Concentration of Solutions Concentrations of Solutions Section 2









![Molar concentration is sometimes indicated by square brackets [ ] i. e [NH 3] Molar concentration is sometimes indicated by square brackets [ ] i. e [NH 3]](https://slidetodoc.com/presentation_image_h2/607ab43a41a1c5ffa32e22d257d358b2/image-10.jpg)













- Slides: 23

Concentration of Solutions

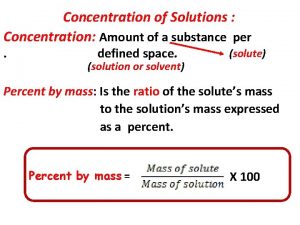
Concentrations of Solutions (Section 2. 5) Concentration = quantity of solute quantity of solution A solution is dilute if there is a relatively small amount of solute per unit volume of solution. A solution is concentrated if there is a relatively large amount of solute per unit volume of solution.

Percentage Concentration


A photographic “stop bath” contains 160 m. L of acetic acid in 600 m. L of solution. What is the (v/v) percentage concentration of acetic acid in the stop bath?

A salt solution is formed by mixing 2. 80 g of Na. Cl in enough water to make exactly 250 m. L of solution. What is the (w/v) percentage concentration of salt in this solution?

Concentrations of very small amounts ppm ppb ppt (parts per million) (parts per billion) (parts per trillion) ppm = milligrams (mg) volume in Litres

The maximum permitted mass of lead in 1. 0 L of public drinking water is 5. 0 X 10 -5 g. What is this concentration in ppm?

Molar Concentration
![Molar concentration is sometimes indicated by square brackets i e NH 3 Molar concentration is sometimes indicated by square brackets [ ] i. e [NH 3]](https://slidetodoc.com/presentation_image_h2/607ab43a41a1c5ffa32e22d257d358b2/image-10.jpg)
Molar concentration is sometimes indicated by square brackets [ ] i. e [NH 3] means the molar concentration of NH 3 [1. 5] means a concentration of 1. 5 mol/L

A solution is prepared by dissolving 1. 68 g of Cu. SO 4 in enough water to make 150 m. L of solution. Calculate the molar concentration of Cu. SO 4.

Sodium carbonate (Na 2 CO 3) is a water softenerr that makes up a significant part of laundry detergent. A student dissolves 5. 00 g of sodium carbonate in enough water to make 250 m. L of solution. What is the molar concentration of Na 2 CO 3 in this case?

A sample of NH 3 solution has a concentration of 14. 8 mol/L. How many moles of NH 3 would be present in a 1. 50 L bottle of this solution?

What volume of a 5. 0 mol/L glucose solution contains 2. 5 mol of glucose?

Diluting Aqueous Solutions A stock solution is a solution whose concentration is precisely known. Dilution is the process of decreasing the concentration of a solution by adding more solvent (usually water).

When more solvent is added to a solution it does not change the amount of solute present. It only changes the volume of the solution. If you double the volume of a 6% H 2 O 2 solution what will the new concentration be? 3% H 2 O 2

There is a fairly simple equation that relates the initial and final concentration and volumes when a solution is diluted. Ci = the initial concentration of the solution Cf = the final concentration of the solution Vi = the initial volume of the solution Vf = the final volume of the solution C i V i = C f. V f

Using this equation you can solve for any of the variables. Just be careful that if you have two concentrations that they are measured in the same unit or if you have two volumes that they are both be measured in the same unit.

Calculate the new concentration (molarity) if enough water is added to 100 m. L of 0. 25 mol/L sodium chloride to make up 1. 5 L.

Calculate the volume to which 500 m. L of 0. 02 mol/L coppper sulfate solution must be diluted to make a new concentration of 0. 001 mol/L.

If I have 340 m. L of a 0. 5 M Na. Br solution, what will the concentration be if I add 560 m. L more water to it?

If I leave 750 m. L of 0. 50 M sodium chloride solution uncovered on a windowsill and 150 m. L of the solvent evaporates, what will the new concentration of the sodium chloride solution be?

To what volume would I need to add water to the evaporated solution in problem 3 to get a solution with a concentration of 0. 25 M?
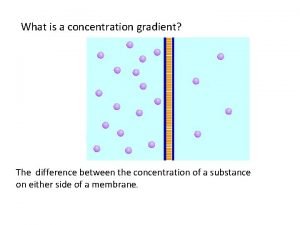
 Whats a concentration gradient
Whats a concentration gradient Movement of high concentration to low concentration
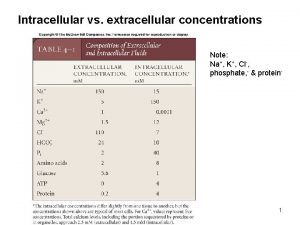
Movement of high concentration to low concentration Intracellular vs extracellular ion concentrations
Intracellular vs extracellular ion concentrations Solute vs solvent
Solute vs solvent Concentration of solutions
Concentration of solutions Modern chemistry chapter 12
Modern chemistry chapter 12 Chapter 12 review solutions section 3
Chapter 12 review solutions section 3 Types of reactions
Types of reactions Chapter 6 section 3 water and solutions
Chapter 6 section 3 water and solutions Dachau visite virtuelle
Dachau visite virtuelle Measures of concentration molarity quiz
Measures of concentration molarity quiz 4 firm concentration ratio
4 firm concentration ratio Concentration= moles/volume
Concentration= moles/volume Selective specialization example
Selective specialization example Fig 3
Fig 3 Mass gfm triangle
Mass gfm triangle Warburg-christian method
Warburg-christian method Dilution equation
Dilution equation Molar concentration unit
Molar concentration unit Percent by mass
Percent by mass Concentration ratio
Concentration ratio Concentration formula
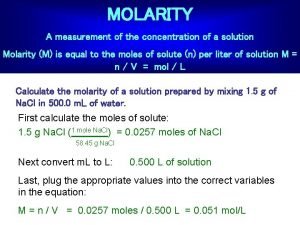
Concentration formula What is molarity a measurement of
What is molarity a measurement of Molarity measurement
Molarity measurement