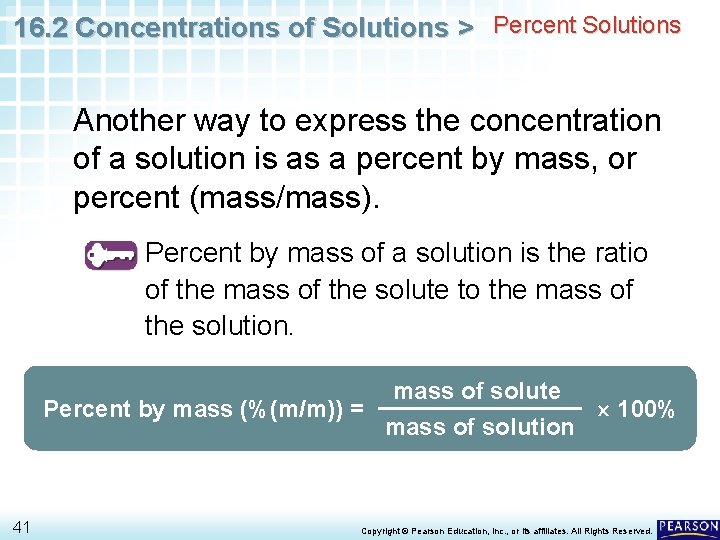
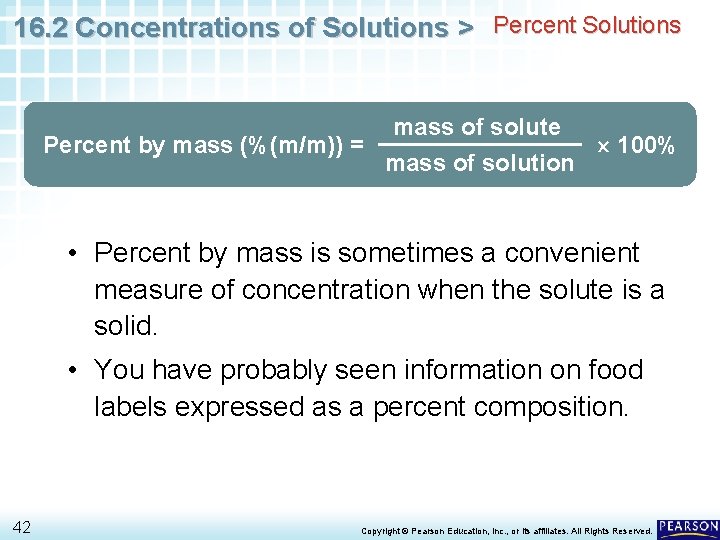
16. 2 Concentrations of Solutions > Percent Solutions Another way to express the concentration of a solution is as a percent by mass, or percent (mass/mass). Percent by mass of a solution is the ratio of the mass of the solute to the mass of the solution. mass of solute Percent by mass (%(m/m)) = 100% mass of solution 41 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Percent Solutions Percent by mass (%(m/m)) = mass of solute mass of solution 100% • Percent by mass is sometimes a convenient measure of concentration when the solute is a solid. • You have probably seen information on food labels expressed as a percent composition. 42 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > CHEMISTRY & YOU What are three ways to calculate the concentration of a solution? 43 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > CHEMISTRY & YOU What are three ways to calculate the concentration of a solution? The concentration of a solution can be calculated in moles solute per liter of solvent, or molarity (M), percent by volume (%(v/v)), or percent by mass (%(m/m)). 44 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

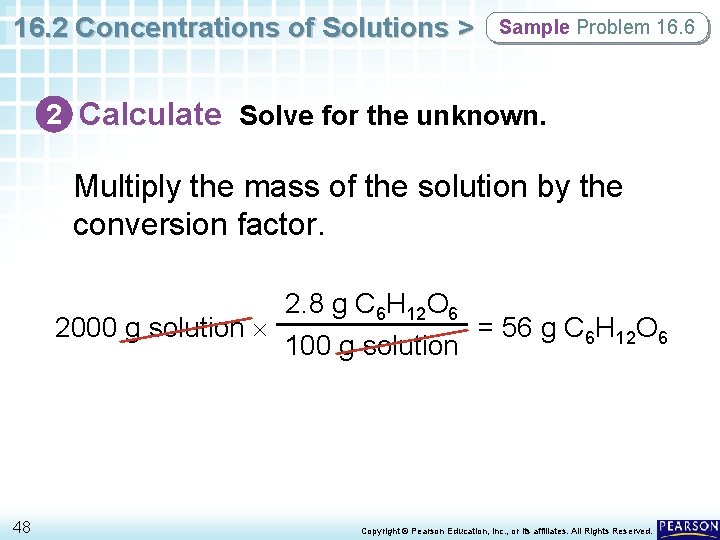
16. 2 Concentrations of Solutions > Sample Problem 16. 6 Using Percent by Mass as a Conversion Factor How many grams of glucose (C 6 H 12 O 6) are needed to make 2000 g of a 2. 8% glucose (m/m) solution? 45 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

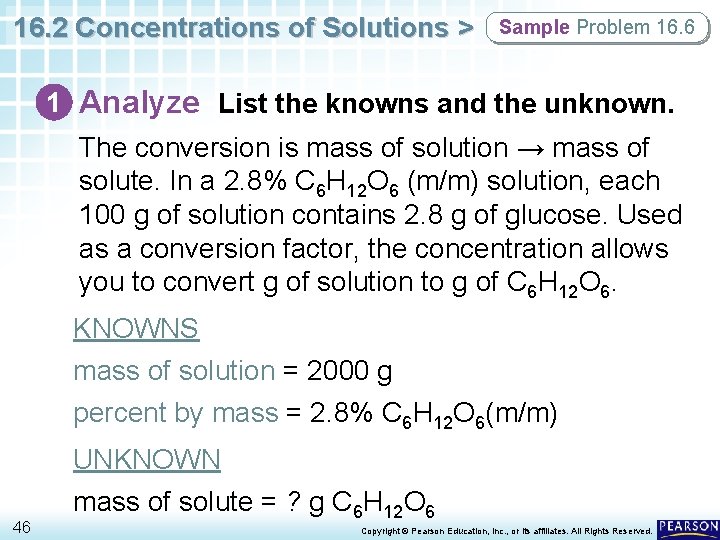
16. 2 Concentrations of Solutions > Sample Problem 16. 6 1 Analyze List the knowns and the unknown. The conversion is mass of solution → mass of solute. In a 2. 8% C 6 H 12 O 6 (m/m) solution, each 100 g of solution contains 2. 8 g of glucose. Used as a conversion factor, the concentration allows you to convert g of solution to g of C 6 H 12 O 6. KNOWNS mass of solution = 2000 g percent by mass = 2. 8% C 6 H 12 O 6(m/m) 46 UNKNOWN mass of solute = ? g C 6 H 12 O 6 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

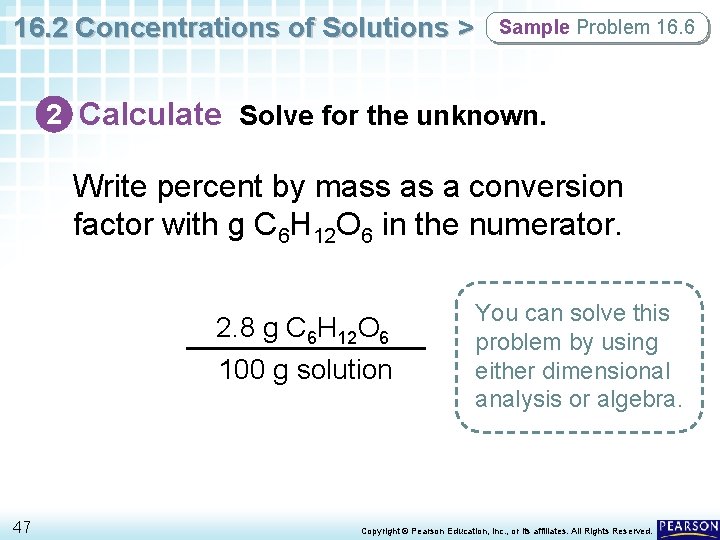
16. 2 Concentrations of Solutions > Sample Problem 16. 6 2 Calculate Solve for the unknown. Write percent by mass as a conversion factor with g C 6 H 12 O 6 in the numerator. 2. 8 g C 6 H 12 O 6 100 g solution 47 You can solve this problem by using either dimensional analysis or algebra. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Sample Problem 16. 6 2 Calculate Solve for the unknown. Multiply the mass of the solution by the conversion factor. 2. 8 g C 6 H 12 O 6 2000 g solution = 56 g C 6 H 12 O 6 100 g solution 48 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

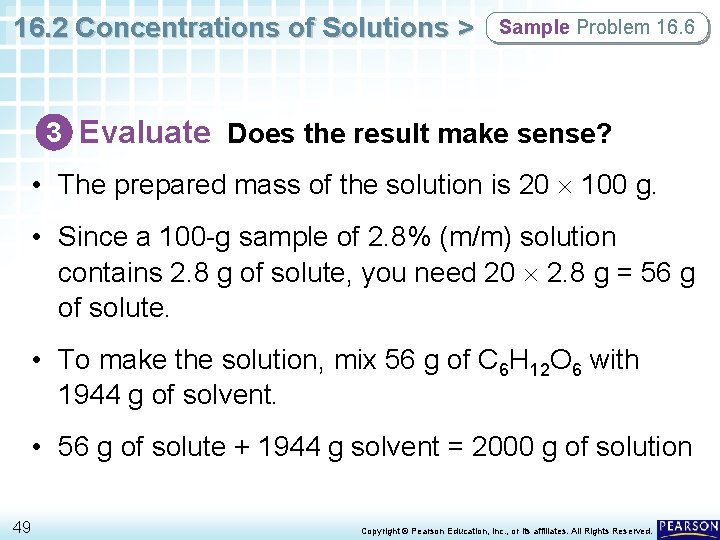
16. 2 Concentrations of Solutions > Sample Problem 16. 6 3 Evaluate Does the result make sense? • The prepared mass of the solution is 20 100 g. • Since a 100 -g sample of 2. 8% (m/m) solution contains 2. 8 g of solute, you need 20 2. 8 g = 56 g of solute. • To make the solution, mix 56 g of C 6 H 12 O 6 with 1944 g of solvent. • 56 g of solute + 1944 g solvent = 2000 g of solution 49 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > What is the mass of water in a 2000 g glucose (C 6 H 12 O 6) solution that is labeled 5. 0% (m/m)? 50 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > What is the mass of water in a 2000 g glucose (C 6 H 12 O 6) solution that is labeled 5. 0% (m/m)? % (m/m) = mass of glucose 100% mass of solution (% (m/m)) mass of solution mass of glucose = 100% mass of glucose = 2000 g 0. 050 = 100 g C 6 H 12 O 6 mass of water = 2000 g – 100 g = 1900 g H 2 O 51 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

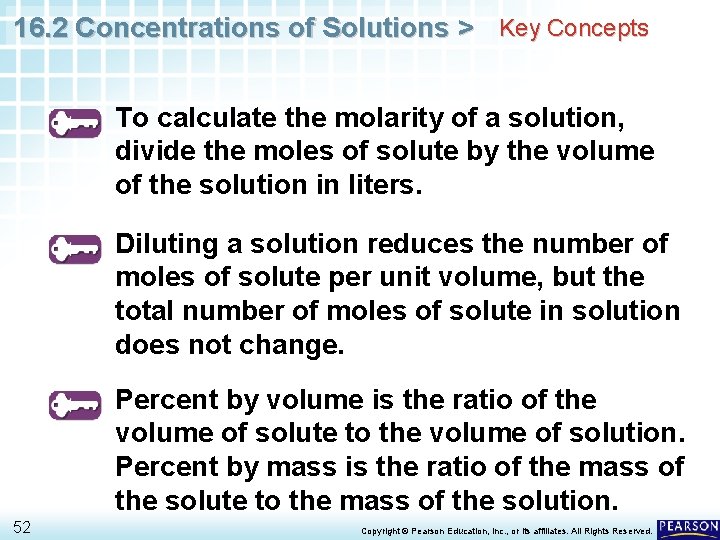
16. 2 Concentrations of Solutions > Key Concepts To calculate the molarity of a solution, divide the moles of solute by the volume of the solution in liters. Diluting a solution reduces the number of moles of solute per unit volume, but the total number of moles of solute in solution does not change. Percent by volume is the ratio of the volume of solute to the volume of solution. Percent by mass is the ratio of the mass of the solute to the mass of the solution. 52 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

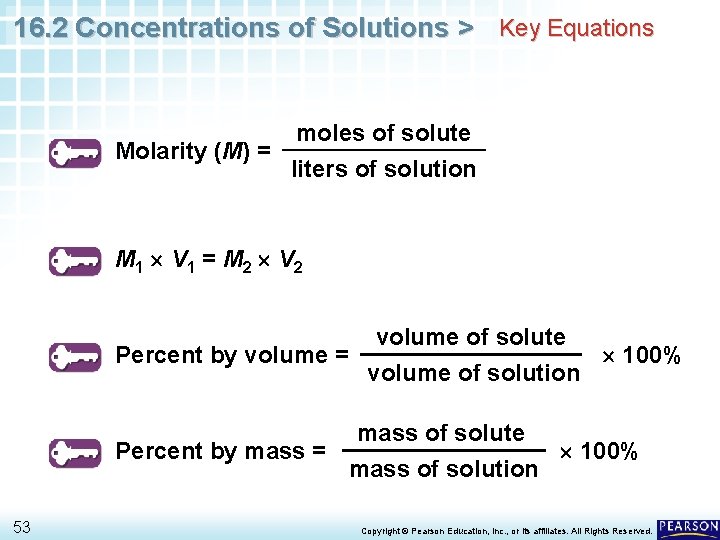
16. 2 Concentrations of Solutions > Key Equations Molarity (M) = moles of solute liters of solution M 1 V 1 = M 2 V 2 volume of solute Percent by volume = 100% volume of solution mass of solute Percent by mass = 100% mass of solution 53 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > Glossary Terms • concentration: a measurement of the amount of solute that is dissolved in a given quantity of solvent; usually expressed as mol/L • dilute solution: a solution that contains a small amount of solute • concentrated solution: a solution containing a large amount of solute • molarity (M): the concentration of solute in a solution expressed as the number of moles of solute dissolved in 1 liter of solution 54 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

16. 2 Concentrations of Solutions > END OF 16. 2 55 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.