Section 4 2 Precipitation Reactions Aqueous Reactions Precipitation















- Slides: 27

Section 4. 2 Precipitation Reactions Aqueous Reactions

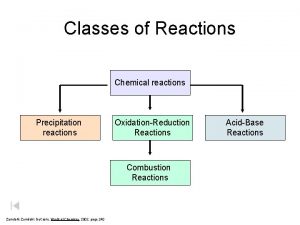
Precipitation Reactions Mixing ions that form insoluble compounds The insoluble solid formed is a precipitate Aqueous Reactions

Solubility • The amount of substance that can dissolve in a given quantity of solvent at a given temperature Aqueous Reactions

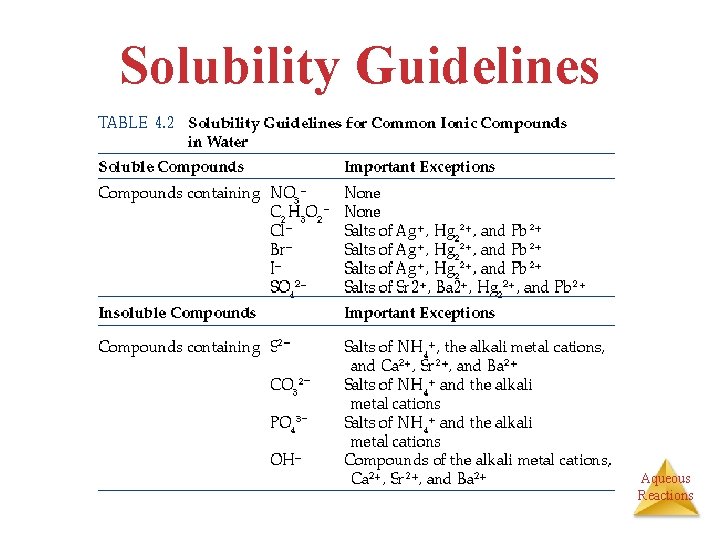
Solubility Guidelines Aqueous Reactions

Steps for Predicting Precipitation 1. Note ions present in reaction 2. Consider possible combinations of cations and anions 3. Use Table 4. 1 to determine if any of those combinations are insoluble Aqueous Reactions

Example • Will a precipitate form when solutions of Mg(NO 3)2 and Na. OH are mixed? Aqueous Reactions

Step 1: Ions present • • Mg 2+ NO 3 – Na + OH - Aqueous Reactions

Step 2: Possible combinations • Mg 2+ with OH – • Na + with NO 3 – Aqueous Reactions

Step 3: Table 4. 1 • Hydroxides generally insoluble, and Mg is not an exception • Na. NO 3 is soluble Mg(NO 3)2 (aq) + 2 Na. OH (aq) Mg(OH)2 (s) + 2 Na. NO 3 (aq) Aqueous Reactions

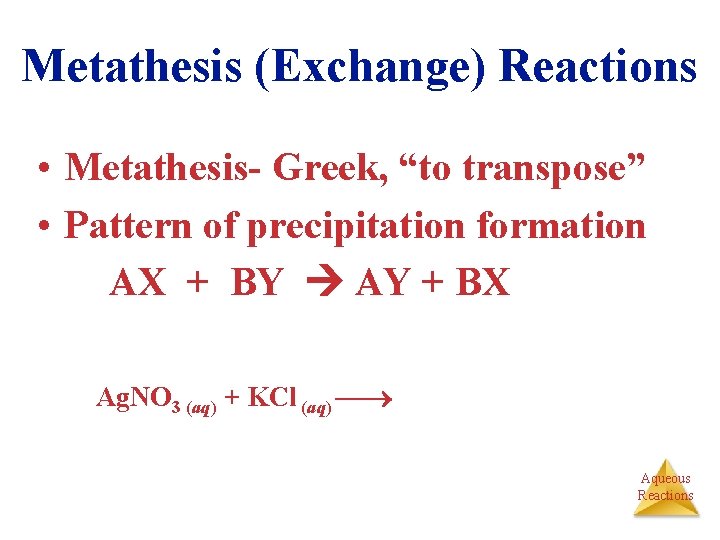
Metathesis (Exchange) Reactions • Metathesis- Greek, “to transpose” • Pattern of precipitation formation AX + BY AY + BX Ag. NO 3 (aq) + KCl (aq) Ag. Cl (s) + KNO 3 (aq) Aqueous Reactions

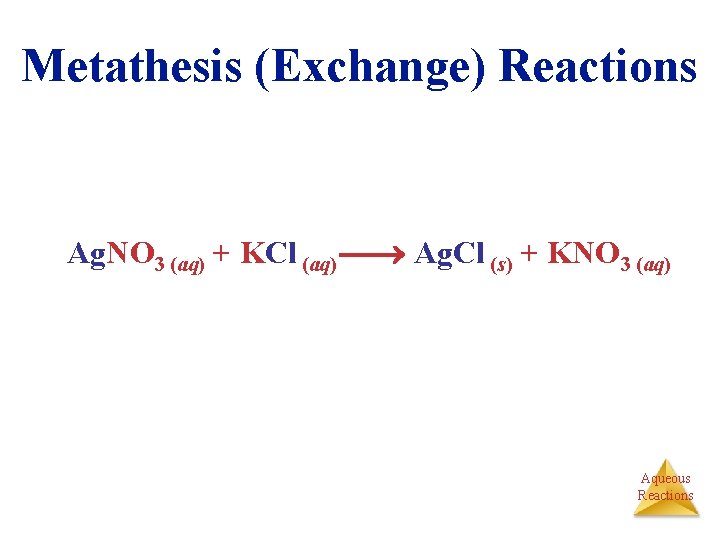
Metathesis (Exchange) Reactions Ag. NO 3 (aq) + KCl (aq) Ag. Cl (s) + KNO 3 (aq) Aqueous Reactions

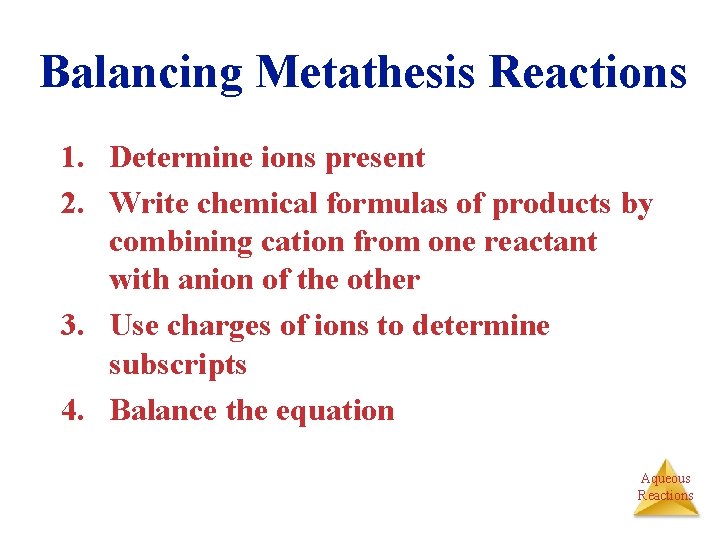
Balancing Metathesis Reactions 1. Determine ions present 2. Write chemical formulas of products by combining cation from one reactant with anion of the other 3. Use charges of ions to determine subscripts 4. Balance the equation Aqueous Reactions

Sample Problem • Predict the identity of the precipitate that forms when solutions of Ba. Cl 2 and K 2 SO 4 are mixed. Aqueous Reactions

Step 1: Determine ions • Ba. Cl 2 and K 2 SO 4 are mixed: • Ba 2+ • Cl • K+ • SO 4 2 Aqueous Reactions

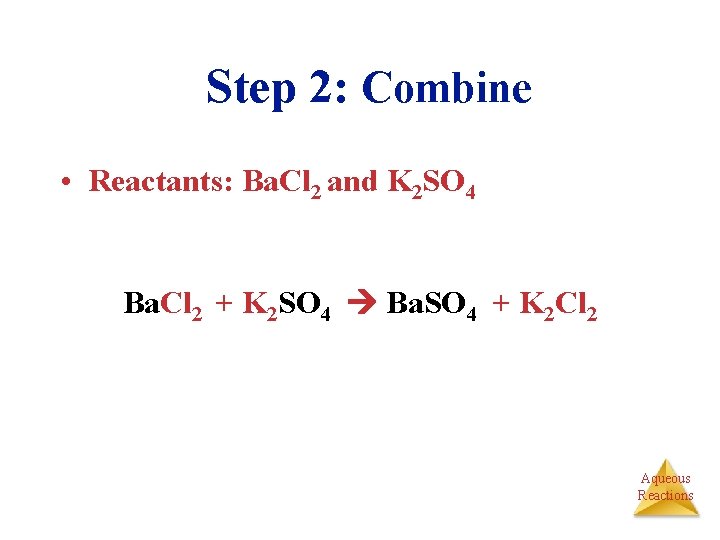
Step 2: Combine • Reactants: Ba. Cl 2 and K 2 SO 4 Ba. Cl 2 + K 2 SO 4 Ba. SO 4 + K 2 Cl 2 Aqueous Reactions

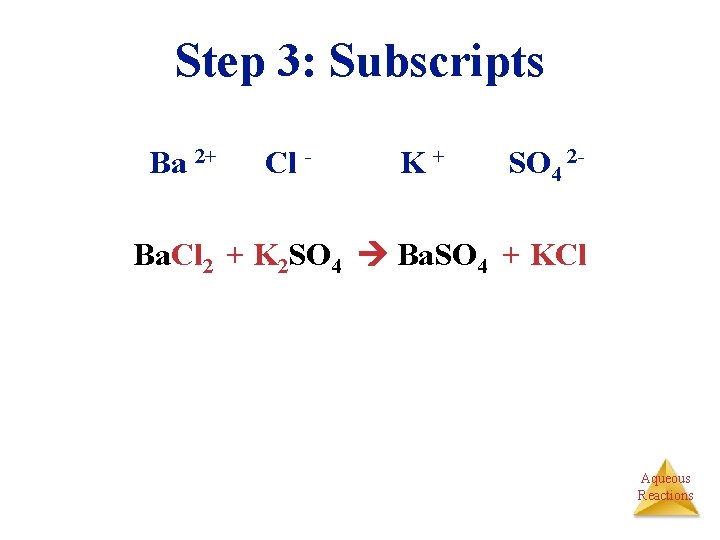
Step 3: Subscripts Ba 2+ Cl - K+ SO 4 2 - Ba. Cl 2 + K 2 SO 4 Ba. SO 4 + KCl Aqueous Reactions

Step 4: Balance Ba. Cl 2 + K 2 SO 4 Ba. SO 4 + 2 KCl Did a precipitate form? Aqueous Reactions

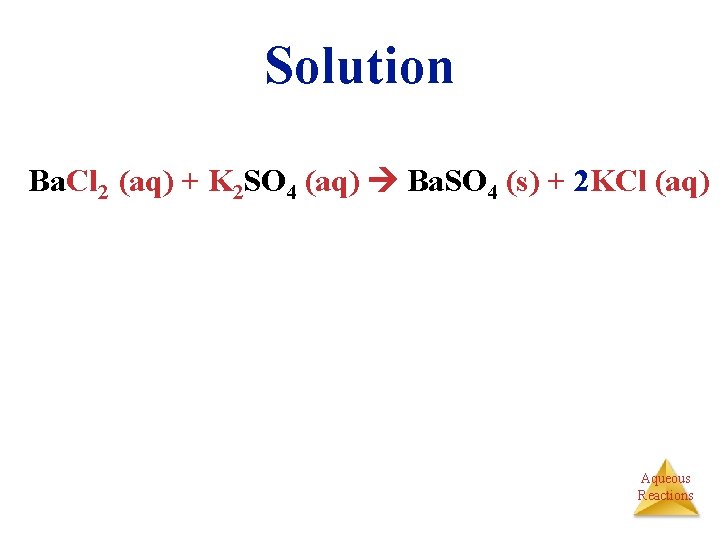
Solution Ba. Cl 2 (aq) + K 2 SO 4 (aq) Ba. SO 4 (s) + 2 KCl (aq) Aqueous Reactions

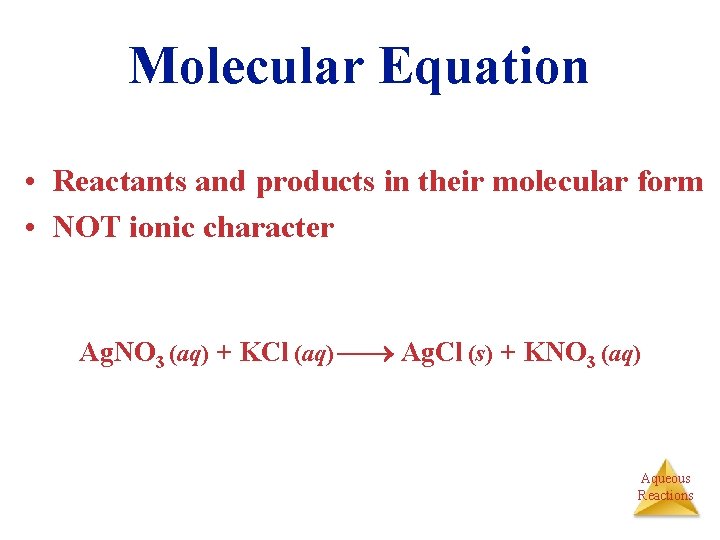
Molecular Equation • Reactants and products in their molecular form • NOT ionic character Ag. NO 3 (aq) + KCl (aq) Ag. Cl (s) + KNO 3 (aq) Aqueous Reactions

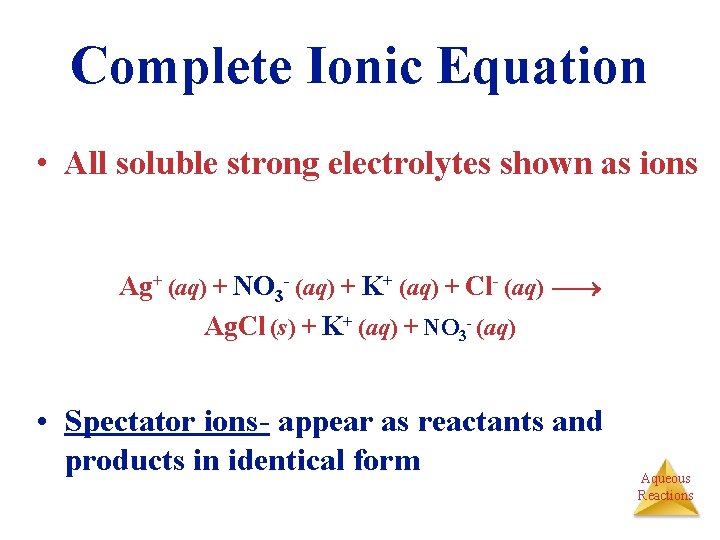
Complete Ionic Equation • All soluble strong electrolytes shown as ions Ag+ (aq) + NO 3 - (aq) + K+ (aq) + Cl- (aq) Ag. Cl (s) + K+ (aq) + NO 3 - (aq) • Spectator ions- appear as reactants and products in identical form Aqueous Reactions

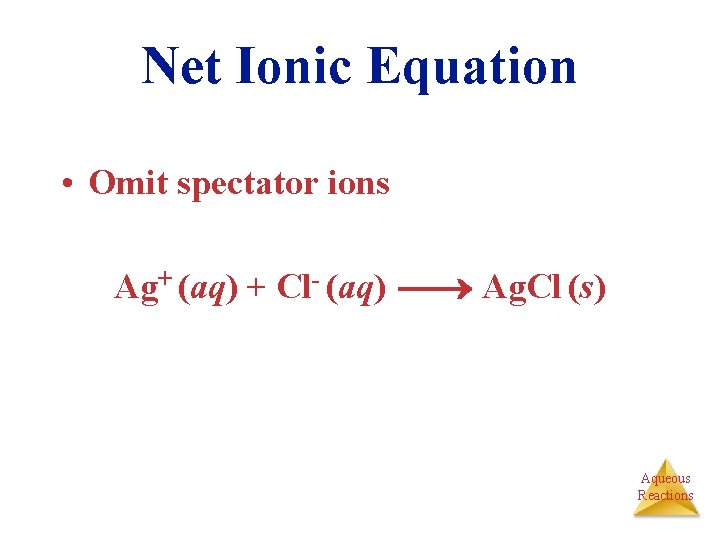
Net Ionic Equation • Omit spectator ions Ag+ (aq) + Cl- (aq) Ag. Cl (s) Aqueous Reactions

Writing Net Ionic Equations 1. Write a balanced molecular equation. 2. Dissociate all strong electrolytes. 3. Identify and cancel spectator ions Aqueous Reactions

Sample Problem • Write the net ionic equation for mixing calcium chloride and sodium carbonate. Aqueous Reactions

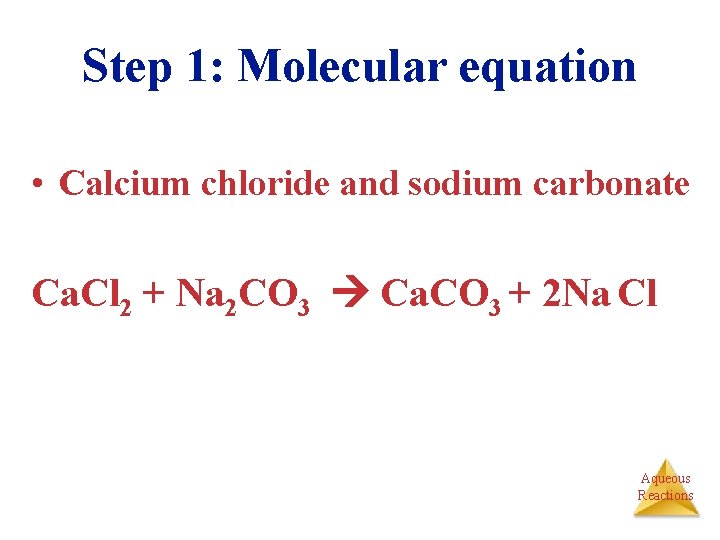
Step 1: Molecular equation • Calcium chloride and sodium carbonate Ca. Cl 2 + Na 2 CO 3 Ca. CO 3 + 2 Na Cl Aqueous Reactions

Step 2: Dissociate strong electrolytes Ca 2+ + 2 Cl- + 2 Na+ + CO 3 2 - Ca. CO 3 + 2 Na+ + 2 Cl- All are strong electrolytes, but Ca. CO 3 is insoluble in water Aqueous Reactions

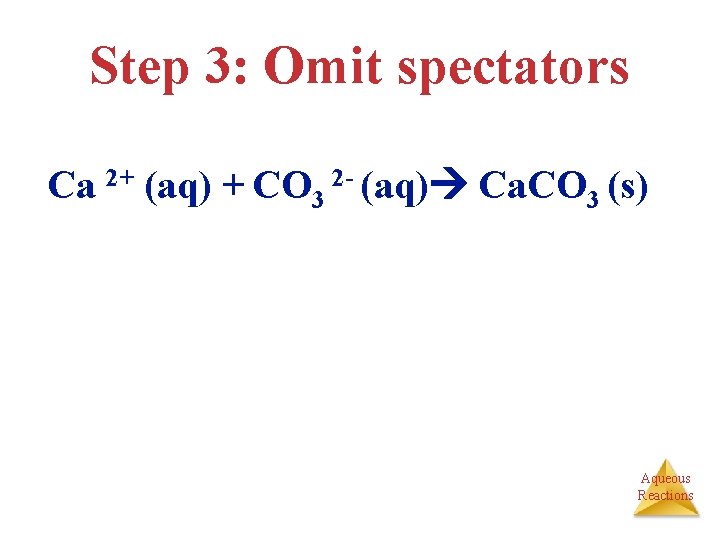
Step 3: Omit spectators Ca 2+ (aq) + CO 3 2 - (aq) Ca. CO 3 (s) Aqueous Reactions

Homework • 4. 19 -4. 24 on page 158 Aqueous Reactions
 Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Reactions in aqueous solutions
Reactions in aqueous solutions Aqueous reactions and solution stoichiometry
Aqueous reactions and solution stoichiometry Aqueous solution practice problems
Aqueous solution practice problems Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Chapter 4 reactions in aqueous solutions worksheet answers
Chapter 4 reactions in aqueous solutions worksheet answers Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions
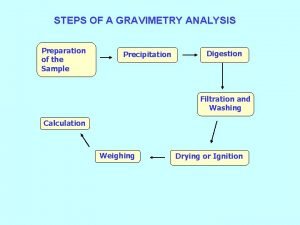
Section 2 classifying chemical reactions Gravimetry steps
Gravimetry steps Co precipitation and post precipitation
Co precipitation and post precipitation Lesson 90 solid evidence precipitation reactions
Lesson 90 solid evidence precipitation reactions Predicting precipitation reactions
Predicting precipitation reactions Are precipitation reactions double replacement
Are precipitation reactions double replacement Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Section 3 acid precipitation
Section 3 acid precipitation Section 3 clouds and precipitation
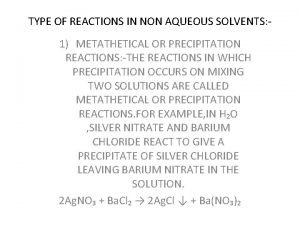
Section 3 clouds and precipitation Types of reaction in non aqueous solvents
Types of reaction in non aqueous solvents Clarity of aqueous extract test
Clarity of aqueous extract test Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Pelarut non air yang dapat bercampur dengan air adalah
Pelarut non air yang dapat bercampur dengan air adalah Non aqueous solvents classification
Non aqueous solvents classification Chapter 13 ions in aqueous solutions
Chapter 13 ions in aqueous solutions Aqueous solutions module
Aqueous solutions module Solid liquid, gas aqueous chart
Solid liquid, gas aqueous chart