Precipitation Reactions Introduction to precipitates Discuss key ideas






- Slides: 12

Precipitation Reactions

Introduction to precipitates Ø Discuss key ideas: Ø Ø Define salts (including polyatomic ions) Soluble insoluble Electrostatic attraction of water Define precipitate - insoluble salt. Ø Activity: Ø Read Science Search 4 p 156 & 157. Ø Do Copy & Complete on p 157 Ø Answer questions: 1, 2, 4 & 8

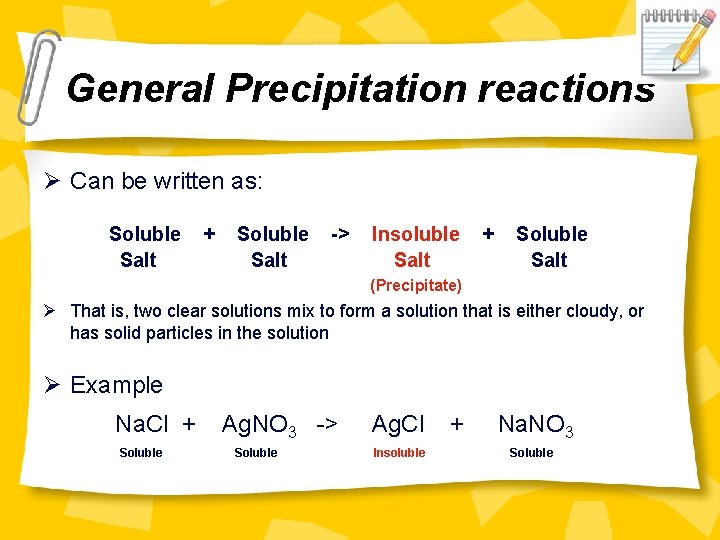
General Precipitation reactions Ø Can be written as: Soluble Salt + Soluble Salt -> Insoluble Salt + Soluble Salt (Precipitate) Ø That is, two clear solutions mix to form a solution that is either cloudy, or has solid particles in the solution Ø Example Na. Cl + Soluble Ag. NO 3 -> Soluble Ag. Cl Insoluble + Na. NO 3 Soluble

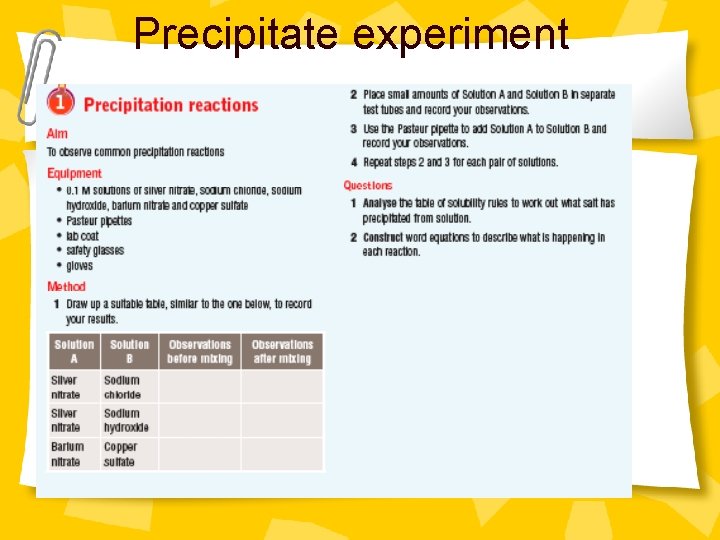
Precipitation Experiment Ø Read handout and complete experiment. Ø Answer question on solubility rules.

Precipitate handout

Precipitate experiment

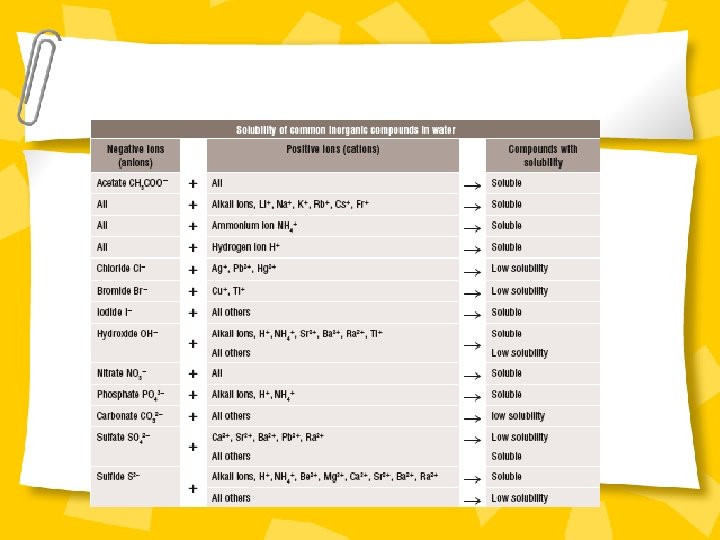
Questions / discussion • Write your own table of results for the experiment. • Analyse the table of solubility rules to work out what salt has precipitated from solutions • Construct word equations to describe what is happening in each reaction


Checklist Ø Line 17 - 3/5 - not balancing equations or theories and laws Ø Line 18 - 3/5 - Experiment combining solutions. Ø Line 19 - 20/5 - Precipitation

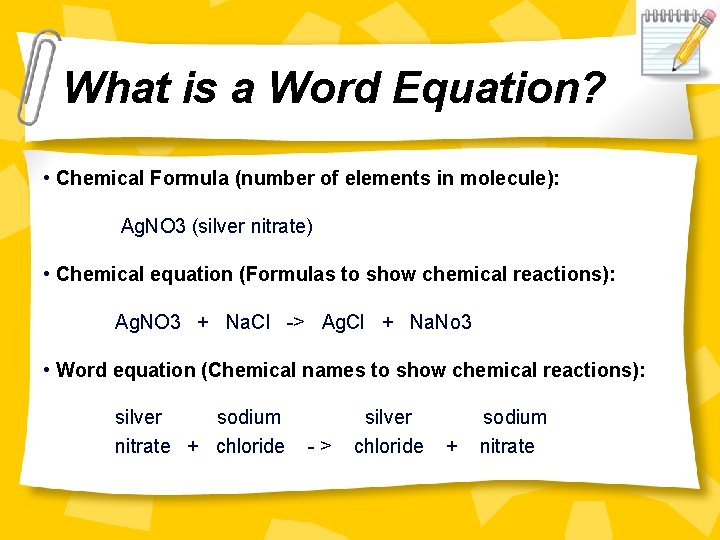
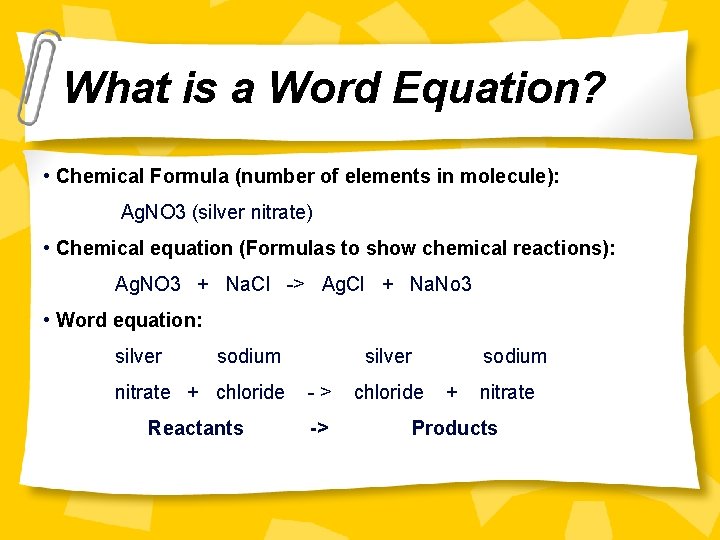
What is a Word Equation? • Chemical Formula (number of elements in molecule): Ag. NO 3 (silver nitrate) • Chemical equation (Formulas to show chemical reactions): Ag. NO 3 + Na. Cl -> Ag. Cl + Na. No 3 • Word equation (Chemical names to show chemical reactions): silver sodium nitrate + chloride -> silver chloride + sodium nitrate

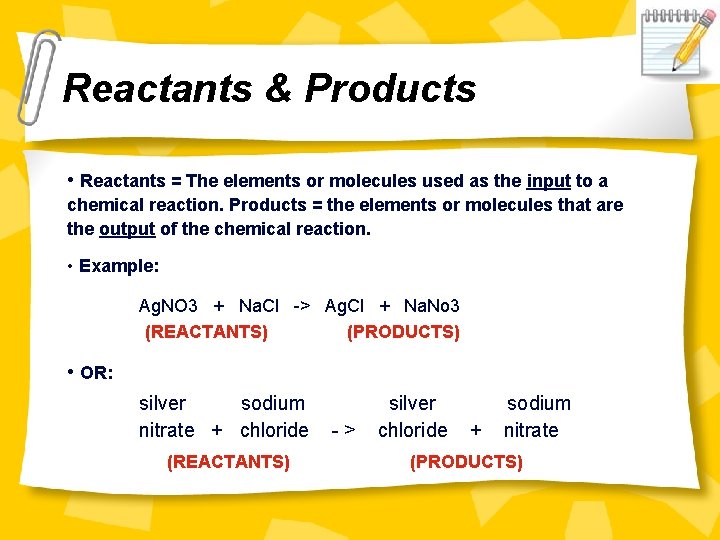
Reactants & Products • Reactants = The elements or molecules used as the input to a chemical reaction. Products = the elements or molecules that are the output of the chemical reaction. • Example: Ag. NO 3 + Na. Cl -> Ag. Cl + Na. No 3 (REACTANTS) (PRODUCTS) • OR: silver sodium nitrate + chloride (REACTANTS) -> silver chloride + sodium nitrate (PRODUCTS)

What is a Word Equation? • Chemical Formula (number of elements in molecule): Ag. NO 3 (silver nitrate) • Chemical equation (Formulas to show chemical reactions): Ag. NO 3 + Na. Cl -> Ag. Cl + Na. No 3 • Word equation: silver sodium silver nitrate + chloride -> Reactants -> sodium chloride + nitrate Products
 Keratic precipitates
Keratic precipitates Occlusio pupillae definition
Occlusio pupillae definition Lesson 90 solid evidence precipitation reactions
Lesson 90 solid evidence precipitation reactions Co precipitation and post precipitation
Co precipitation and post precipitation Co precipitation and post precipitation
Co precipitation and post precipitation Potassium chloride precipitate
Potassium chloride precipitate Double replacement precipitation
Double replacement precipitation Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Oxidation half reaction
Oxidation half reaction Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Amateurs talk tactics professionals talk logistics
Amateurs talk tactics professionals talk logistics