Physiology of Aging Nisha Rughwani MD Assistant Professor























- Slides: 48

Physiology of Aging Nisha Rughwani MD Assistant Professor Mount Sinai School of Medicine http: //www. anti-aging. org/blog/aging_3. jpg

Objectives u Discuss the Characteristics of Aging u Review the various Theories of Aging u Explore the effects of Aging on the different organ systems

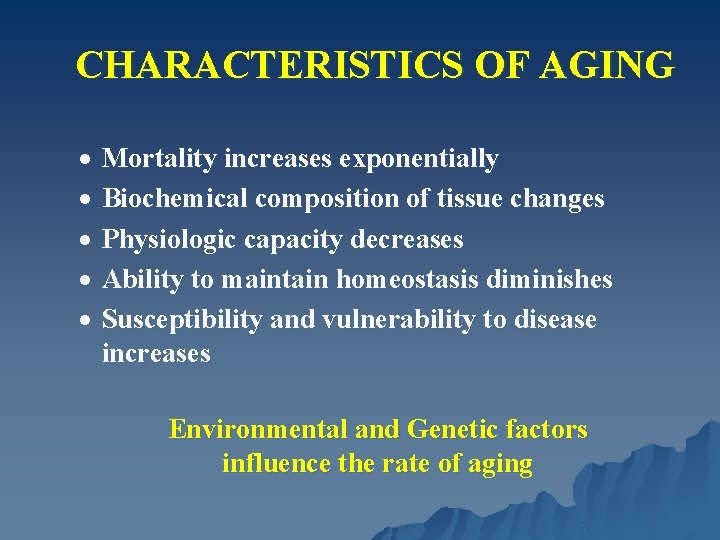
CHARACTERISTICS OF AGING · Mortality increases exponentially · Biochemical composition of tissue changes · Physiologic capacity decreases · Ability to maintain homeostasis diminishes · Susceptibility and vulnerability to disease increases Environmental and Genetic factors influence the rate of aging

CHARACTERISTICS OF AGING · Loss of physiologic reserve and decreased homeostatic control may result from: Allostatic load (persistent activation of normal neuroendocrine, immune, and autonomic responses to stress) u Development of homeostenosis (altered response to physiologic stresses) u · Changes are generally irreversible

THEORIES OF AGING: OXIDATIVE STRESS Synopsis: Oxygen converted during metabolism causes protein, lipid, and DNA damage over time In support: u Mutations in oxidative stress pathway can extend life span u Mutations in other pathways that increase longevity resist oxidative damage In opposition: Antioxidants do not delay human senescence or disease

THEORIES OF AGING: CHROMOSOMAL ALTERATIONS Synopsis: Age-acquired chromosomal instabilities contribute to gene silencing or expression of diseaserelated genes (e. g. cancer genes) In support: u u u Damage by free radicals causes mitochondrial DNA (mt. DNA) mutations in muscle and brain Defective mitochondrial respiration and further oxidant injury creates a cycle of damage Mitochondrial mutations and defective respiration have been linked to neurodegeneration In opposition: The practical impact on non-diseased aging appears to be minimal

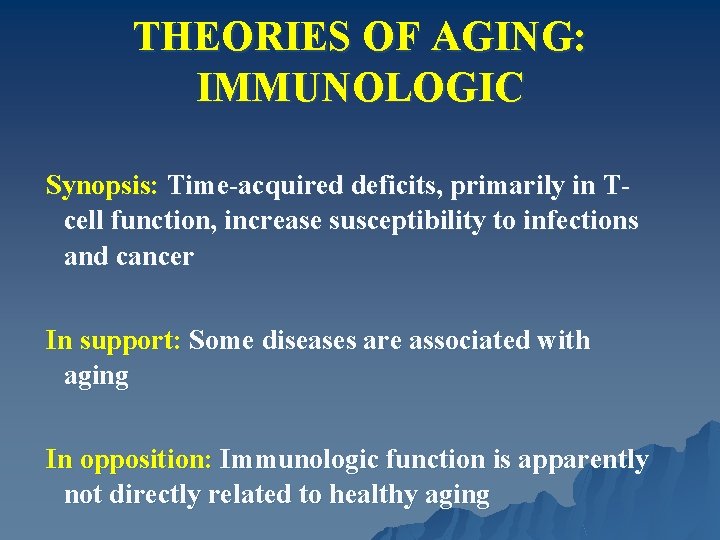
THEORIES OF AGING: IMMUNOLOGIC Synopsis: Time-acquired deficits, primarily in Tcell function, increase susceptibility to infections and cancer In support: Some diseases are associated with aging In opposition: Immunologic function is apparently not directly related to healthy aging

THEORIES OF AGING: NEUROENDOCRINOLOGIC Synopsis: Hypothalamic and pituitary responses are altered (TRH, GNRH, GHRH, TSH, LH, FSH, GH, ACTH) In support: No direct support as causative of healthy aging, supplementation does not alter aging in humans

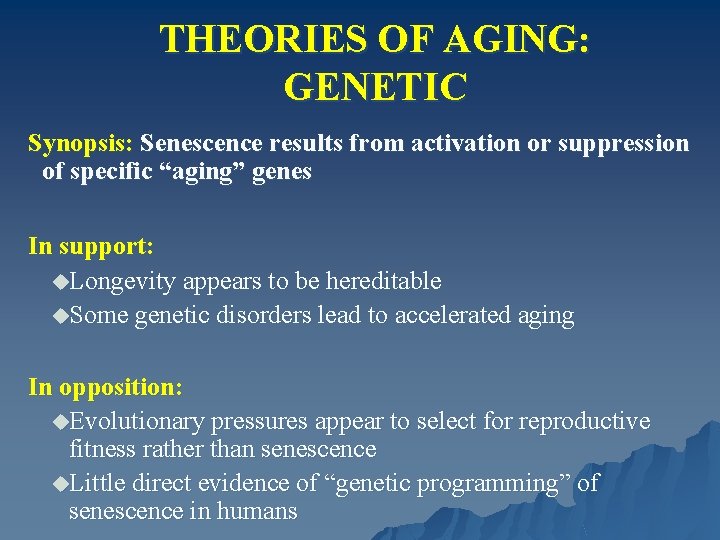
THEORIES OF AGING: GENETIC Synopsis: Senescence results from activation or suppression of specific “aging” genes In support: u. Longevity appears to be hereditable u. Some genetic disorders lead to accelerated aging In opposition: u. Evolutionary pressures appear to select for reproductive fitness rather than senescence u. Little direct evidence of “genetic programming” of senescence in humans

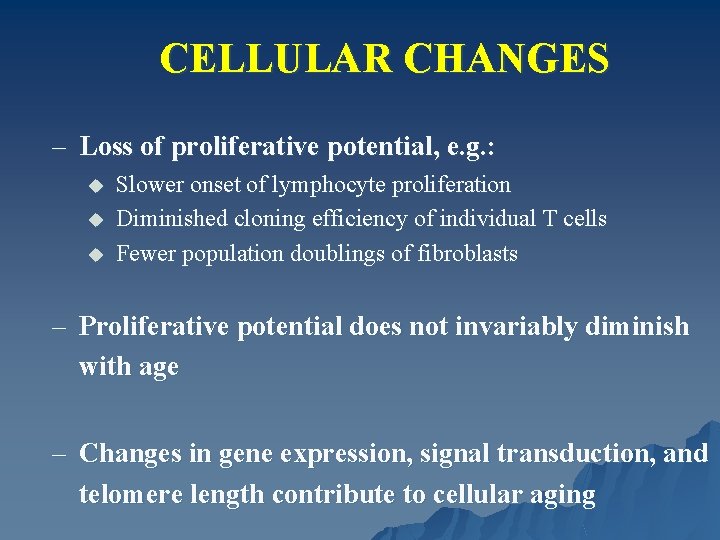
CELLULAR CHANGES – Loss of proliferative potential, e. g. : u u u Slower onset of lymphocyte proliferation Diminished cloning efficiency of individual T cells Fewer population doublings of fibroblasts – Proliferative potential does not invariably diminish with age – Changes in gene expression, signal transduction, and telomere length contribute to cellular aging

CELL DEATH – Age-dependent problems with apoptosis could result in leukemias, lymphomas, and abnormal tissue repair – Apoptosis may play a role in age-related neurodegeneration, e. g. : u u Neuronal loss in Alzheimer’s disease may be due to cytotoxicity of -amyloid, which can induce apoptosis in cultured cells Putative toxins such as free radicals have been implicated in neuronal loss in Parkinson’s disease

Aging effects on organ systems

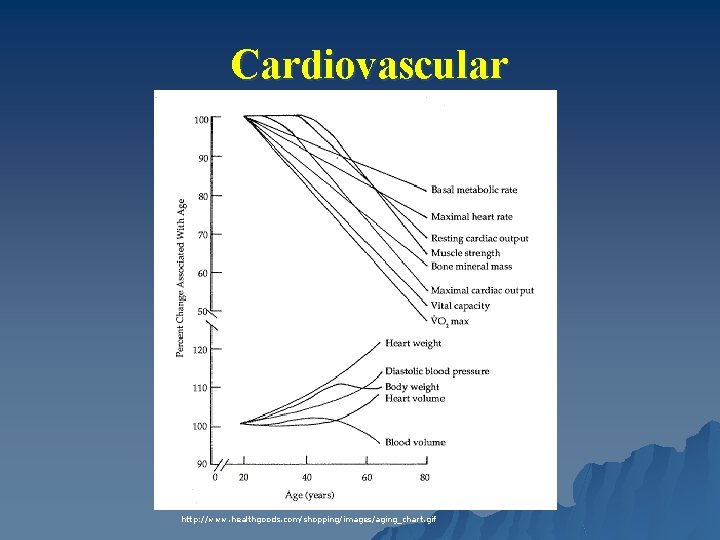
Cardiovascular http: //www. healthgoods. com/shopping/images/aging_chart. gif

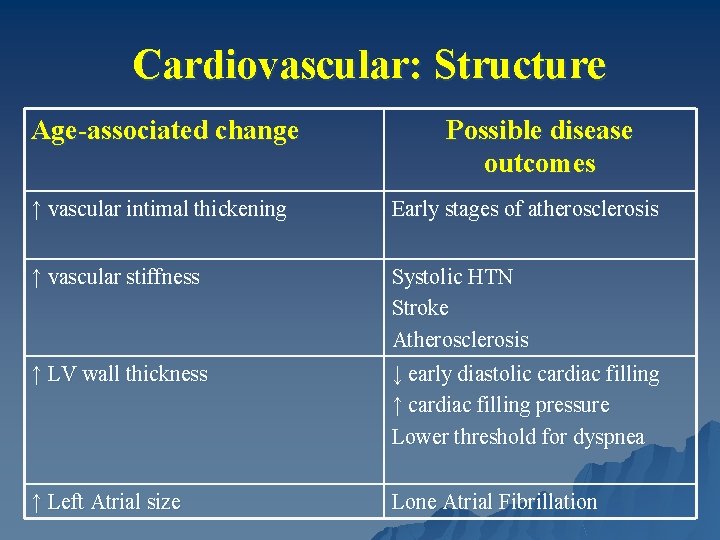
Cardiovascular: Structure Age-associated change Possible disease outcomes ↑ vascular intimal thickening Early stages of atherosclerosis ↑ vascular stiffness Systolic HTN Stroke Atherosclerosis ↓ early diastolic cardiac filling ↑ cardiac filling pressure Lower threshold for dyspnea ↑ LV wall thickness ↑ Left Atrial size Lone Atrial Fibrillation

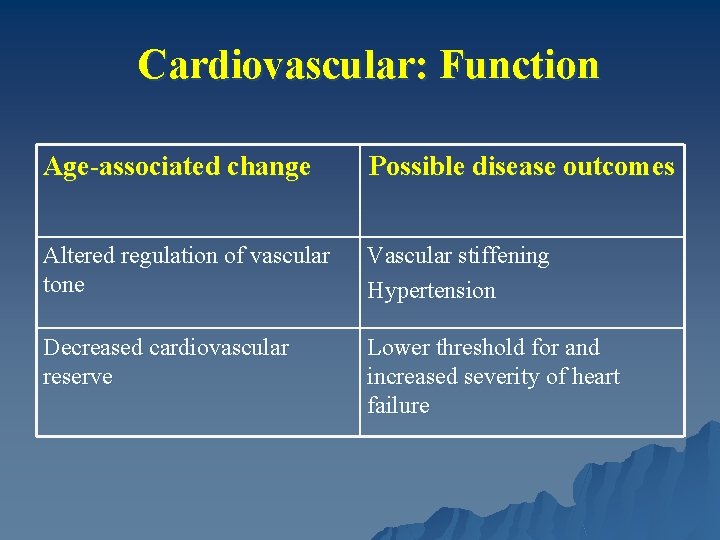
Cardiovascular: Function Age-associated change Possible disease outcomes Altered regulation of vascular tone Vascular stiffening Hypertension Decreased cardiovascular reserve Lower threshold for and increased severity of heart failure

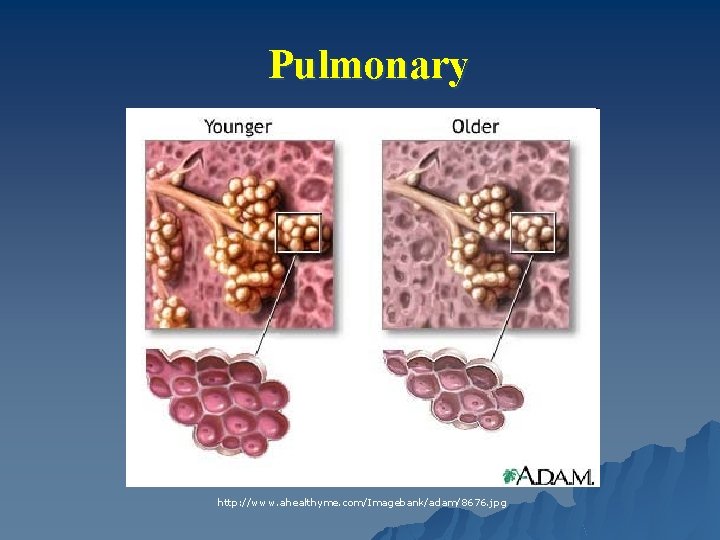
Pulmonary http: //www. ahealthyme. com/Imagebank/adam/8676. jpg

Pulmonary - Structure ↓ Alveoli and lung capillaries u ↓ Number and elasticity of parenchymal elastic fibers → Gradual loss of elastic recoil of the lungs u Airway size ↓ u Ciliary action less effective u Chest wall stiffens u Respiratory muscles weaken u ↓ lung mass u Diaphragm weakens by 25% u

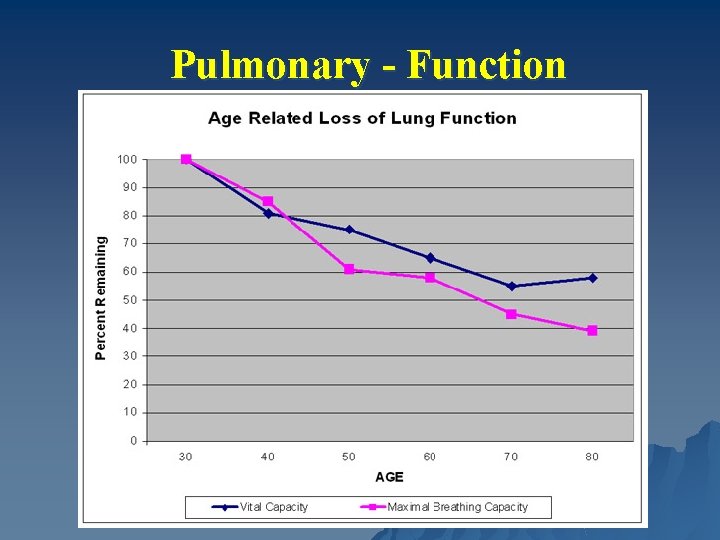
Pulmonary - Function

Pulmonary – Function u ↓ FEV 1 and FVC u ↑ Residual volume u Ventilation-Perfusion mismatching causes ↓ Pa. O 2 [100 – (0. 32 * age)] u Maximum inspiratory and expiratory pressures ↓ u Diffusion of CO decreased u Decreased ventilatory response to hypercapnia

Question u An 85 -year-old man has had increasingly severe shortness of breath on exertion over the past 3 months. For the past 20 years, he has walked 30 minutes three times weekly at a fairly rapid pace without symptoms. He has no chest pain, wheezing, or cough. Blood pressure is 140/85 mm Hg. On examination, the lungs are clear and there is no evidence of wheezing. Radiographs of the chest and an electrocardiogram show normal findings. Which of the following additional findings would require further evaluation? u (A) Arterial PO 2 of 80 mm Hg (B) Decreased cardiac output on ultrasonography (C) Decreased maximum heart rate on stress testing (D) Decreased vital capacity on pulmonary function testing (E) Presence of an S 4 gallop u u

u Cardiac output both at rest and with moderate exercise does not change with age. A finding of ↓ cardiac output in this patient would suggest an underlying disease. u Arterial PO 2 ↓ with age because of an age-related increase in ventilation-perfusion mismatch. The age-expected normal Pa O 2 is determined by calculating 100 minus (0. 325 × patient age). u Vital capacity ↓ with age. Total lung capacity does not change with age. Residual volume ↑ substantially in older persons. u An S 4 gallop may occur without underlying disease in persons older than 80 years. ↓ early ventricular filling velocities are caused by aged, less compliant ventricles.

Renal

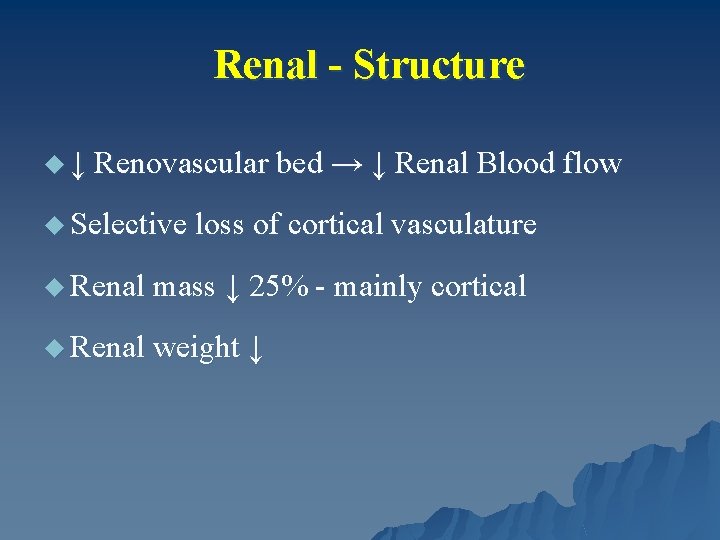
Renal - Structure u ↓ Renovascular bed → ↓ Renal Blood flow u Selective loss of cortical vasculature u Renal mass ↓ 25% - mainly cortical u Renal weight ↓

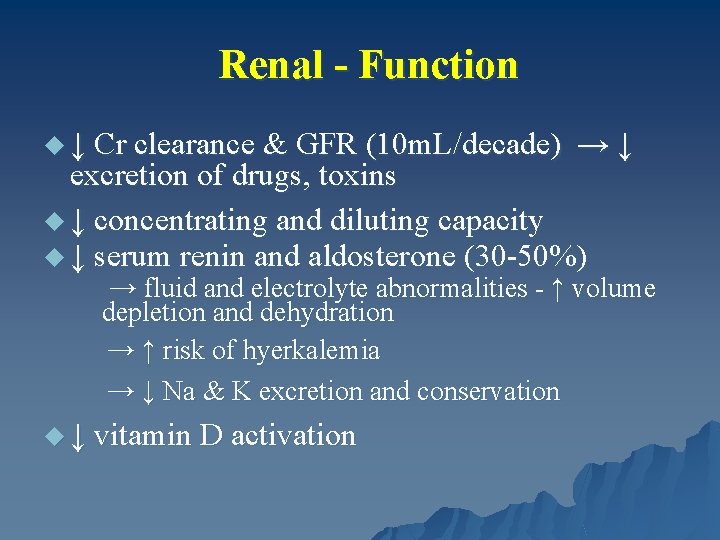
Renal - Function u ↓ Cr clearance & GFR (10 m. L/decade) → ↓ excretion of drugs, toxins u ↓ concentrating and diluting capacity u ↓ serum renin and aldosterone (30 -50%) → fluid and electrolyte abnormalities - ↑ volume depletion and dehydration → ↑ risk of hyerkalemia → ↓ Na & K excretion and conservation u ↓ vitamin D activation

GI

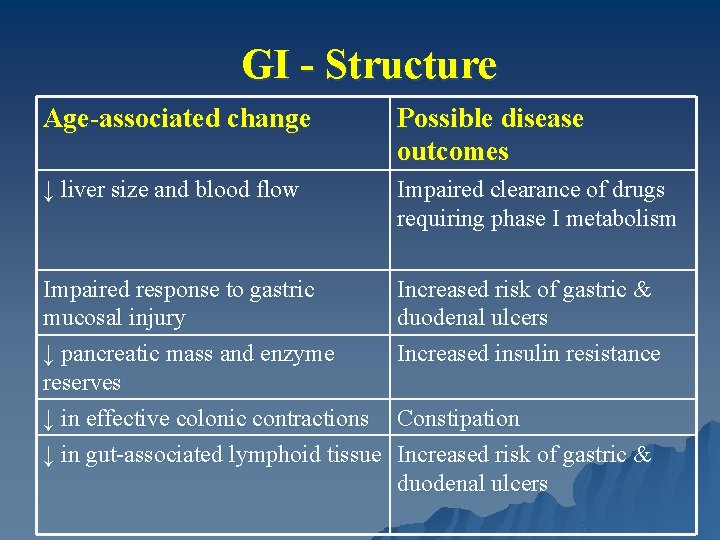
GI - Structure Age-associated change Possible disease outcomes ↓ liver size and blood flow Impaired clearance of drugs requiring phase I metabolism Impaired response to gastric mucosal injury ↓ pancreatic mass and enzyme reserves ↓ in effective colonic contractions ↓ in gut-associated lymphoid tissue Increased risk of gastric & duodenal ulcers Increased insulin resistance Constipation Increased risk of gastric & duodenal ulcers

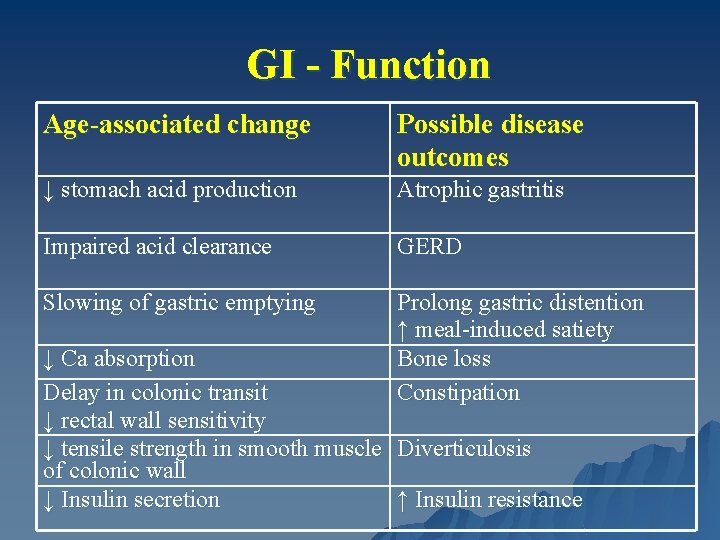
GI - Function Age-associated change Possible disease outcomes ↓ stomach acid production Atrophic gastritis Impaired acid clearance GERD Slowing of gastric emptying Prolong gastric distention ↑ meal-induced satiety Bone loss Constipation ↓ Ca absorption Delay in colonic transit ↓ rectal wall sensitivity ↓ tensile strength in smooth muscle Diverticulosis of colonic wall ↓ Insulin secretion ↑ Insulin resistance

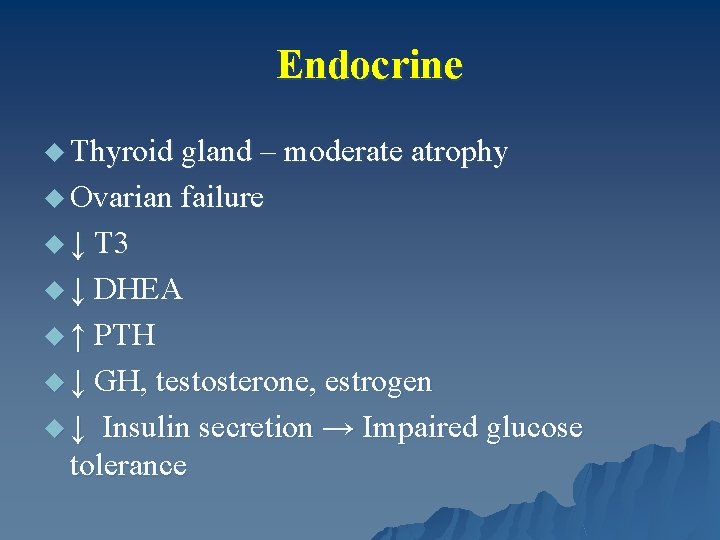
Endocrine u Thyroid gland – moderate atrophy u Ovarian failure u ↓ T 3 u ↓ DHEA u ↑ PTH u ↓ GH, testosterone, estrogen u ↓ Insulin secretion → Impaired glucose tolerance

Hematology u % of marrow space occupied by hematopoietic tissue declines u ↓ stem cells in marrow u Slowed erythropoiesis ↓ incorporation of iron into RBC u Average values of Hb and hematocrit ↓ slightly

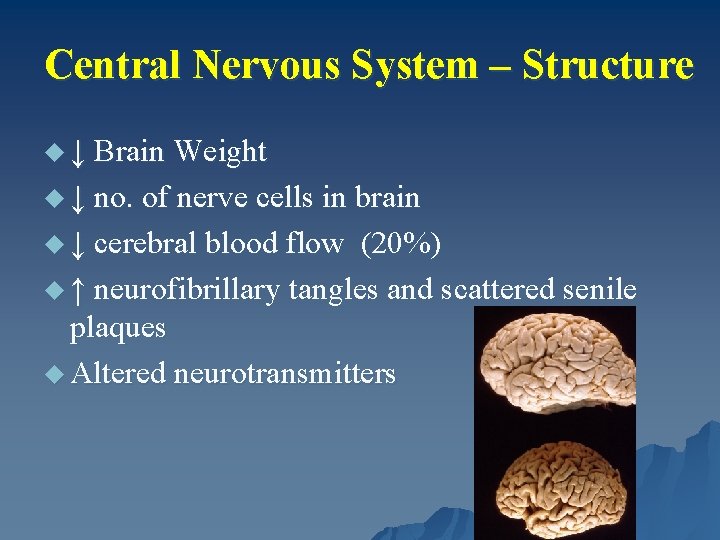
Central Nervous System – Structure u ↓ Brain Weight u ↓ no. of nerve cells in brain u ↓ cerebral blood flow (20%) u ↑ neurofibrillary tangles and scattered senile plaques u Altered neurotransmitters

Central Nervous System - Function Intellect u Maintained until at least age 80 u Slowing in central processing → Tasks take longer to perform Verbal skills u Maintained until age 70 u Gradually ↓ in vocabulary, ↑ semantic errors and abnormal prosody Mentation u Difficulty learning, especially languages and forgetfulness in non-critical areas – doesn’t impair recall of important memories or affect function

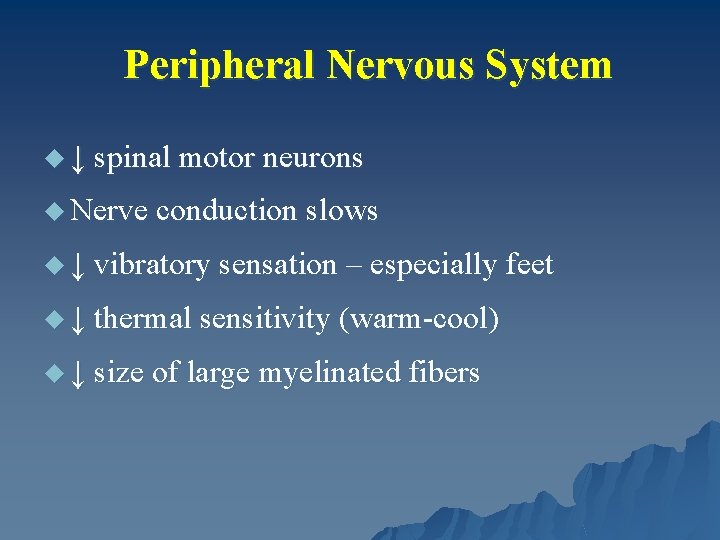
Peripheral Nervous System u ↓ spinal motor neurons u Nerve conduction slows u ↓ vibratory sensation – especially feet u ↓ thermal sensitivity (warm-cool) u ↓ size of large myelinated fibers

Musculoskeletal - Muscle u ↓ muscle fibers → ↓ muscle mass (sarcopenia) → lean body mass u Infiltration of fat into muscle bundles u ↑ Fatigability u ↓ Basal metabolic rate (4%/decade after age 50)

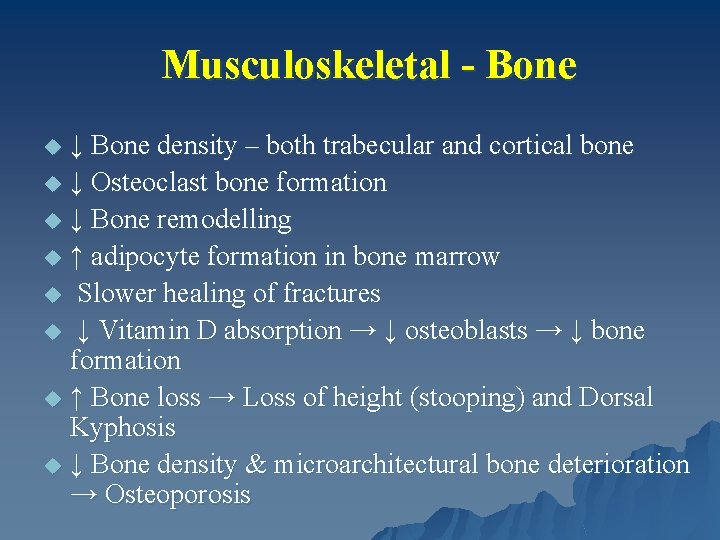
Musculoskeletal - Bone ↓ Bone density – both trabecular and cortical bone u ↓ Osteoclast bone formation u ↓ Bone remodelling u ↑ adipocyte formation in bone marrow u Slower healing of fractures u ↓ Vitamin D absorption → ↓ osteoblasts → ↓ bone formation u ↑ Bone loss → Loss of height (stooping) and Dorsal Kyphosis u ↓ Bone density & microarchitectural bone deterioration → Osteoporosis u

Musculoskeletal - Joints Non-articular cartilage grows throughout life Articular cartilage does change u ↓ thickness of cartilage u ↓ chondrocytes u Collagen becomes stiffer → Disordered cartilage matrix u As a result, less able to handle mechanical stress

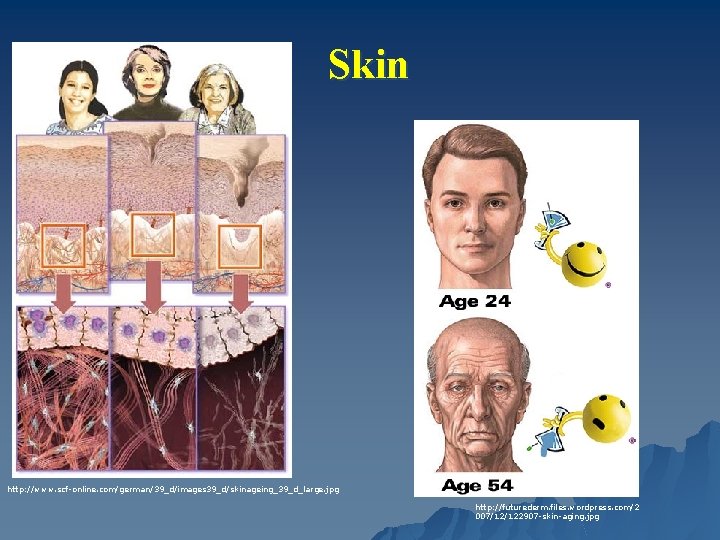
Skin http: //www. scf-online. com/german/39_d/images 39_d/skinageing_39_d_large. jpg http: //futurederm. files. wordpress. com/2 007/12/122907 -skin-aging. jpg

Skin - Structure Epidermal changes Melanocytes u ↓ 15%/decade u Density doubles on sun-exposed skin u ↑ lentigines u u Langerhans cells ↓ density ↓ responsiveness Dermal Changes u ↓ collagen – 1% annual decline, altered fibers u ↓ density u Progressive loss of elastic tissue in the papillary dermis

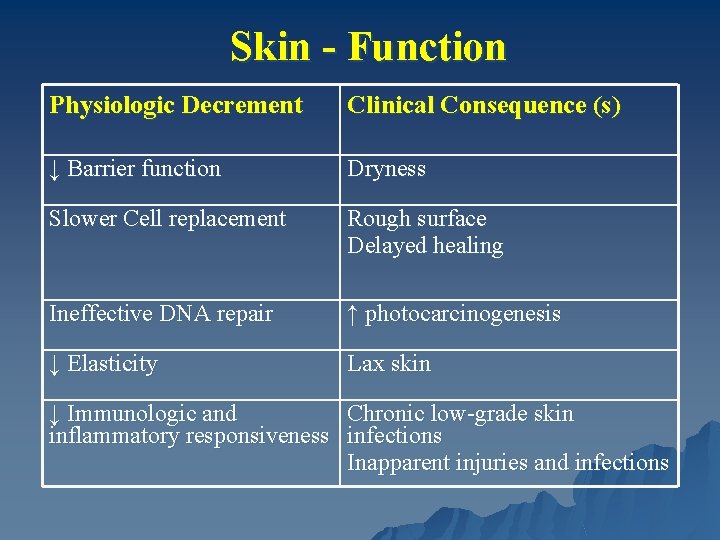
Skin - Function Physiologic Decrement Clinical Consequence (s) ↓ Barrier function Dryness Slower Cell replacement Rough surface Delayed healing Ineffective DNA repair ↑ photocarcinogenesis ↓ Elasticity Lax skin ↓ Immunologic and Chronic low-grade skin inflammatory responsiveness infections Inapparent injuries and infections

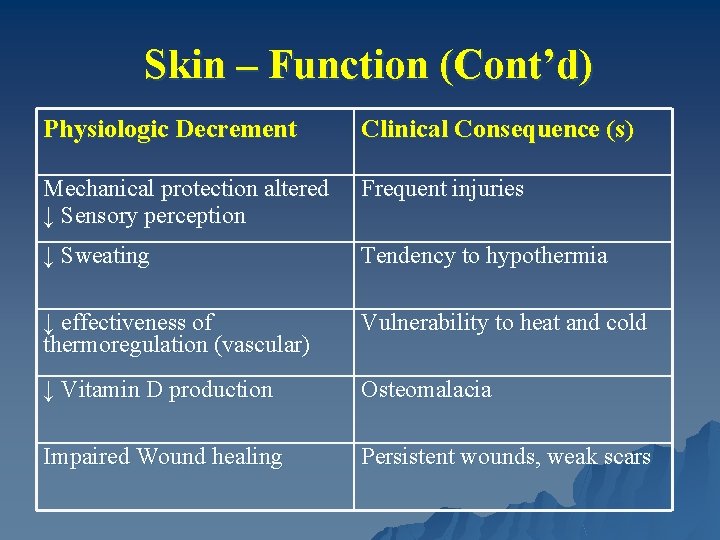
Skin – Function (Cont’d) Physiologic Decrement Clinical Consequence (s) Mechanical protection altered ↓ Sensory perception Frequent injuries ↓ Sweating Tendency to hypothermia ↓ effectiveness of thermoregulation (vascular) Vulnerability to heat and cold ↓ Vitamin D production Osteomalacia Impaired Wound healing Persistent wounds, weak scars

Immune system u u u u ↓ Cell-Mediated immunity Lower affinity antibody production ↓ delayed-type hypersensitivity ↑ Interferon-gamma, TGF-beta, TNF, IL-6, IL-1 production causing impaired macrophage function ↑ circulating IL-6 ↓ IL-2 release and IL-2 responsiveness ↓ production of B cells by bone marrow Resulting in ↓ immunity contributing to ↓ susceptibility to infections and malignancy

Sensory - Vision u Yellowing of lens u Impaired dark adaptation u Inability to focus on near items (Presbyopia) u ↓ Contrast sensitivity u ↓ Lacrimation → Dry eyes

Sensory – Smell and Thirst Smell u Detection ↓ by 50% Thirst u ↓ thirst drive u Impaired control of thirst by endorphins

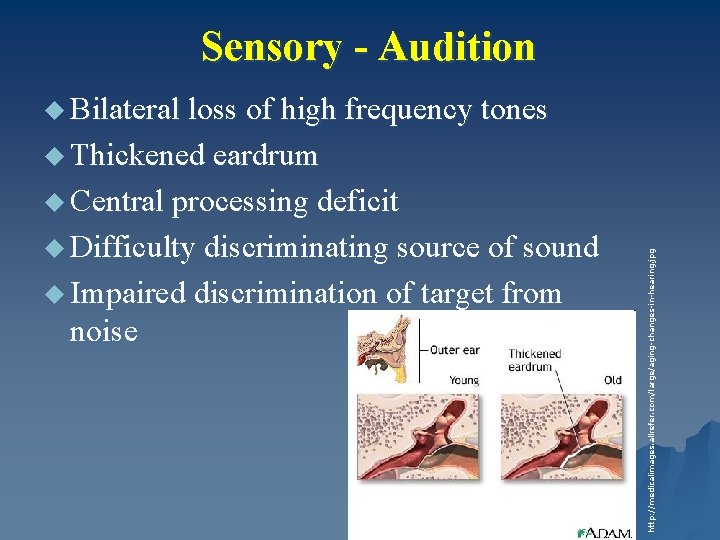
Sensory - Audition u Bilateral loss of high frequency tones u Thickened eardrum u Difficulty discriminating source of sound u Impaired discrimination of target from noise http: //medicalimages. allrefer. com/large/aging-changes-in-hearing. jpg u Central processing deficit

Question A healthy 80 -year-old woman presents for her annual physical examination. She has mild osteoarthritis of her knees, but she is fully functional and plays golf weekly. She takes no medications. A routine chemistry panel is ordered. Which of the following laboratory findings should lead to further work-up? u u u (A) Erythrocyte sedimentation rate: 45 mm/h (B) Alkaline phosphatase: 130 U/L (C) Total cholesterol: 210 mg/d. L (D) 1 -hour postprandial glucose: 160 mg/d. L (E) Magnesium: 1. 4 mg/d. L

u Mg - 1. 4 mg/d. L represents a ↓ beyond the amount expected with normal aging and hence should prompt further work-up. u Serum Mg ↓ by about 15% between the 20 s and 70 s. . u All the other laboratory values listed for this patient ↑ normally with age. – ESR tends to ↑ about 10 to 13 mm/h between the ages of 20 and 80 years and is associated with a similar ↑ in C-reactive protein. – The alkaline phosphatase level ↑ by 20% between the 20 s and 70 s, a change that is unassociated with clinical problems. – The total cholesterol level ↑ with age, with a more important ↓ in high-density lipoprotein cholesterol in women as they lose estrogen protection. – The postprandial glucose level ↑ with age, a change also reflected in ↑ levels of hemoglobin A 1 c.

References u Merck Manual of Geriatrics Beers, Mark (2000) u Merck Manual of Health and Aging (2004) u GRS 6 th edition – American Geriatrics Society u Geriatric Medicine Cassel, Leipzig, Cohen, Larson, Meier (4 th edition – 2003)


Questions? ? ?
 Dr nisha rughwani
Dr nisha rughwani Edwin walker acl
Edwin walker acl Promotion from assistant to associate professor
Promotion from assistant to associate professor Fok ping kwan
Fok ping kwan Name of technique
Name of technique Nisha patel md
Nisha patel md Dr nisha patel
Dr nisha patel Nisha singh md
Nisha singh md Nisha nagarajan
Nisha nagarajan Nisha patel md
Nisha patel md Frandec company manufactures
Frandec company manufactures Nisha patel md
Nisha patel md Integumentary system effects of aging
Integumentary system effects of aging Aging better together conference
Aging better together conference Subeom
Subeom Symbolic interaction theory of aging
Symbolic interaction theory of aging Biological theory of aging
Biological theory of aging Garontology
Garontology Pr��ca it projektov�� mana����r
Pr��ca it projektov�� mana����r Middle age
Middle age Dane county aging and disability resource center
Dane county aging and disability resource center Types of makeup in theatre
Types of makeup in theatre Conclusion of aging
Conclusion of aging Csi office on aging
Csi office on aging Sherwin williams murray ky
Sherwin williams murray ky Memory aging and brain maintenance
Memory aging and brain maintenance Environmental press model
Environmental press model Aging aircraft solutions
Aging aircraft solutions Aging algorithm page replacement
Aging algorithm page replacement Idaho commission on aging
Idaho commission on aging George carlin old
George carlin old Belady's anomaly example
Belady's anomaly example George carlin age
George carlin age Aging in thailand
Aging in thailand Wisconsin institute for healthy aging
Wisconsin institute for healthy aging Jessie emily schofield lyrics
Jessie emily schofield lyrics How are aging schedules used?
How are aging schedules used? Dr eric berg quackwatch
Dr eric berg quackwatch Lion aging
Lion aging Managing the aging workforce challenges and solutions
Managing the aging workforce challenges and solutions Aging
Aging Disengagement theory of aging examples
Disengagement theory of aging examples Markets
Markets Non stochastic theory of aging
Non stochastic theory of aging Idaho commission on aging
Idaho commission on aging Contoh aging schedule piutang
Contoh aging schedule piutang Office of aging and adults services
Office of aging and adults services Ricoh uky
Ricoh uky Which aging empires suffered from nationalism
Which aging empires suffered from nationalism