PT PT PT 200 200 400 400 600






















































- Slides: 70



PT PT PT $200 $200 $400 $400 $600 $600 $800 $800 $1000 $1000

Category 1 Category 2 Category 3 Category 4 Category 5 Category 6 $400 $400 $800 $800 $1200 $1200 $1600 $1600 $2000 $2000

Final Jeopardy Category

Final Jeopardy answer question

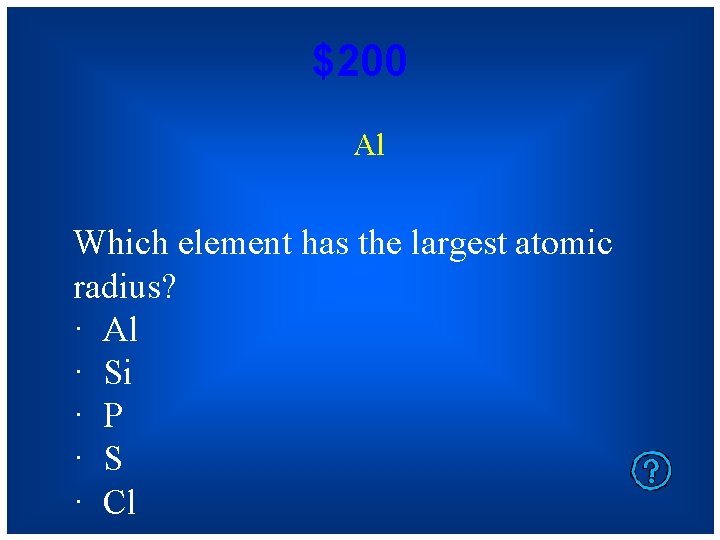
$200 Al Which element has the largest atomic radius? · Al · Si · P · S · Cl

$400 c. 7 How many electrons are in the outer electron orbit of the most electronegative element? a. 1 b. 2 c. 7 d. 8

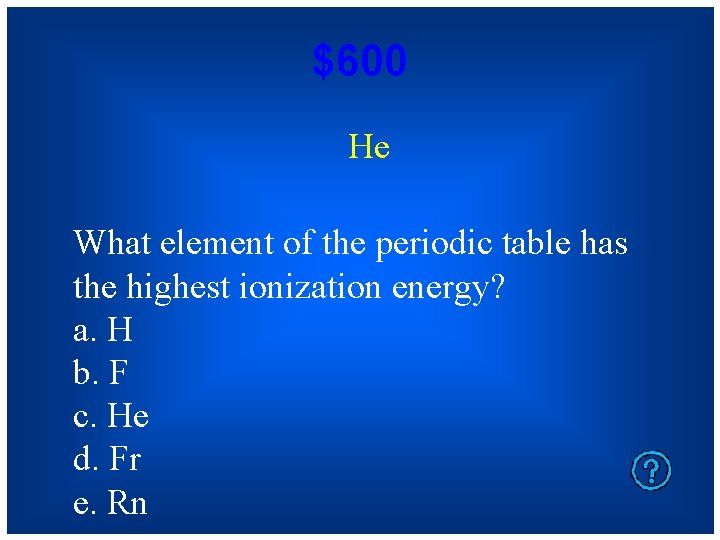
$600 He What element of the periodic table has the highest ionization energy? a. H b. F c. He d. Fr e. Rn

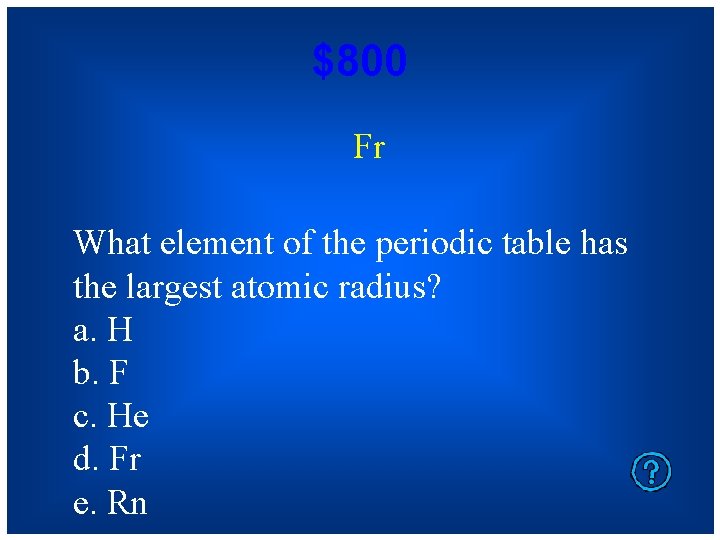
$800 Fr What element of the periodic table has the largest atomic radius? a. H b. F c. He d. Fr e. Rn

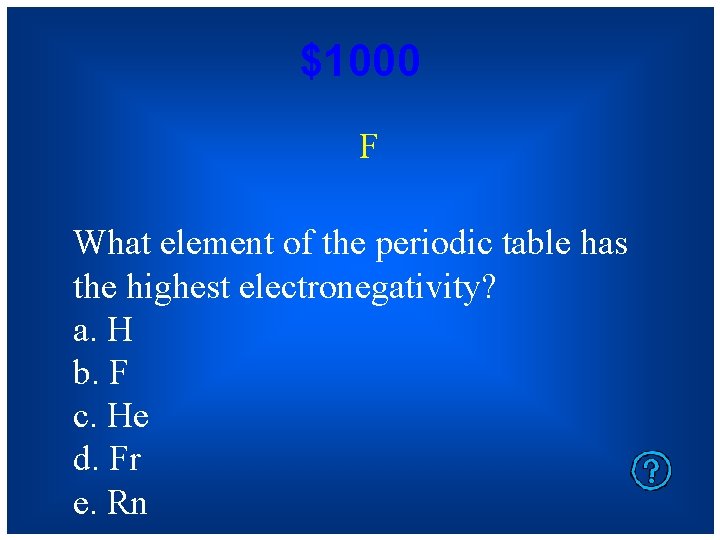
$1000 F What element of the periodic table has the highest electronegativity? a. H b. F c. He d. Fr e. Rn

$200 increases For the elements of Group 1 on the Periodic Table, as the atomic radius increases, the ionization energy a. decreases b. increases c. remains the same

$400 c. nitrogen Which of these elements has the least attraction for electrons in a chemical bond? a. oxygen b. fluorine c. nitrogen d. chlorine

$600 b. Sr Which of the following atoms requires the least energy for the removal of an outer electron? a. Sn b. Sr c. Be d. Br

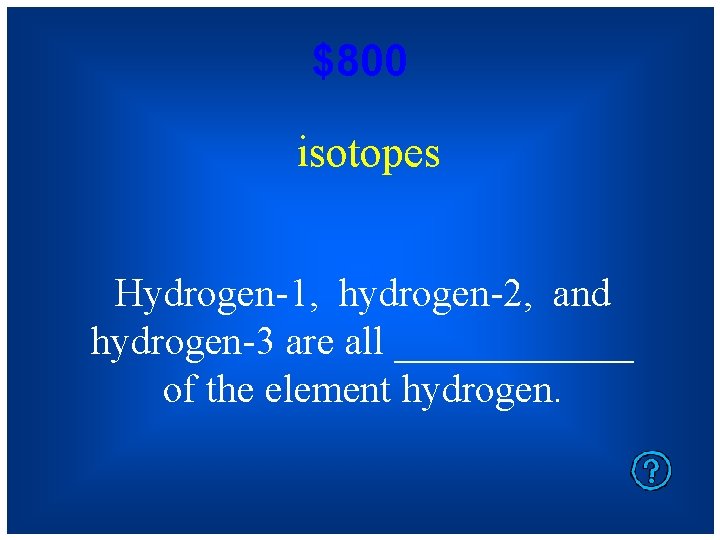
$800 isotopes Hydrogen-1, hydrogen-2, and hydrogen-3 are all ______ of the element hydrogen.

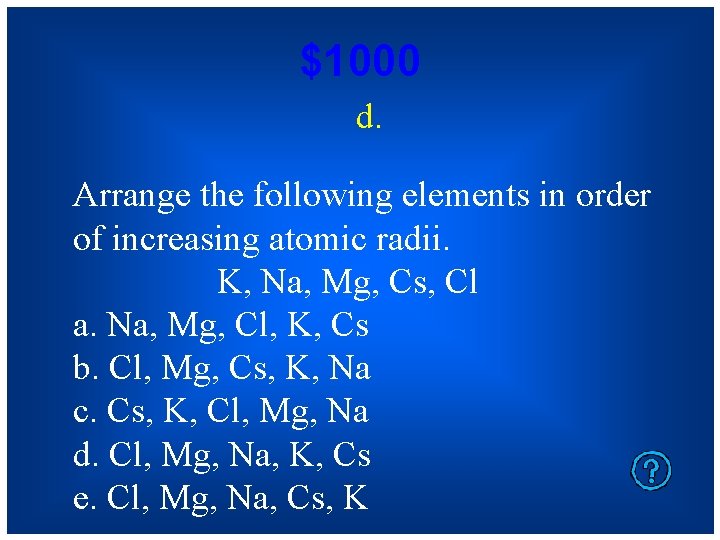
$1000 d. Arrange the following elements in order of increasing atomic radii. K, Na, Mg, Cs, Cl a. Na, Mg, Cl, K, Cs b. Cl, Mg, Cs, K, Na c. Cs, K, Cl, Mg, Na d. Cl, Mg, Na, K, Cs e. Cl, Mg, Na, Cs, K

$200 c. fluorine Atoms of which element have the greatest tendency to gain electrons? a. bromine b. chlorine c. fluorine d. iodine

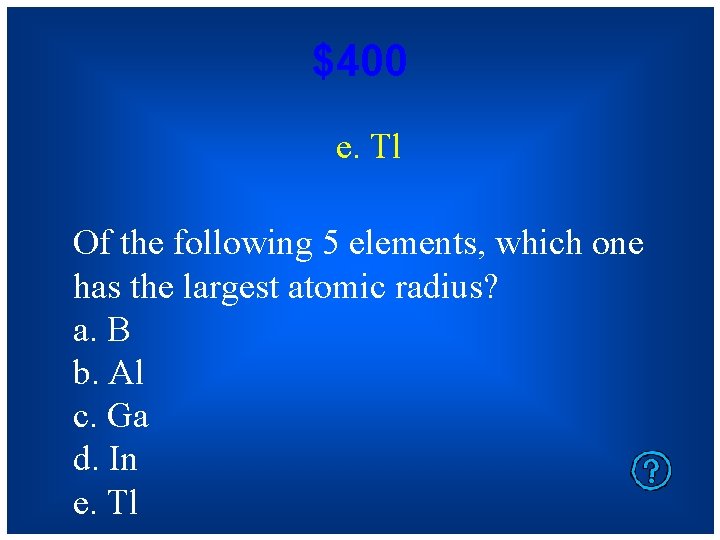
$400 e. Tl Of the following 5 elements, which one has the largest atomic radius? a. B b. Al c. Ga d. In e. Tl

$600 a. Al Of the following 5 elements, which one has the largest atomic radius? a. Al b. Si c. P d. S e. Cl

$800 e. Se Of the following 5 elements, which one has the smallest radius? a. Ca b. Ga c. Ge d. As e. Se

$1000 a. F Of the following 5 elements, which one has the smallest radius? a. F b. Cl c. Br d. I e. At

$200 a. Be Of the following 5 elements, which one has the lowest first ionization energy? a. Be b. B c. C d. N e. O

$400 b. strontium Which of the following elements has the least tendency to gain electrons? a. tin b. strontium c. iodine d. nickel

$600 b. K and Br Which of following pairs of elements would have the greatest difference in electronegativity? a. Cl and O b. K and Br c. Na and Rb d. I and F

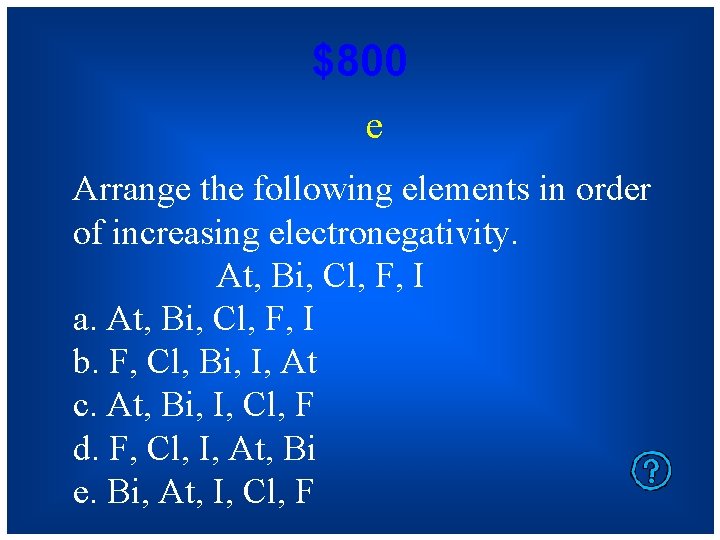
$800 e Arrange the following elements in order of increasing electronegativity. At, Bi, Cl, F, I a. At, Bi, Cl, F, I b. F, Cl, Bi, I, At c. At, Bi, I, Cl, F d. F, Cl, I, At, Bi e. Bi, At, I, Cl, F

$1000 c. None of these Which of the following trends have the same pattern as atomic radius? a. electronegativity. b ionization energy c. none of these d. both of these

$200 a. krypton Which element is a noble gas? a. krypton b. chlorine c. antimony d. nobelium

$400 c. ionization energy The amount of energy required to remove the outermost electron from an atom is called a. electron affinity b. activation energy c. ionization energy d. electronegativity

$600 d. their outer orbitals have the same number of electrons Why do Na and K have similar chemical properties? a. they are in the same row b. they are in the same period c. their 2 nd electron orbitals are complete d. their outer orbitals have the same number of electrons

$800 d. argon atom When potassium reacts to become stable, its electron configuration will look like a a. potassium atom b. sodium ion c. neon atom d. argon atom e. calcium atom

a. low first ionization energy and low $1000 electronegativity Which two characteristics are associated with metals? a. low first ionization energy and low electronegativity b. low first ionization energy and high electronegativity c. high first ionization energy and low electronegativity d. high first ionization energy and high electronegativity

$200 a. 5 What is the total number of valence electrons in an atom of arsenic? a. 5 b. 15 c. 33 d. 75 e. None of the above

$400 a. metal The element in Period 4 and Group 1 of the Periodic Table would be classified as a a. metal b. metalloid c. nonmetal d. noble gas e. none of the above

$600 c. 8 valence electrons . An atom of argon rarely bonds to an atom of another element because an argon atom has a. 3 electron shells b. 2 electrons in the first shell c. 8 valence electrons d. 22 neutrons

$800 Which element has both metallic and nonmetallic properties? a. Rb b. Rn c. Si d. Sr

b $1000 How do the energy and the location of an electron in the third shell (orbit) of an atom compare to the energy and location of an electron in the first shell of the same atom? a. In the third shell, an electron has more energy and is closer to the nucleus. b. In the third shell, an electron has more energy and is farther from the nucleus. c. In the third shell, an electron has less energy and is closer to the nucleus. d. In the third shell, an electron has less energy and is farther from the nucleus.

$400 c. neon In the compound sodium fluoride, Na. F, the sodium atom loses one electron and the fluorine atom gains one electron to form ions that have electron configurations similar to a. helium. c. neon. b. oxygen. d. calcium.

$800 b. noble gas The elements of the _____ group satisfy the octet rule without forming compounds. a. Halide c. alkali metal b. noble gas d. alkaline-earth metal

$1200 d. The correct Lewis structure for a Group 18 (VIII) atom has a. one pair of valence electrons and one single valence electron. b. two pairs of valence electrons and one single valence electron. c. three pairs of valence electrons and one single valence electron. d. four pairs of valence electrons.

$1600 c. The correct Lewis structure for the oxygen atom has a. one pair of valence electrons and one single valence electron. b. two pairs of valence electrons and one single valence electron c. two pairs of valence electrons and two single valence electrons. d. three pairs of valence electrons.

$2000 b. What is the formula for the compound formed by aluminum (III) and the sulfate ion, SO 42–? a. Al. SO 4 b. Al 2(SO 4)3 c. Al 2 SO 4 d. Al(SO 4)3

$400 ion An atom or group of atoms that has an electrical charge because it has either lost or gained one or more electrons is called a(n) __________.

$800 cation An ion that has a positive charge is a(n) __________.

$1200 anion An ion that has a negative charge is a(n) __________.

$1600 oxide The name of the ion O 2– is the __________ ion

$2000 hydrogen In the compound HCl, the atom of the element __________ carries a partial positive charge

$400 b. length The meter is a unit of a) volume b) length c) temperature d) energy

$800 c. The number of millimeters in 0. 532 meters is a. 5. 32 x 10 -3 mm b. 5. 32 x 104 mm c. 5. 32 x 102 mm d. 5. 32 x 103 mm

$1200 b. Express 1870000 in scientific notation. a. 1. 87 x 10 -6 b. 1. 87 x 106 c. 187 x 106 d. 187 x 104

$1600 electron Which particle has the least mass? neutron proton electron helium nucleus hydrogen atom

$2000 atom The fundamental “particle” of a chemical element according to Dalton’s theory is the electron molecule atom compound

$400 c) burning wood Which of the following involves a chemical change? a) freezing water b) breaking ice c) burning wood d) boiling water

$800 sodium Which of the following is an element? air water salt sodium sugar

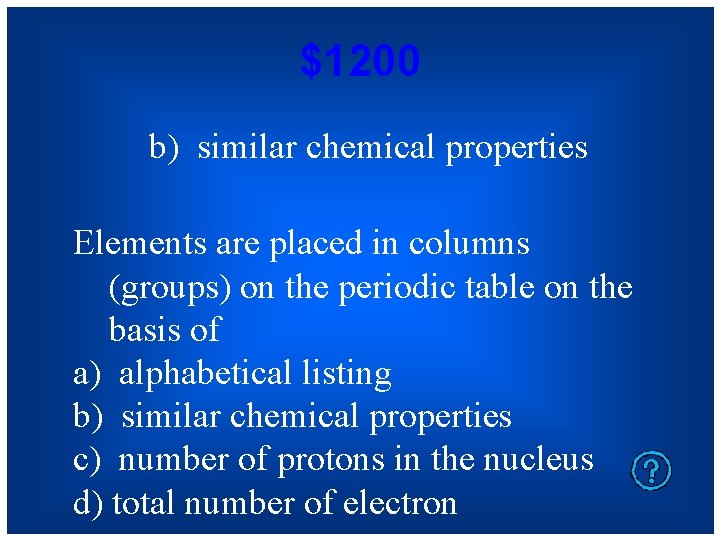
$1200 b) similar chemical properties Elements are placed in columns (groups) on the periodic table on the basis of a) alphabetical listing b) similar chemical properties c) number of protons in the nucleus d) total number of electron

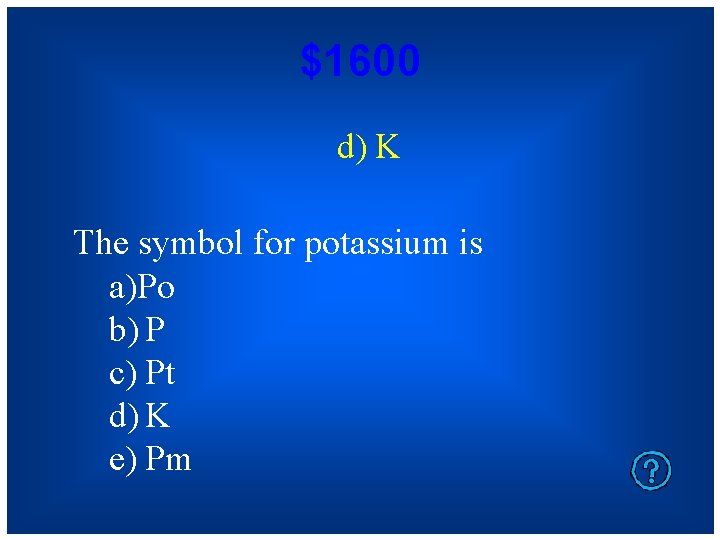
$1600 d) K The symbol for potassium is a)Po b) P c) Pt d) K e) Pm

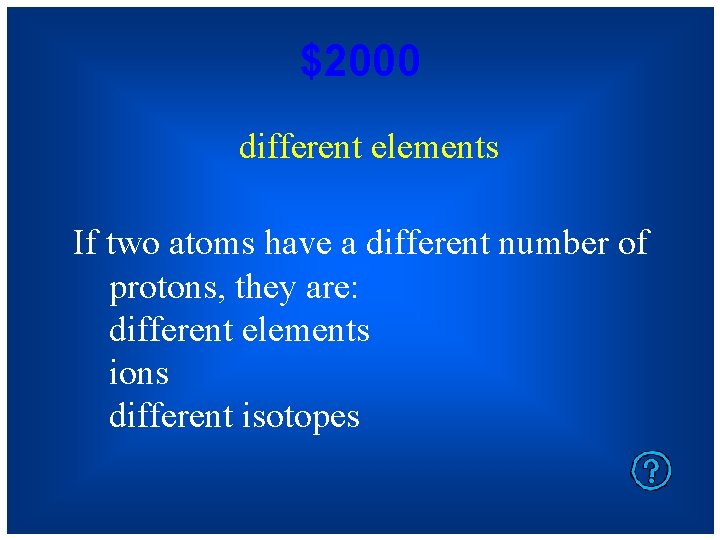
$2000 different elements If two atoms have a different number of protons, they are: different elements ions different isotopes

$400 d) The mass number of an atom equals a) the number of neutrons b) the atomic number of the element c) the number of protons plus the number of electrons d) the number of protons plus the number of neutrons

$800 gas The state of matter for an object that has neither definite shape nor definite volume is solid liquid gas elemental

$1200 carbon Which of the following is a nonmetal? cerium cesium carbon calcium copper

$1600 7 How many hydrogen atoms are indicated in the formula NH 4 C 2 H 3 O 2?

$2000 calcium fluoride The compound Ca. F 2 is called difluorocalcium fluoride calcium fluorine dicalcium fluoride monocalcium difluoride

$400 aluminum Which would be an example of a element? a) the air b) oily water c) sodium chloride d) aluminum

$800 sodium chloride Which would be an example of a compound? the air oily water sodium chloride aluminum

$1200 the air Which would be an example of a homogeneous mixture? the air oily water sodium chloride aluminum

$1600 none Which would be an example of a heterogeneous mixture? the air sodium chloride dissolved in water aluminum A solution of Kool. Aid none of the above

$2000 c) Theory 1. If a hypothesis has been supported by many experiments, it is a a) Fact b) Plan c) Theory d) Symbol

Daily Double answer Draw the Lewis structure for H 2 O

Daily Double answer Draw the Lewis structure for N 2

Daily Double answer Draw the Lewis structure for CO 2

The Jeopardy champion!