Reaction Investigation 9 Part 2 Limewater Reaction Part














































- Slides: 75

Reaction Investigation 9 Part 2

Limewater Reaction �Part 2

Limewater Reaction �In an earlier investigation, you worked with substances to identify a mystery mixture. Part of the evidence you used to solve the puzzle was the bubbles that formed when pairs of substances were mixed. �Today we will take a second look at gases and bubbles.

Limewater Reaction �Limewater is a saturate solution of calcium hydroxide. Its chemical formula is Ca(OH)2 �How many atoms are in one particle of Ca(OH)2? �Atoms in a hydroxide group have a strong attraction for one another.

Limewater Reaction �Draw a representation of a calcium hydroxide particle.

Pumping Air through Limewater �Did anything happen? �Did you notice any change in the limewater when we bubbled air through it?

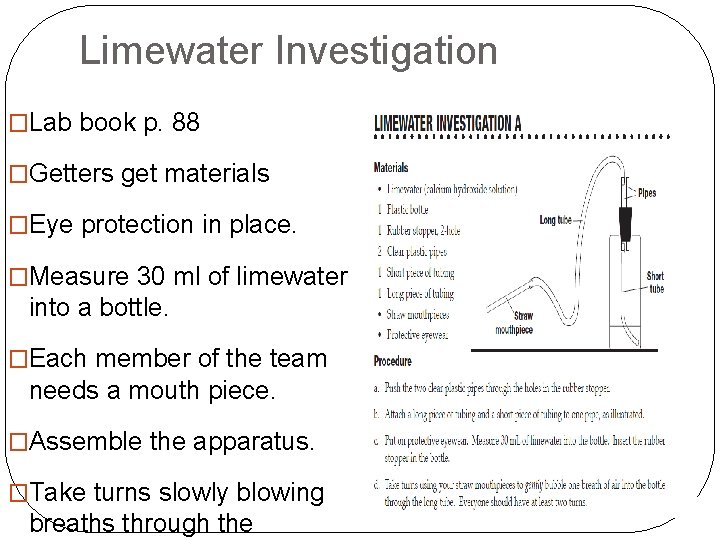
Limewater Investigation �Lab book p. 88 �Getters get materials �Eye protection in place. �Measure 30 ml of limewater into a bottle. �Each member of the team needs a mouth piece. �Assemble the apparatus. �Take turns slowly blowing breaths through the Lab book p. 88

Limewater Investigation �Write your results and conclusion at the bottom of your lab book. (3 minutes) �Share results. �Starting substances change into new substances during a chemical reaction. �Do you think a reaction occurred in the bottle? Why or why not?

�The limewater changed from clear to murky. A precipitate formed. �A precipitate is a new substance – it is evidence of a chemical reaction.

Clean up �Throw away straws. �Use the acid wash to clean the bottles. �Pour the acid wash into the waste bucket and finish by rinsing the bottles with clean water. �Return all materials to the materials station.

Assignment �Read How Do Atoms Rearrange? in you Resource Books.

Break Point

Review �What happened when air was pumped through the limewater? �What happened when exhaled breath was blown through the limewater? �What caused the change? �Breath reacted with limewater, but air didn’t. What was different about exhaled air that produced a reaction?

Atom Tiles �These atom tiles can be used to figure out how atoms might go together in a particle of a substance. �You will work with a partner on this activity.

Ca(OH)2 �Limewater is calcium hydroxide dissolved in water. �When you get your bag of atom tiles, work together to make a model of a particle of calcium hydroxide. �When you and your partner are satisfied with your model of calcium hydroxide, draw it in the first section of Lab book p. 89.

�When a chemical reaction occurs, the atoms of the starting substances rearrange to form new substances. The starting substances are called reactants; the new substances are called products. �Work with your partner to answer question #2. use your atom tiles to create representations of the reactants. One is the compound calcium hydroxide. �Then, reorganize the atom tiles to make representations of the products of the reactants. One of them is a white precipitate.

Discuss Reaction (hints) �The second reactant was in your breath. �The white precipitate formed because it was insoluble in water. What white powder do you know that does not dissolve in water? �Calcium Carbonate – Ca. CO 3 �No atoms or created or destroyed during reactions – only reorganized.

Reaction

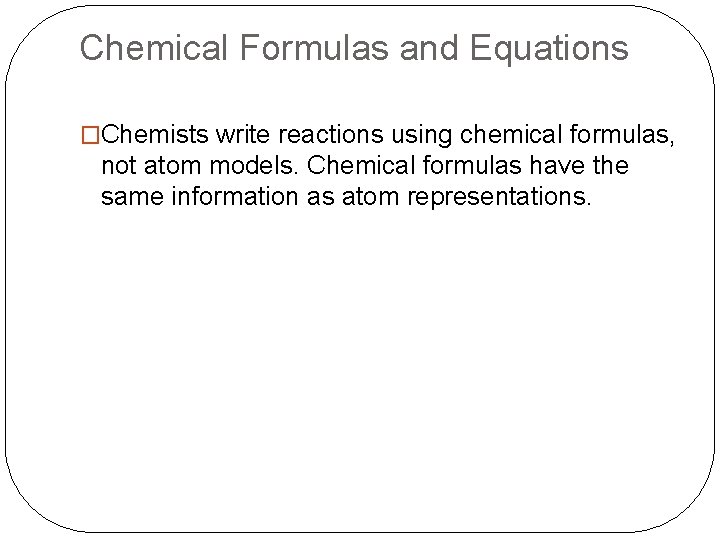
Chemical Formulas and Equations �Chemists write reactions using chemical formulas, not atom models. Chemical formulas have the same information as atom representations.

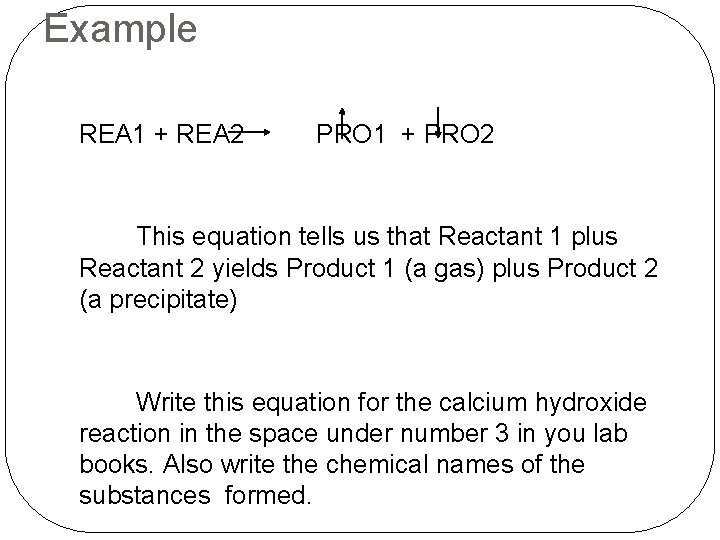
Conventions for Writing Chemical Equations �Reactants are written as chemical formulas on the left hand side of the equation. They are separated from each other by a plus sign. �Products are written as chemical formulas on the right hand side of the equation. They are separated from each other by a plus sign. �An arrow pointing to the right is used in place of an equal sign. The arrow is read as “yields”. �If a product is a gas, an arrow pointing up follows its chemical formula. �If a product appears as a precipitate, an arrow

Example REA 1 + REA 2 PRO 1 + PRO 2 This equation tells us that Reactant 1 plus Reactant 2 yields Product 1 (a gas) plus Product 2 (a precipitate) Write this equation for the calcium hydroxide reaction in the space under number 3 in you lab books. Also write the chemical names of the substances formed.

Assignment �Complete the last 3 questions on the Limewater Investigation B. �Turn in �Finish reading How Do Atoms Rearrange? in you Resource Books. �Lab book p. 91 – Answer the questions and turn in.

Baking Soda and Acid �Part 3

Review �We bubbled carbon dioxide into limewater. Did a reaction occur? How do you know? �Precipitate �What were the reactants? �Calcium Hydroxide and Carbon Dioxide �What were the products? �Calcium Carbonate and Water

�Reproduce the equation for the reaction using atomic representation. �Write the equation using chemical formulas and conventional symbols.

Model Volcanoes �What is the chemical name for baking soda? �Sodium bicarbonate �What is the chemical formula? �Na. HCO 3 �What is vinegar? �Acetic acid �Do vinegar and baking soda react when they are mixed? �Yes

Hydrochloric Acid �This is a different kind of acid called hydrochloric acid. It is a strong acid and must be handled with care. �Hydrochloric acid is important to each of us. Do you know why? What role does it play in your life? �It is stomach acid, and plays an important role in food digestion. �This acid is 10 times more concentrated than your stomach acid.

What do you think would happen if you mixed baking soda with hydrochloric acid instead of acetic acid?

Hydrochloric acid and Baking soda Demo

The Reaction �The chemical formula for hydrochloric acid is HCl. �Work with your partner to use the atomic tiles to make representations of the two reactants, hydrochloric acid and sodium bicarbonate. �Then rearrange the atoms to figure out what products form when these two substances react.

Hints �What happened immediately when the acid was added to the sodium bicarbonate? �Did a new substance form? �Was there any white substance in the bottle after the reaction stopped?

The Reaction �Atomic Representation Click to edit the outline text format Second Outline Level �Chemical Formula and Third Outline Names Level Fourth Outline Level Fifth Eighth Outline Level Ninth Outline Level. Click to edit Master text styles Click to edit the outline text format Second Outline Level Third Outline Level Fourth Outline Level Fifth

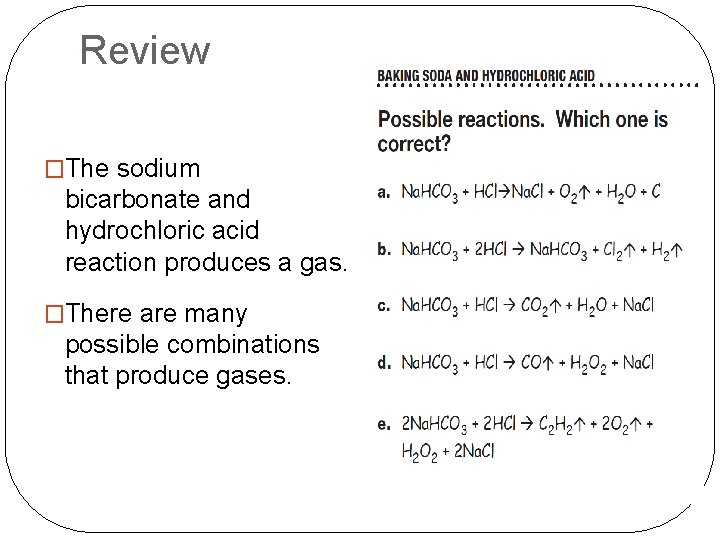
Review �The sodium bicarbonate and hydrochloric acid reaction produces a gas. �There are many possible combinations that produce gases.

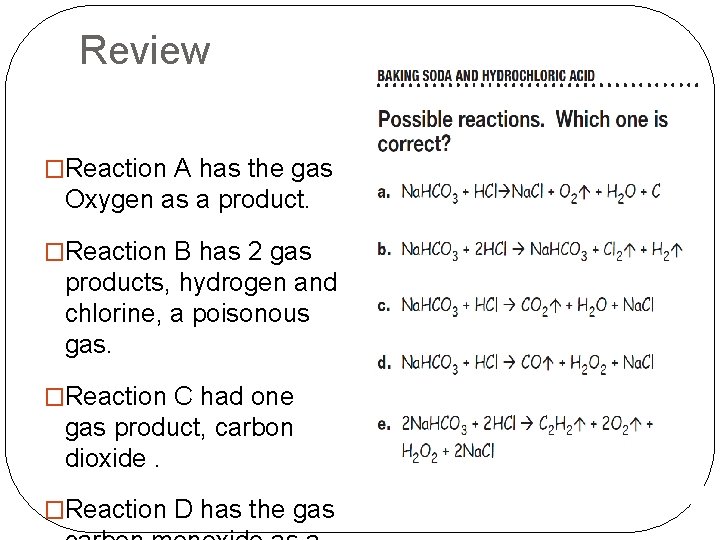
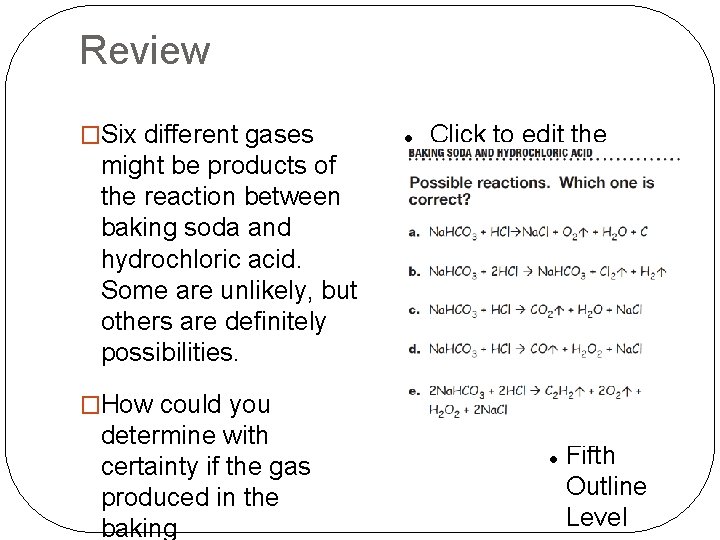
Review �Reaction A has the gas Oxygen as a product. �Reaction B has 2 gas products, hydrogen and chlorine, a poisonous gas. �Reaction C had one gas product, carbon dioxide. �Reaction D has the gas

Review �Six different gases might be products of the reaction between baking soda and hydrochloric acid. Some are unlikely, but others are definitely possibilities. �How could you determine with certainty if the gas produced in the baking soda/hydrochloric acid reaction is indeed carbon dioxide?

Click icon to add picture Break Point

Review �Six different gases might be products of the reaction between baking soda and hydrochloric acid. Some are unlikely, but others are definitely possibilities. �How could you determine with certainty if the gas produced in the baking Click to edit the outline text format Second Outline Level Third Outline Level Fourth Outline Level Fifth Outline Level

Experimental Ideas �Take 4 minutes to think about the elements of an experiment to test the gas with lime water. �Important elements should include: �Reacting the soda and acid in such a way as to capture the gas �Bubbling the gas through the limewater �Looking for a precipitate

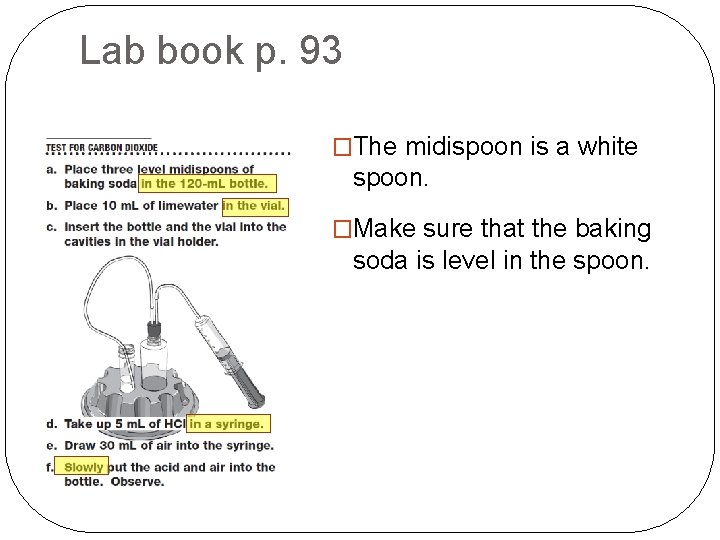
Lab book p. 93 �The midispoon is a white spoon. �Make sure that the baking soda is level in the spoon.

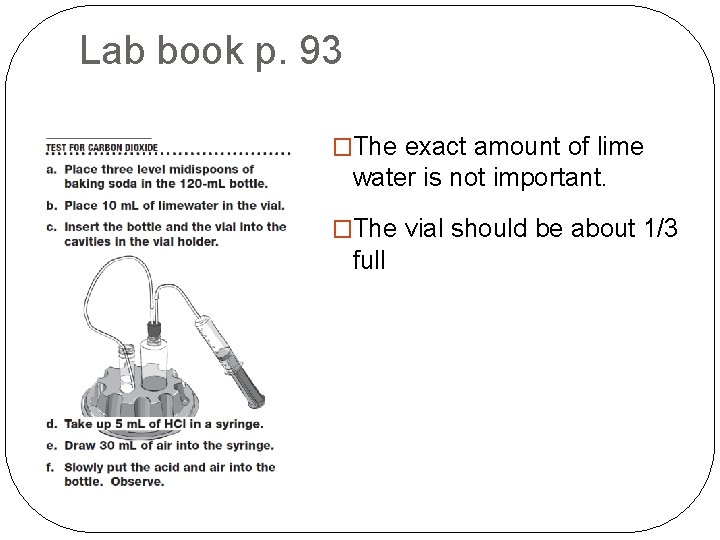
Lab book p. 93 �The exact amount of lime water is not important. �The vial should be about 1/3 full

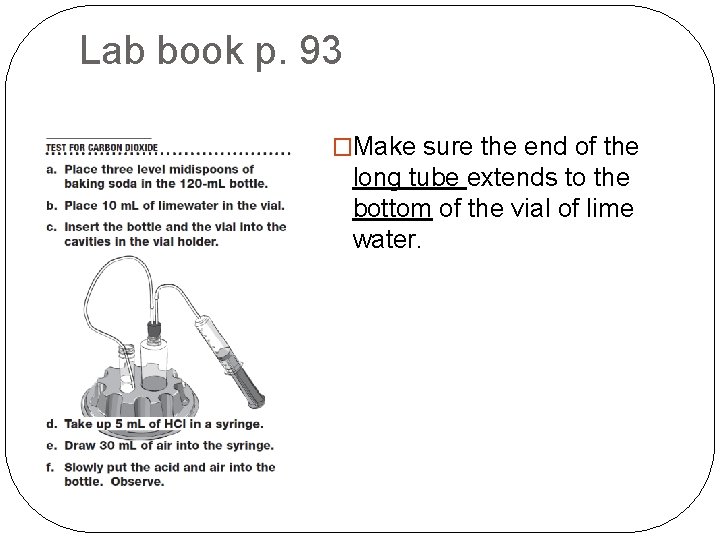
Lab book p. 93 �Make sure the end of the long tube extends to the bottom of the vial of lime water.

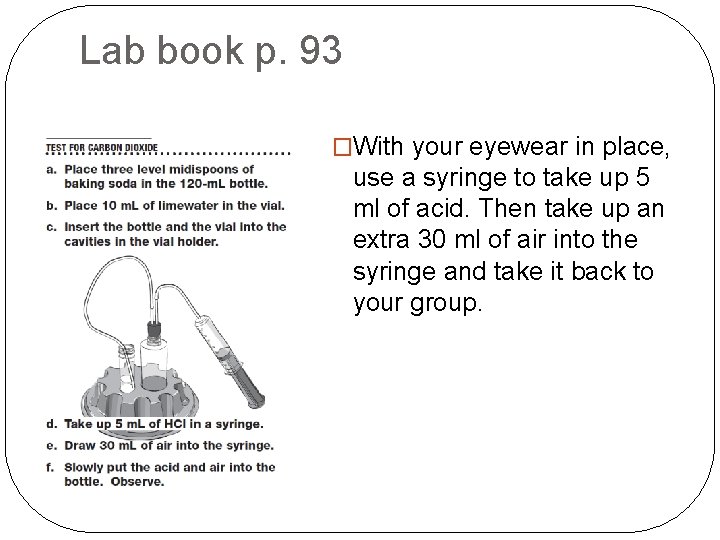
Lab book p. 93 �With your eyewear in place, use a syringe to take up 5 ml of acid. Then take up an extra 30 ml of air into the syringe and take it back to your group.

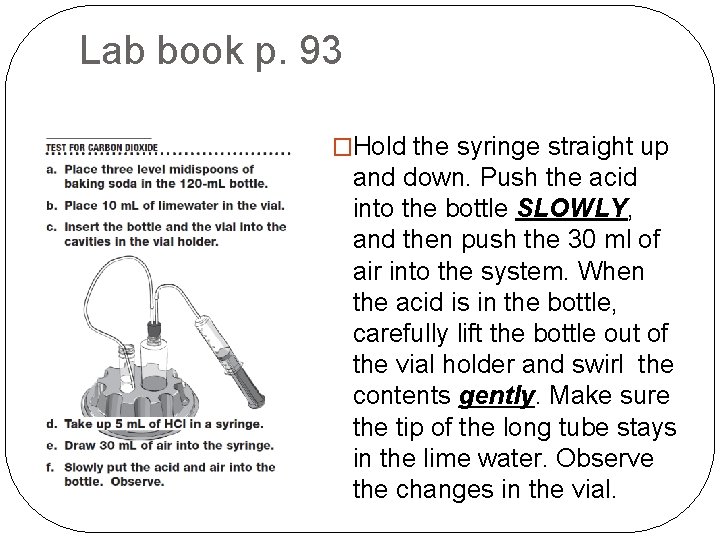
Lab book p. 93 �Hold the syringe straight up and down. Push the acid into the bottle SLOWLY, and then push the 30 ml of air into the system. When the acid is in the bottle, carefully lift the bottle out of the vial holder and swirl the contents gently. Make sure the tip of the long tube stays in the lime water. Observe the changes in the vial.

Conducting the Reaction �Go over the procedure again BEFORE you begin. �You have 15 minutes to conduct the experiment and make your observations. �Refer to your lab book if you have any questions on the procedure. �You may use atom tiles to help you answer the 3

Discuss �Were you able to confirm all the products that formed during the reaction between the baking soda and the hydrochloric acid? �What is your evidence that the gas produced was carbon dioxide?

Na. HCO 3 + HCl CO 2 + Na. Cl + H 2 O �If sodium chloride is a product of this reaction, where is it? �Dissolved in solution. �How could you confirm that the sodium chloride is dissolved in solution? �Evaporation.

Set up Evaporation �Write your group names on a sticky note and attach it to the tray �Use a pipette to place 5 drops – no more – in well #1 �Place the tray carefully at the evaporation area. �Clean up – remember to use the acid wash for the vial. Return all materials.

Assigment �Read p. 69 -72 in Resource Book �Lab book p. 97

Break Point

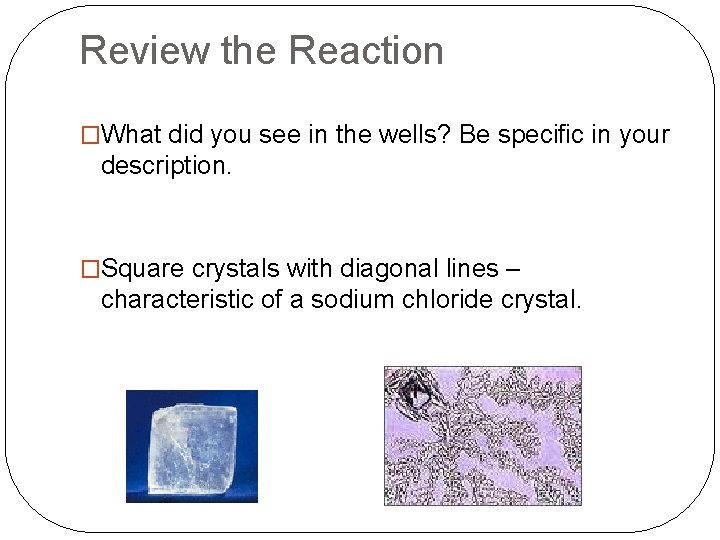
Review the Reaction �Retrieve the well trays. DO NOT touch the contents of the well. �Use your hand lenses to observe the contents.

Review the Reaction �What did you see in the wells? Be specific in your description. �Square crystals with diagonal lines – characteristic of a sodium chloride crystal.

Review the Reaction �How did the sodium chloride get into the well tray? �It was dissolved in the water left in the bottle after the reaction.

Review the Reaction �We didn’t put any sodium chloride in the bottle. Where did the sodium chloride come from? �Atoms in the reactants rearranged to make sodium chloride, one of the products.

Review the Reaction �We put hydrochloric acid and sodium bicarbonate in the reaction bottle. Where are these two substances now? �Gone. They no longer exist. They have been changes into new materials.

Important Point!!!!! �In a chemical reaction, the original substances – the reactants – are disassembled, so they cease to exist. �The parts reassemble in new ways, producing new substances – the products.

When an acid (or base) is changed into a new substance during a reaction, it is referred to as neutralizing the acid (or base).

Assignment �Complete the Response Sheet – Reaction p. 95 in your lab book. - together �Turn in Response Sheet when complete.

Naming Compounds and Balancing Equations Video – Discovery Ed Balancing Equations PPT Jefferson Lab – Balancing Act

Assignment �Read Antoine-Laurent Lavoisier in your resource book. �Complete p. 97 Lavoisier Questions in your lab book and turn in.

Antacid �Part 4

Review Response Sheet �Explain why Beth thought the antacid tablet might help grandmother.

Review Response Sheet �Use chemical formulas to write the equation for the reaction.

Antacid Testing �Come up with a plan to determine how much acid one antacid tablet will neutralize. (4 minutes) �Plan should include: �Placing one tablet in a cup �Add acid in small increments, looking for evidence of a reaction �Record results after each ml of acid is added �Continue until additional acid no longer reacts

Lab book p. 99 �You have 3 minutes to write your procedure. �How will you know when the antacid has neutralized as much acid as it can? �Be sure to subtract the last unreacted ml of acid from your total ml added to determine the amount of acid that is neutralized.

Investigation �Getters get 1 antacid tablet and a cup. �Put on protective eyewear. �Another team member carefully get 15 ml of dilute hydrochloric acid in a syringe. �Add the acid to the tablet 1 ml at a time, swirling the cup and observing the fizzing. �Record observations after each ml is added. (use lab book p. 98)

Clean up �Dispose of unused acid in the waste container as well as the neutralized acid. �Rinse out cup and syringes and return to the materials station.

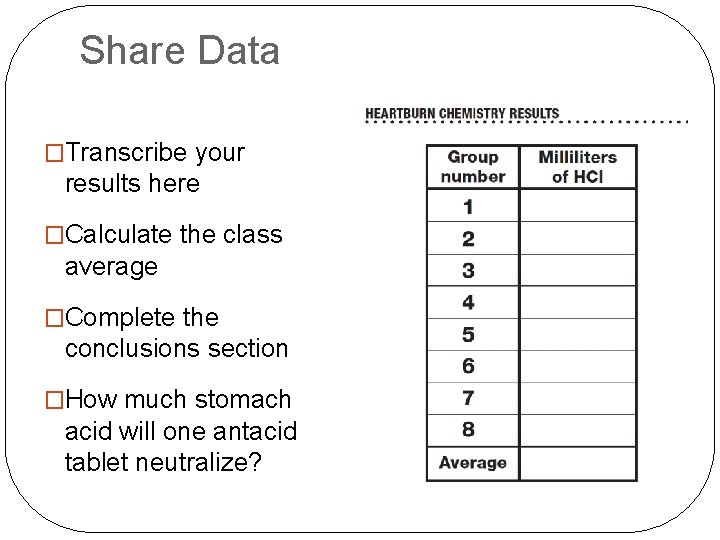
Share Data �Transcribe your results here �Calculate the class average �Complete the conclusions section �How much stomach acid will one antacid tablet neutralize?

Vocabulary Quiz 1. The fundamental particle of an element. Every element has its own kind of atom is __________. 2. An attractive force that acts between atoms to hold particles together is called a _________.

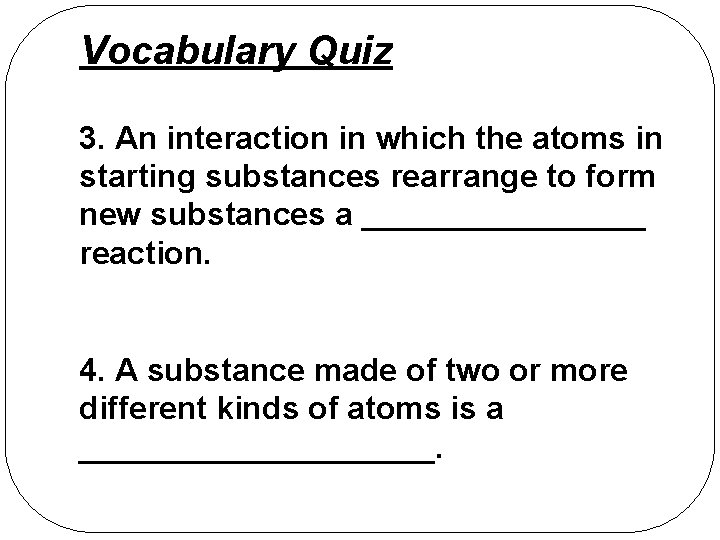
Vocabulary Quiz 3. An interaction in which the atoms in starting substances rearrange to form new substances a ________ reaction. 4. A substance made of two or more different kinds of atoms is a __________.

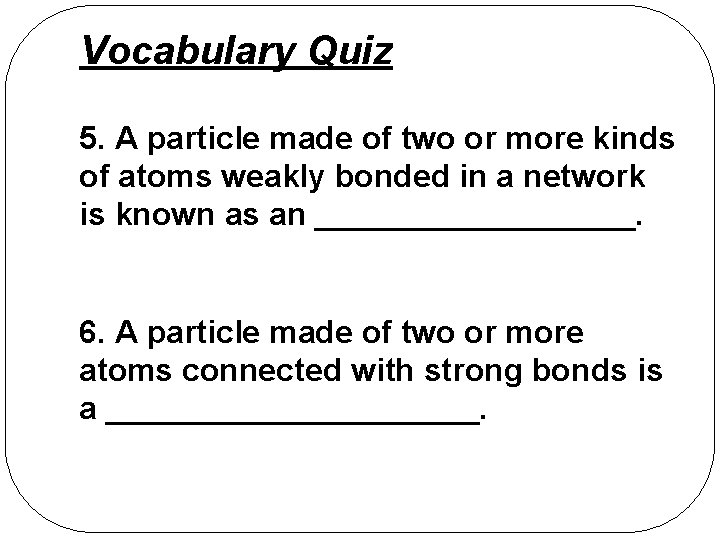
Vocabulary Quiz 5. A particle made of two or more kinds of atoms weakly bonded in a network is known as an _________. 6. A particle made of two or more atoms connected with strong bonds is a ___________.

Vocabulary Quiz 7. A substance that is created during a chemical reaction is a _________. 8. A starting substance in a chemical reaction ___________.

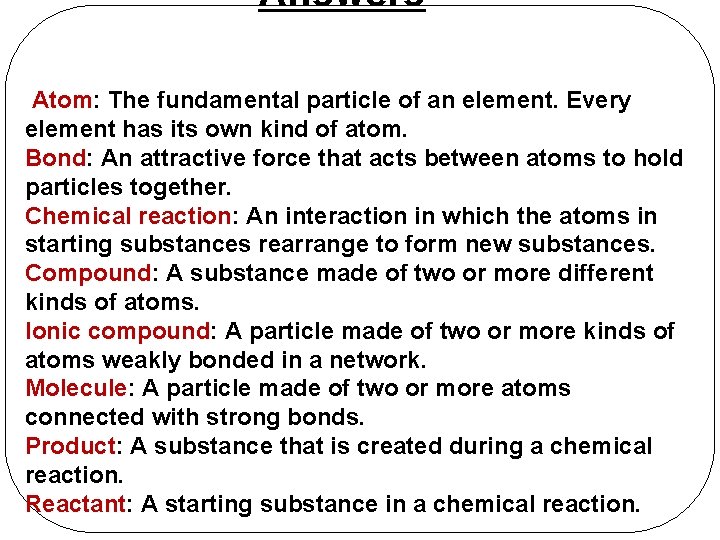
Answers Atom: The fundamental particle of an element. Every element has its own kind of atom. Bond: An attractive force that acts between atoms to hold particles together. Chemical reaction: An interaction in which the atoms in starting substances rearrange to form new substances. Compound: A substance made of two or more different kinds of atoms. Ionic compound: A particle made of two or more kinds of atoms weakly bonded in a network. Molecule: A particle made of two or more atoms connected with strong bonds. Product: A substance that is created during a chemical reaction. Reactant: A starting substance in a chemical reaction.

Assignment �Midsummative Exam 9 �Read in your Resource Books �Organic Compounds �Dr. Donna Nelson – Chemist

�Atom �Compound �Bond �Chemical Reaction �Product �Reactant �Ionic Compound

 Physical properties of oxygen
Physical properties of oxygen Reaction rate equation
Reaction rate equation Addition reaction and substitution reaction
Addition reaction and substitution reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Half-life formula
Half-life formula Euclidean geometry rules
Euclidean geometry rules Summarize locard's principle of exchange
Summarize locard's principle of exchange Kidnap for ransom investigation
Kidnap for ransom investigation Stages of investigation
Stages of investigation Continuous mortality investigation
Continuous mortality investigation Vce physics poster
Vce physics poster Fulton county child protective services
Fulton county child protective services Internal corporate investigation
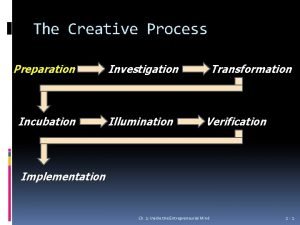
Internal corporate investigation Transformation in creative process
Transformation in creative process Pascal's identity
Pascal's identity Objectives of investigation
Objectives of investigation Formulas for career success
Formulas for career success During a recent police investigation
During a recent police investigation Historical scene investigation
Historical scene investigation Investigation of megaloblastic anemia
Investigation of megaloblastic anemia Tripod beta method
Tripod beta method What is system investigation
What is system investigation Holistic approach in qualitative research
Holistic approach in qualitative research Independent investigation method
Independent investigation method Security incident investigation
Security incident investigation Investigation for reservoir planning
Investigation for reservoir planning Clinical investigation plan
Clinical investigation plan How to solve special right triangles 45 45 90
How to solve special right triangles 45 45 90 A systematic investigation
A systematic investigation Weather investigation
Weather investigation Démarche d'investigation
Démarche d'investigation Extended investigation vce
Extended investigation vce Investigation procedures
Investigation procedures English language nea investigation
English language nea investigation Causes of macrocytic anaemia
Causes of macrocytic anaemia Arson evidence collection and analysis
Arson evidence collection and analysis Corpus delicti
Corpus delicti History scene investigation
History scene investigation Vcaa chemistry study design
Vcaa chemistry study design Personal investigation a level art
Personal investigation a level art 2106 crime scene investigation
2106 crime scene investigation Vce scientific poster
Vce scientific poster Parts of an investigation
Parts of an investigation Practice 7-3 special right triangles
Practice 7-3 special right triangles Statistical investigation process
Statistical investigation process Spacing of boreholes for soil investigation
Spacing of boreholes for soil investigation Carrying out an investigation
Carrying out an investigation System planning and initial investigation
System planning and initial investigation Lockheed martin investigation
Lockheed martin investigation 5-9 accident investigation data answers
5-9 accident investigation data answers Ex officio investigation
Ex officio investigation Career investigation lcvp
Career investigation lcvp George h w bush apush
George h w bush apush Factors of 150
Factors of 150 Mhra oos
Mhra oos Feasibility study phase of sdlc
Feasibility study phase of sdlc Investigation task
Investigation task Systematic investigation example
Systematic investigation example Oot investigation flowchart
Oot investigation flowchart Criminal investigation lesson plans
Criminal investigation lesson plans Stretching and shrinking investigation 2 answers
Stretching and shrinking investigation 2 answers Svg
Svg Accident investigation data analysis
Accident investigation data analysis Jenis investigasi
Jenis investigasi Lockdown ive
Lockdown ive Note taking crime scene investigation
Note taking crime scene investigation How to use tems investigation
How to use tems investigation Ctive
Ctive Tcole 2106
Tcole 2106 Special right triangles (radical answers)
Special right triangles (radical answers) Ggara
Ggara Orensic
Orensic Secondary hypothyroidism
Secondary hypothyroidism Tcole crime scene investigation
Tcole crime scene investigation 385+40
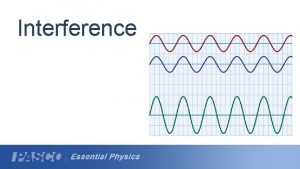
385+40 Wave interference investigation
Wave interference investigation