Reaction in Aqueous Solution Chapter 4 Precipitation reactions


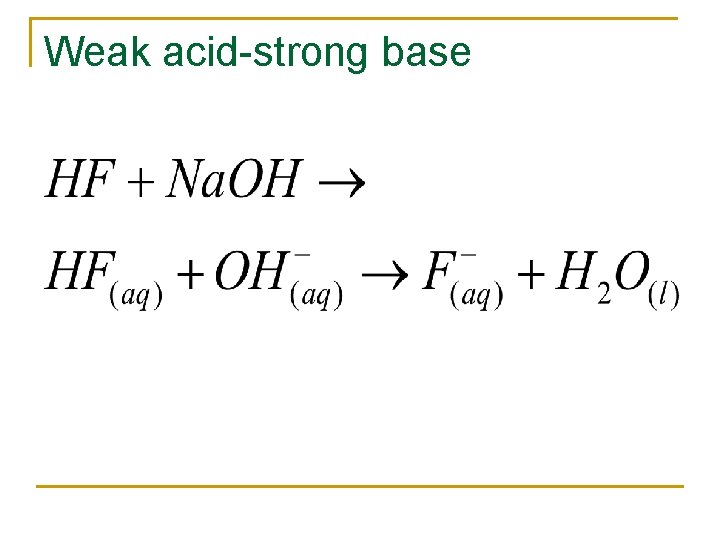



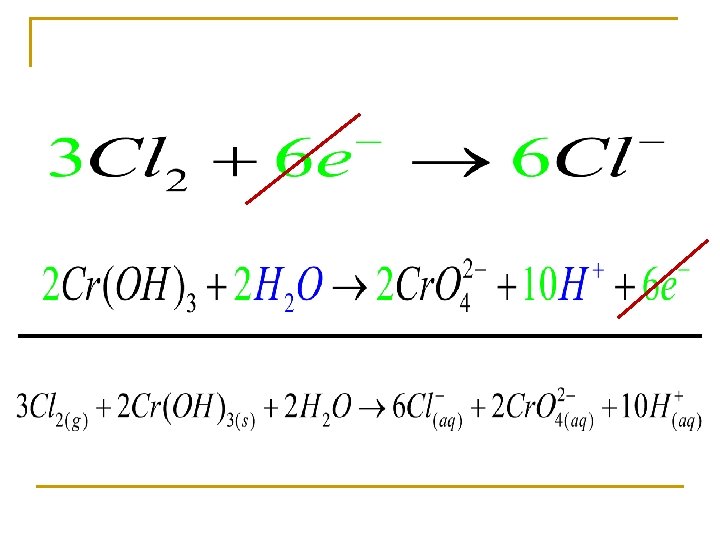

- Slides: 24

Reaction in Aqueous Solution Chapter 4

Precipitation reactions n n Form a solid---precipitate! Remember: Solubility Rules Found on page 91 figure 4. 2 Or on the web Or you saved them from last year Yep ya gotta know em

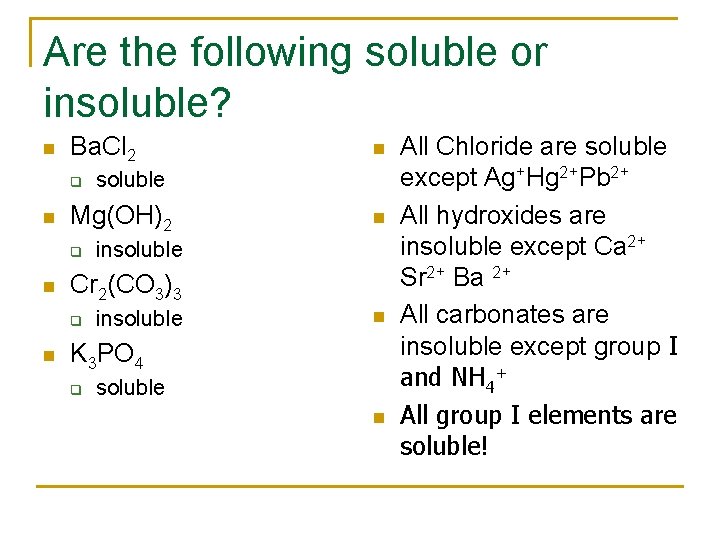
Are the following soluble or insoluble? n Ba. Cl 2 q n n n insoluble Cr 2(CO 3)3 q n soluble Mg(OH)2 q n insoluble n K 3 PO 4 q soluble n All Chloride are soluble except Ag+Hg 2+Pb 2+ All hydroxides are insoluble except Ca 2+ Sr 2+ Ba 2+ All carbonates are insoluble except group I and NH 4+ All group I elements are soluble!

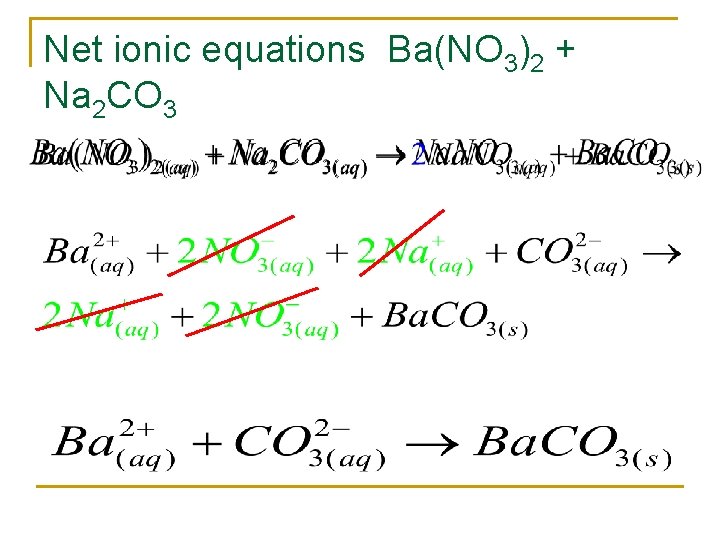
Net ionic equations Ba(NO 3)2 + Na 2 CO 3

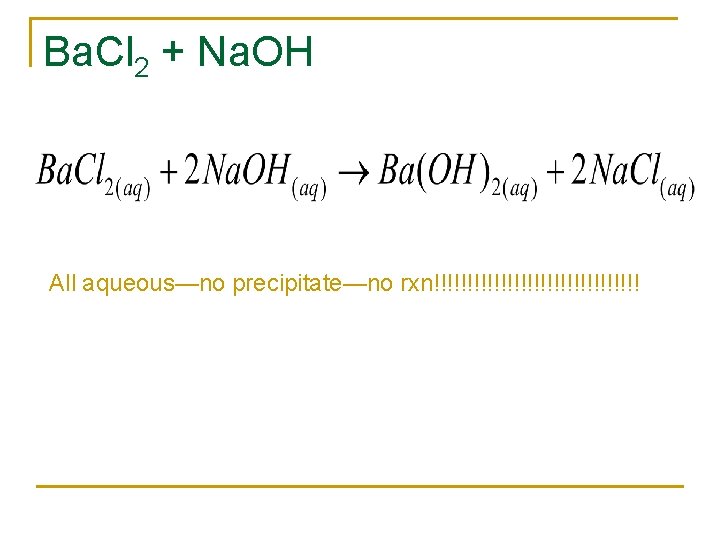
Ba. Cl 2 + Na. OH All aqueous—no precipitate—no rxn!!!!!!!!!!!!!!!!

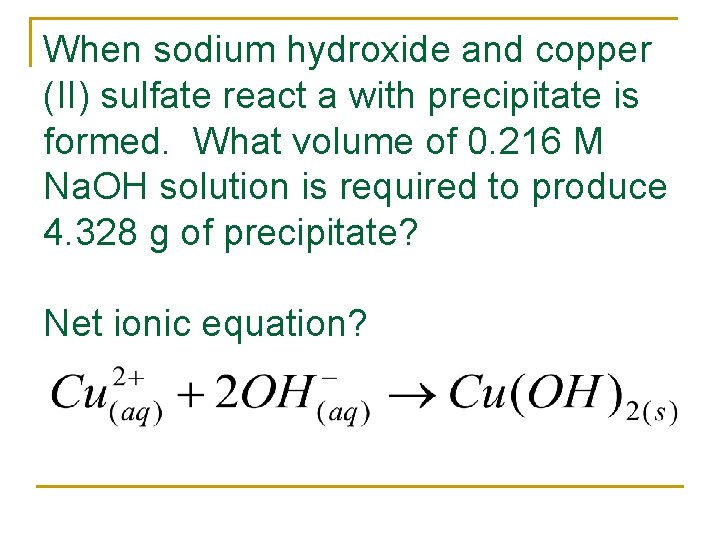
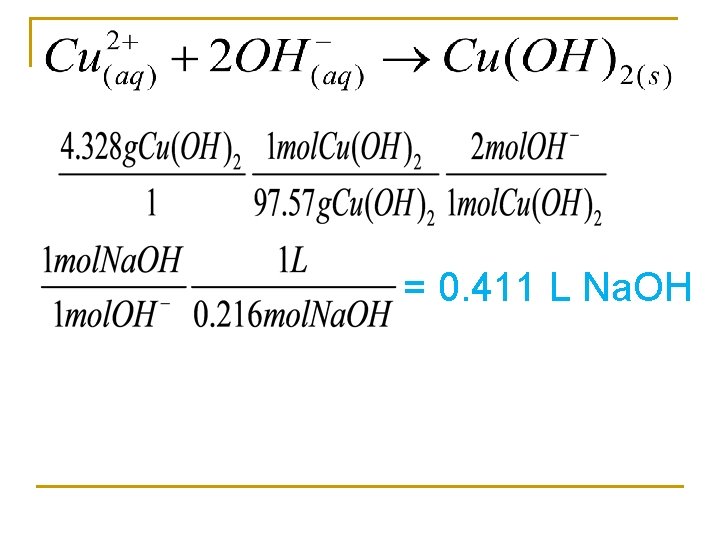
When sodium hydroxide and copper (II) sulfate react a with precipitate is formed. What volume of 0. 216 M Na. OH solution is required to produce 4. 328 g of precipitate? Net ionic equation?

= 0. 411 L Na. OH

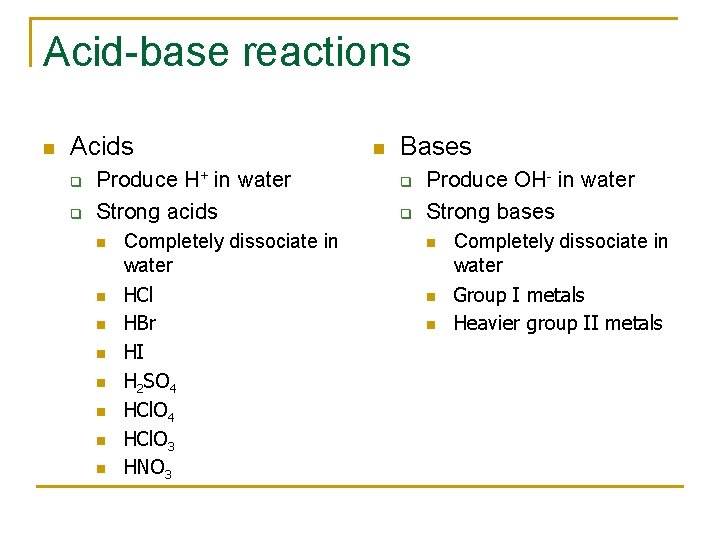
Acid-base reactions n Acids q q Produce H+ in water Strong acids n n n n Completely dissociate in water HCl HBr HI H 2 SO 4 HCl. O 3 HNO 3 n Bases q q Produce OH- in water Strong bases n n n Completely dissociate in water Group I metals Heavier group II metals

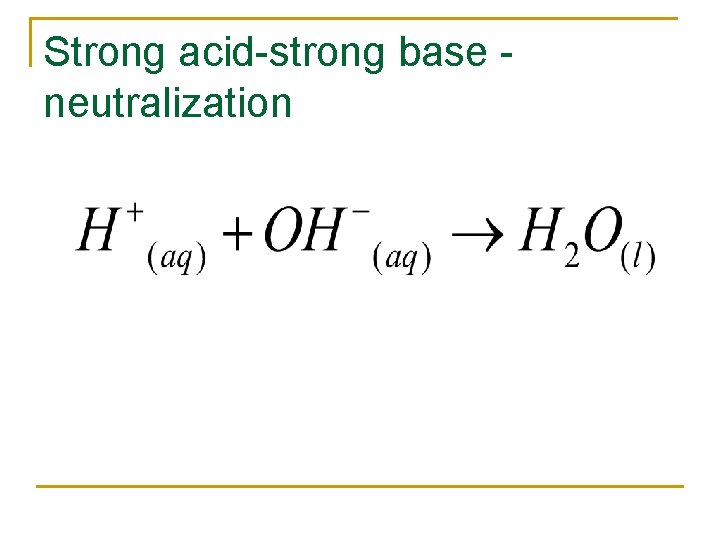
Strong acid-strong base neutralization
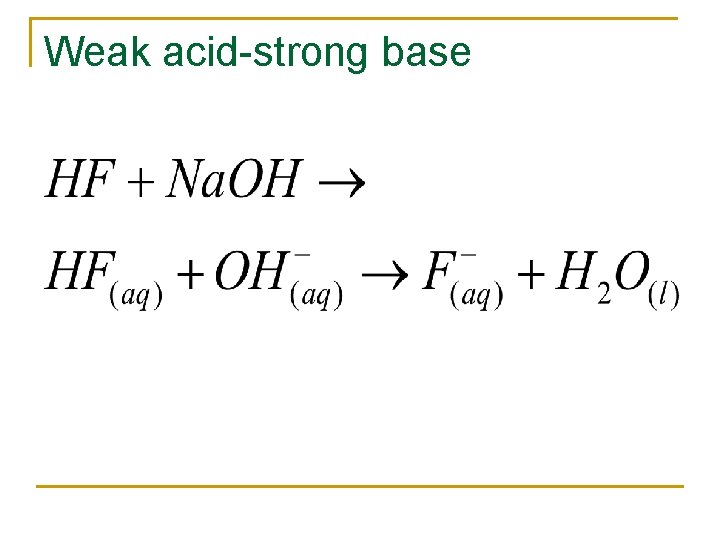
Weak acid-strong base

Strong acid-weak base

Acid –base titrations n n n Used to determine an unknown concentration of either the acid or the base The concentration of the other solution is known and so is the volume of both solutions Equivalence point q q The point at which the reaction is complete Moles of acid = moles of base

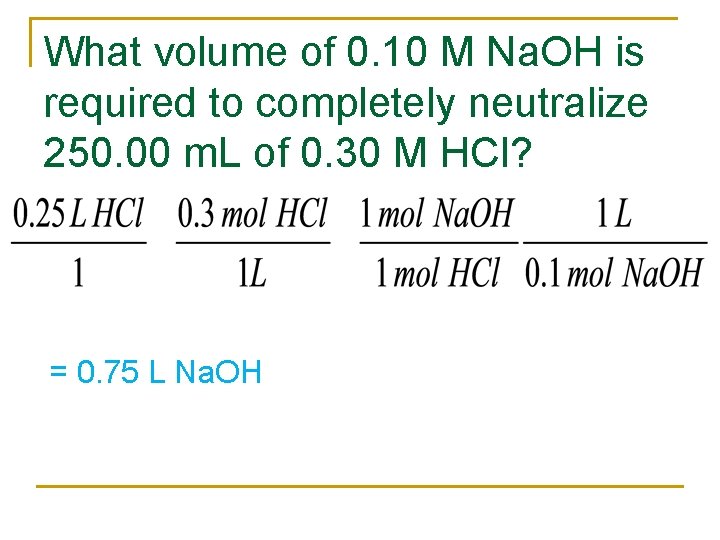
What volume of 0. 10 M Na. OH is required to completely neutralize 250. 00 m. L of 0. 30 M HCl? = 0. 75 L Na. OH

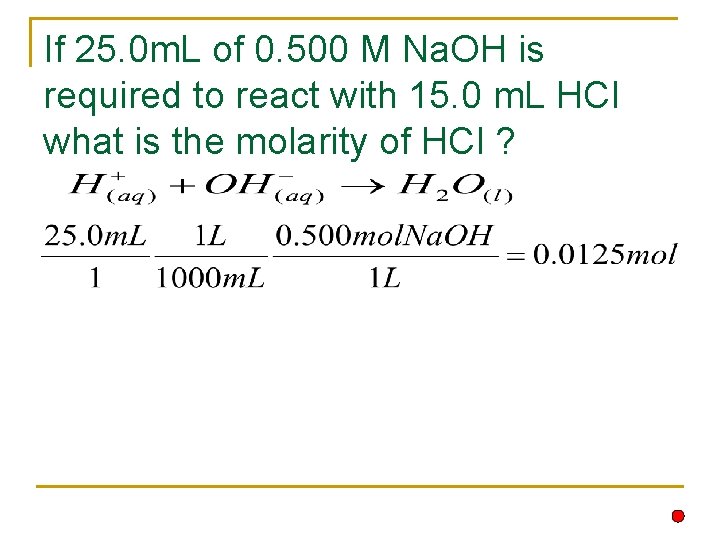
If 25. 0 m. L of 0. 500 M Na. OH is required to react with 15. 0 m. L HCl what is the molarity of HCl ?

Oxidation-reduction reactions n Redox q Oxidation-loss of electrons n q Reduction-gain of electrons n q Oxidation number decreases Oxidizing agent n q Oxidation number increases The element that undergoes reduction Reducing agent n The element that undergoes oxidation

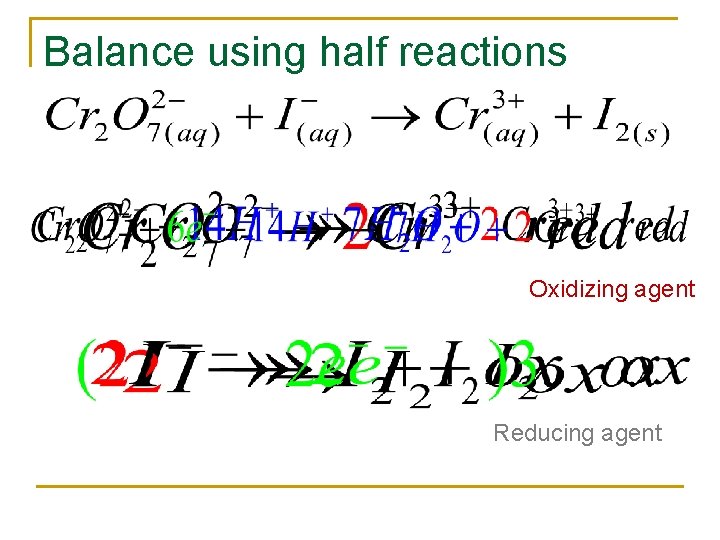
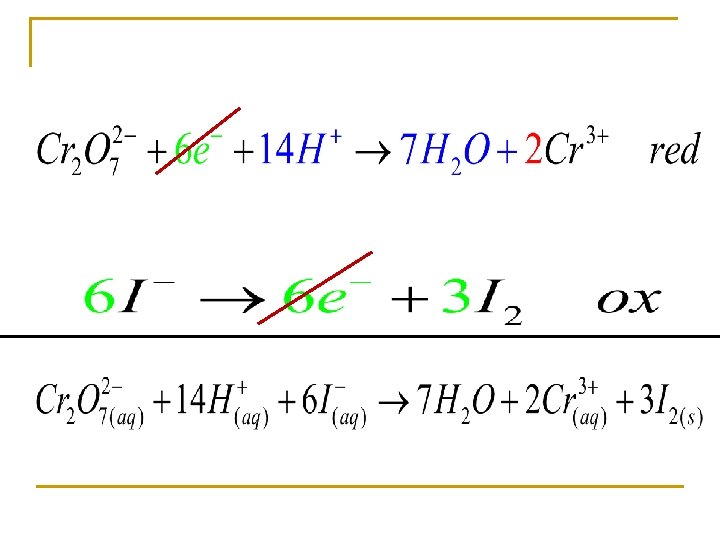
Balancing n n Balance the atoms Break the equation into two half reaction q q n n Oxidation Reduction Balance O’s with H 20 Balance H’s with H+ Balance the charge with electrons If confused do not use the book’s method!

Balance using half reactions Oxidizing agent Reducing agent


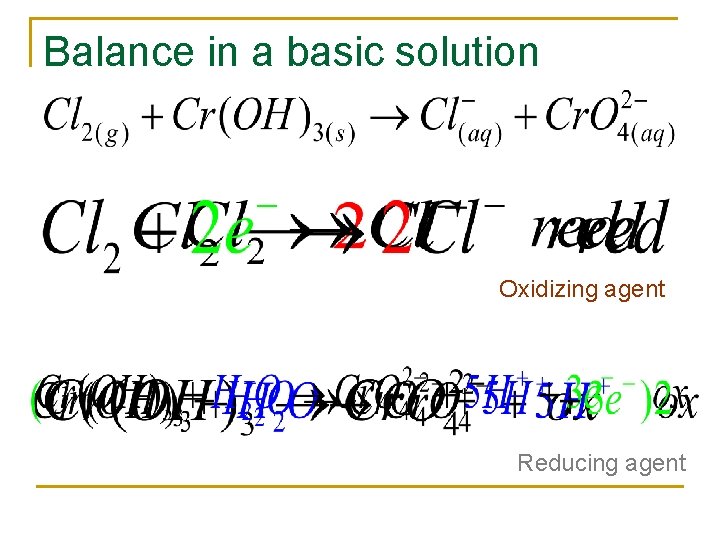
In a basic solution n Balance all redox in acidic solution unless stated

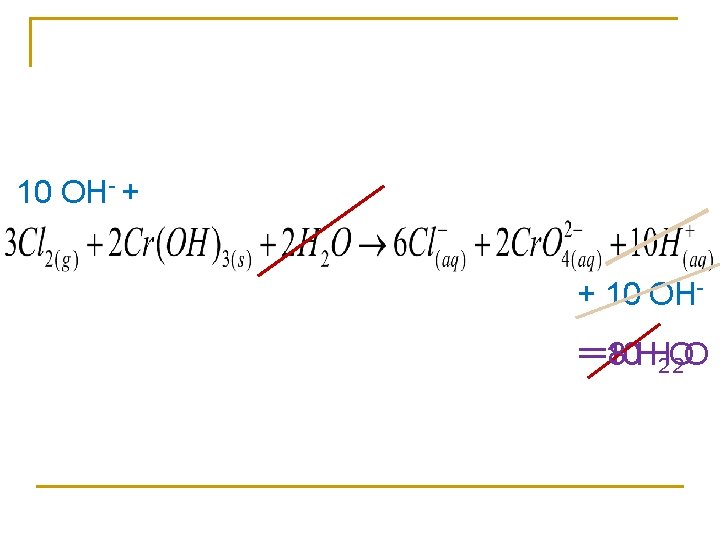
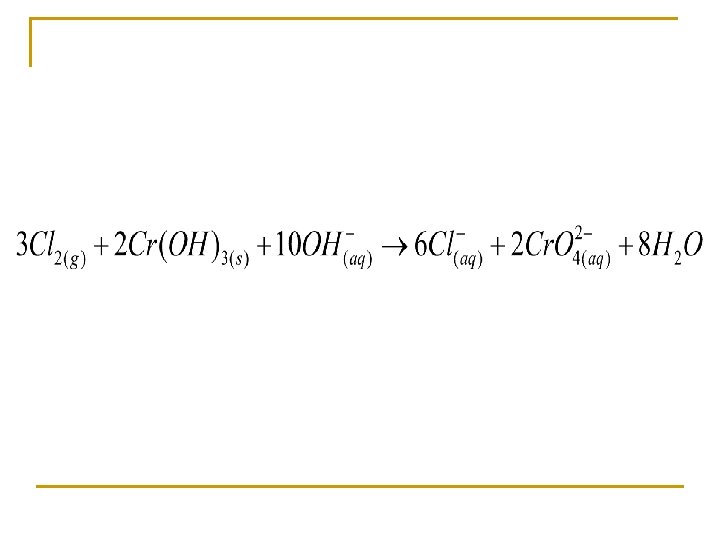
Balance in a basic solution Oxidizing agent Reducing agent
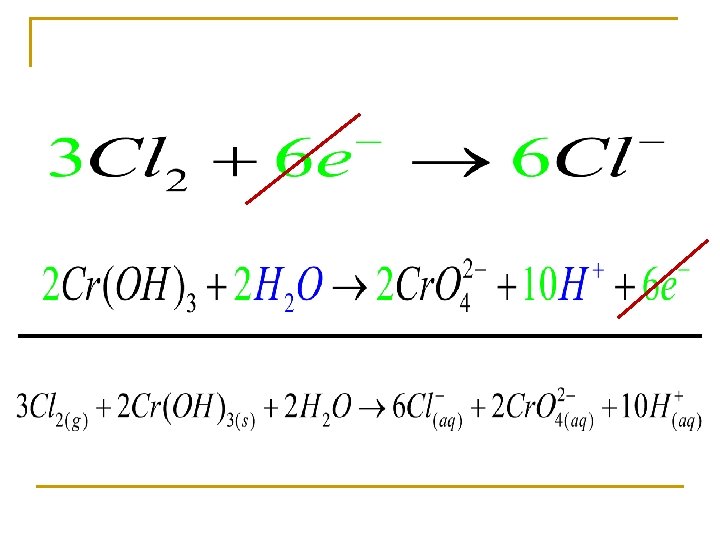

10 OH- + + 10 OH==8 10 HH 2 O 2 O


What volume of 0. 684 M KMn. O 4 solution is required to completely react with 27. 50 m. L of 0. 250 M Fe(NO 3)2