Physics Edexcel International GCSE in Physics 4 PH


















































































- Slides: 110

Physics Edexcel International GCSE in Physics (4 PH 0) First examination June 2013 1

Forces and motion – – Units Movement and position Forces, movement, shape and momentum Astronomy 2

Units 1. 1 use the following units: kilogram (kg), meter (m), meter/second (m/s), meter/second 2 (m/s 2), newton (N), second (s), newton per kilogram (N/kg), kilogram meter/second (kg m/s) – Kilogram (kg) is for mass – Meter (m) is for distance – Meter / second (m/s) is for speed or velocity – Newton (N) is force – Second (s) is for time – Newton per kilogram (N/kg) is for acceleration (same as m/s 2) – Kilogram meter / second (kg m/s) is for momentum 3

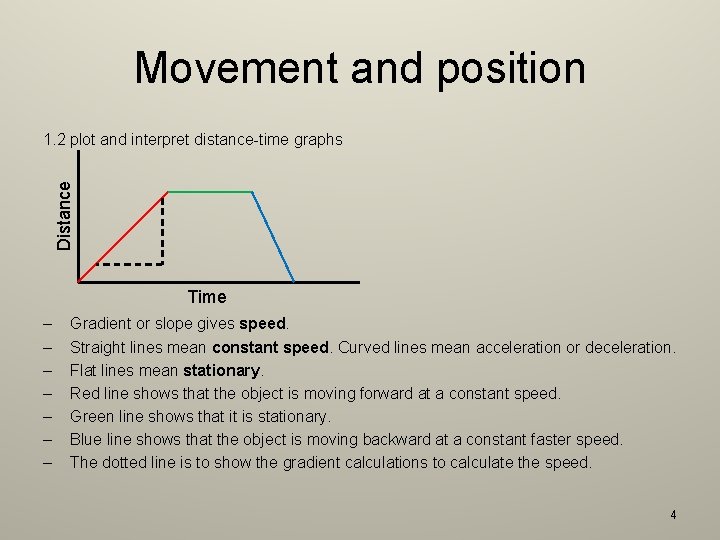
Movement and position Distance 1. 2 plot and interpret distance-time graphs Time – – – – Gradient or slope gives speed. Straight lines mean constant speed. Curved lines mean acceleration or deceleration. Flat lines mean stationary. Red line shows that the object is moving forward at a constant speed. Green line shows that it is stationary. Blue line shows that the object is moving backward at a constant faster speed. The dotted line is to show the gradient calculations to calculate the speed. 4

Movement and position 1. 3 know and use the relationship between average speed, distance moved and time: – Average speed = distance moved / time taken 1. 4 describe experiments to investigate the motion of everyday objects such as toy cars or tennis balls – Experimenting with rolling tennis balls and toy cars, measuring the distance and time taken and working out the average speed. – e. g. A toy car is pushed, it moves 4 meters in 2 seconds. – Distance moved: 4 m – Time taken: 2 s – Calculation: Speed = 4 / 2 – Speed = 2 m/s 5

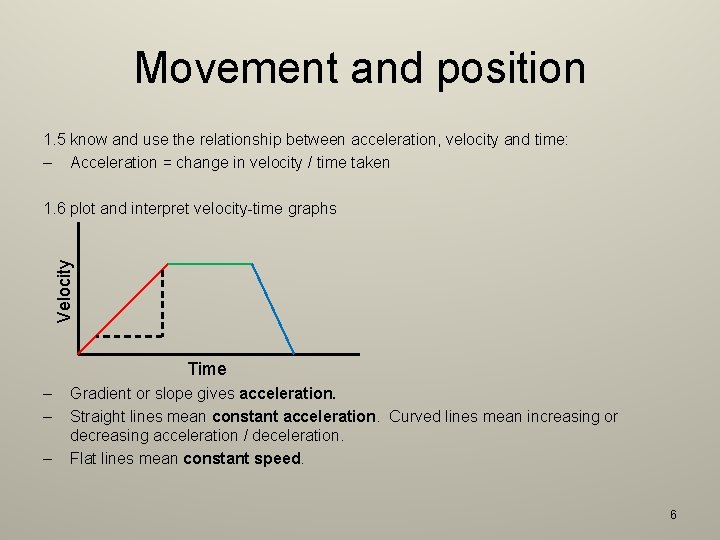
Movement and position 1. 5 know and use the relationship between acceleration, velocity and time: – Acceleration = change in velocity / time taken Velocity 1. 6 plot and interpret velocity-time graphs Time – – – Gradient or slope gives acceleration. Straight lines mean constant acceleration. Curved lines mean increasing or decreasing acceleration / deceleration. Flat lines mean constant speed. 6

Movement and position – – – Red line shows that the object is accelerating constantly. Green line shows that the object is at a constant speed. Blue line shows that the object is decelerating constantly. 1. 7 determine acceleration from the gradient of a velocity-time graph – Acceleration is determined from the gradient or slope of the graph. – The dotted line shows the gradient calculations to calculate acceleration. 1. 8 determine the distance travelled from the area between a velocity-time graph and the time axis. – The area between the line and the axis shows the distance travelled during the motion. 7

Forces, movement, shape and momentum 1. 9 describe the effects of forces between bodies such as changes in speed, shape or direction – When an object is stationary or moving at a constant speed, the forces are balanced. – In the case of the car, the forward and backward forces are equal when the car is at a constant speed. – When the forward forces are greater than the backward forces, the car accelerates. – When the backward forces are greater than the forward forces, the car decelerates. Or, it will change the direction in which the car is going eventually. 8

Forces, movement, shape and momentum 1. 10 identify different types of force such as gravitational or electrostatic – Weight: the force that acts on a body because of gravity – Friction: the force that opposes motion – Air resistance: friction between an object and the air – Viscous drag: similar to air resistance, but between an object and a liquid – Upthrust: the upward force that liquids and gases exert on objects – Magnetic: the forces that magnets exert on other magnets or ferrous materials – Electrostatic: the force between electrically charged objects – Normal reaction: the name for the contact force that acts on an object pressing down on another. – Tension: in strings cable, ropes that are being stretched. 9

Forces, movement, shape and momentum 1. 11 distinguish between vector and scalar quantities – Scalar quantities have only one measurement: – Speed – Distance – Temperature – Vector quantities have a direction: – Displacement (distance in a specified direction) – Velocity (speed in a specified direction) 1. 12 understand that force is a vector quantity – Force has a direction 10

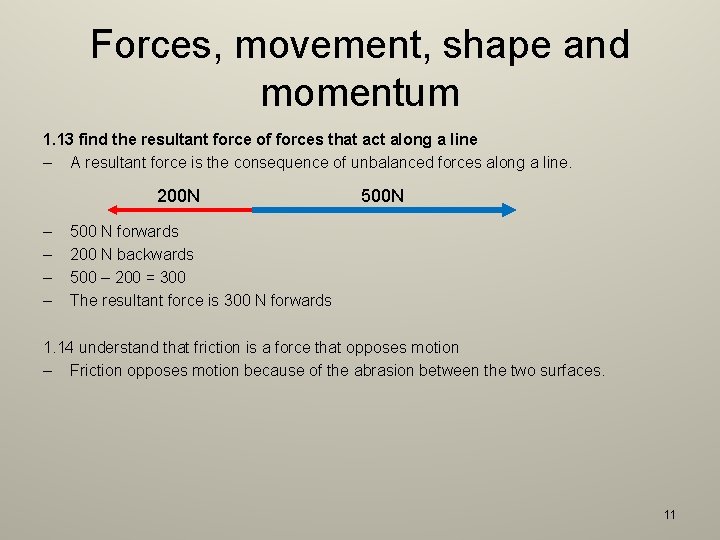
Forces, movement, shape and momentum 1. 13 find the resultant force of forces that act along a line – A resultant force is the consequence of unbalanced forces along a line. 200 N – – 500 N 500 N forwards 200 N backwards 500 – 200 = 300 The resultant force is 300 N forwards 1. 14 understand that friction is a force that opposes motion – Friction opposes motion because of the abrasion between the two surfaces. 11

Forces, movement, shape and momentum 1. 15 know and use the relationship between unbalanced force, mass and acceleration: – Force = mass X acceleration 1. 16 know and use the relationship between weight, mass and g: – Weight = mass X gravitational field strength (g) 1. 17 describe the forces acting on falling objects and explain why falling objects reach a terminal velocity – As the velocity downwards increases, the air resistance increases. This occurs until the air resistance force is equal to the weight force. When the two forces are balanced, the object reaches a constant speed and this is called terminal velocity. 12

Forces, movement, shape and momentum 1. 18 describe experiments to investigate the forces acting on falling objects, such as sycamore seeds or parachutes – When a man jumps off a plane, his weight causes him to accelerate downwards, this continues until the air resistance equals the force of his weight and he reaches a terminal velocity. – When he opens his parachute, he decelerates drastically because the air resistance force is greater than his weight. As he slows down, the air resistance decreases and gradually another terminal velocity is reached. 1. 19 describe the factors affecting vehicle stopping distance including speed, mass, road condition and reaction time – Thinking distance: intoxication, poor visibility, tiredness – Braking distance: poor tires, road surface 13

Forces, movement, shape and momentum 1. 20 know and use the relationship between momentum, mass and velocity: – Momentum = mass X velocity 1. 21 use the idea of momentum to explain safety features – Newton’s second law is force = change in momentum / time taken – The change in momentum is fixed by the speed the car is traveling at and the mass of the car and contents. – Therefore if the equation is rearranged as force X time = change in momentum, force and time is inversely proportional. – It is vital to increase time for the car to come to rest to minimalize the force. Therefore, crumple zones and airbags are used to increase the time. 14

Forces, movement, shape and momentum 1. 22 use the conservation of momentum to calculate the mass, velocity or momentum of objects – Total momentum of the two bodies before collision = total momentum of the two bodies after collision – Elastic collision is which no energy is lost (no energy being converted into heat or sound) – Inelastic collision is when the colliding objects do not rebound at all – – A small pellet of mass 0. 01 kg travelling at 30 m/s towards a stationary wheeled block with a mass of 0. 19 kg. Momentum before collision = 0. 01 kg X 30 m/s = 0. 3 kg m/s Momentum after the collision = (0. 19 kg + 0. 01 kg) X v = 0. 2 X v = 0. 3 kg m/s v is 1. 5 m/s 15

Forces, movement, shape and momentum 1. 24 demonstrate an understanding of Newton’s third law – Newton’s third law is for every action there is a equal and opposite reaction. 1. 25 know and use the relationship between the moment of a force and its distance from the pivot: – Moment = force × perpendicular distance from the pivot 1. 26 recall that the weight of a body acts through its center of gravity – The whole weight of an object acts through one point of the object called its center of gravity. – e. g. If a shape is suspended from a point, so that it can turn, it will come to rest with the center of gravity of the object immediately below the point from which it is suspended. 16

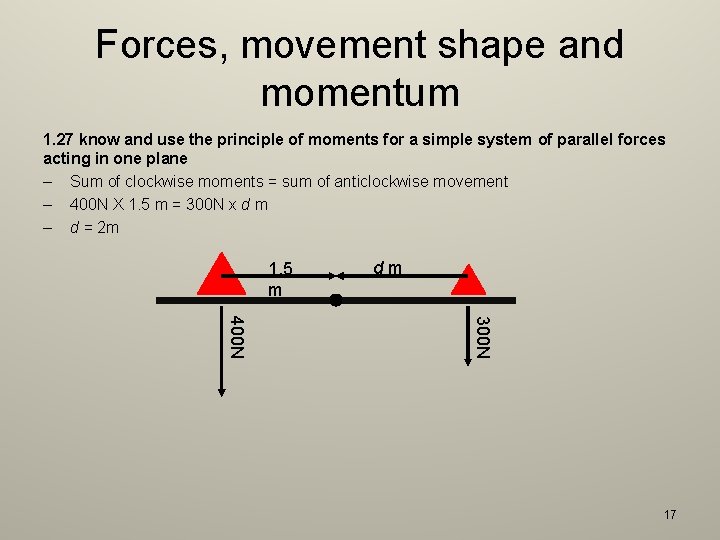
Forces, movement shape and momentum 1. 27 know and use the principle of moments for a simple system of parallel forces acting in one plane – Sum of clockwise moments = sum of anticlockwise movement – 400 N X 1. 5 m = 300 N x d m – d = 2 m 1. 5 m dm 300 N 400 N 17

Forces, movement, shape and momentum 1. 28 understand that the upward forces on a light beam, supported at its ends, vary with the position of a heavy object placed on the beam – When the object is placed in the middle of the beam, the upward forces of the support beams are equal. However, when the object is placed closer to one side, the closer support beam will have a higher upward force, and the further one will have a lower upward force. 1. 29 describe experiments to investigate how extension varies with applied force for helical springs, metal wires and rubber bands – Hanging known masses under the spring or rubber band measuring the length. – The extension of a spring is directly proportional to the force pulling on it until it reaches a elastic limit. – After it is extended beyond the elastic limit, it does not return to its original length. – A rubber band returns to its original length when the stretching force is removed, provided it is not stretched beyond the breaking point. 18

Forces, movement, shape and momentum 1. 30 understand that the initial linear region of a force-extension graph is associated with Hooke’s law – The initial linear region of a force-extension graph follows Hooke’s law. The increase in length of a spring is directly proportional to the force pulling on it. 1. 31 describe elastic behavior as the ability of a material to recover its original shape after the forces causing deformation have been removed. – It returns to its original form, unless it has been stretched beyond its elastic point. – In this case, it returns to a lengthened form. 19

Astronomy 1. 32 understand gravitational field strength, g, and recall that it is different on other planets and the moon from that on the Earth – The strength of the gravity depends on: – The size of the masses involved. – The distance between the masses. 1. 33 explain that gravitational force: – causes moons to orbit planets – causes the planets to orbit the sun – causes artificial satellites to orbit the Earth – causes comets to orbit the sun – Gravity is the force that keeps the moon in orbit around the earth and the moon of other planets in our solar system in their orbits. 20

Astronomy 1. 34 describe the differences in the orbits of comets, moons and planets – Planets orbit the sun in a spherical path. – Comets orbit the sun in an elliptical path. – Moons orbit the planet in an elliptical path. 1. 35 use the relationship between orbital speed, orbital radius and time period: – Orbital speed = 2 X pi X orbital radius / time period 1. 36 understand that: – the universe is a large collection of billions of galaxies – a galaxy is a large collection of billions of stars – our solar system is in the Milky Way galaxy. 21

Electricity – – Units Mains electricity Energy and potential difference in circuits Electric charge 22

Units 2. 1 use the following units: ampere (A), coulomb (C), joule (J), ohm (Ω), second (s), volt (V), watt (W) – Ampere (A) is for current – Coulomb (C) is for charge – Joule (J) is for energy – Ohm (Ω) is for resistance – Second (s) is for time – Volt (V) is for voltage – Watt (W) is for power 23

Mains electricity 2. 2 understand identify the hazards of electricity including frayed cables, long cables, damaged plugs, water around sockets, and pushing metal objects into sockets – Frayed cables can expose live wires. – Long cables are more likely to get damaged or trip people up. – Damaged plugs or insulating casing may expose live wires. – Water around electric sockets. – Pushing metal objects into mains sockets. 2. 3 understand the uses of insulation, double insulation, earthing, fuses and circuit breakers in a range of domestic appliances – Insulation: All mains wiring is double insulated with two layers of insulation. This prevents separate conductors (live, neutral and earth) from touching. – Double Insulated: As well as the wiring being insulated, the outer casing of the appliance is also made of an insulating material. – Earthing: Provides a low resistance path for the current in the event of a fault, it ensure that the outer casing is held at 0 V. 24

Mains electricity – – Fuses are fitted into plugs, they contain a wire designed to melt when a specified current size is exceeded, cutting off the live wire. Circuit breakers are now more commonly used to replace fuses as they are magnetically operated and they can be reset by pressing a button. 2. 5 know and use the relationship: power = current × voltage, and apply the relationship to the selection of appropriate fuses – Power = current X voltage – e. g. An electric drill with a power rating of 750 W is designed to run from the 230 V mains supply. – 750 W / 230 V = 3. 26 A – The threshold of the fuse has to be larger than 3. 26 A. 2. 6 use the relationship between energy transferred, current, voltage and time: – Energy transferred = current X voltage X time 25

Mains electricity 2. 7 understand the difference between mains electricity being alternating current (a. c. ) and direct current (D. C. ) being supplied by a cell or battery. – Direct current is when the electricity flows in one direction only. – Alternating current is when the current changes continuously, with electricity flowing one direction the other. 26

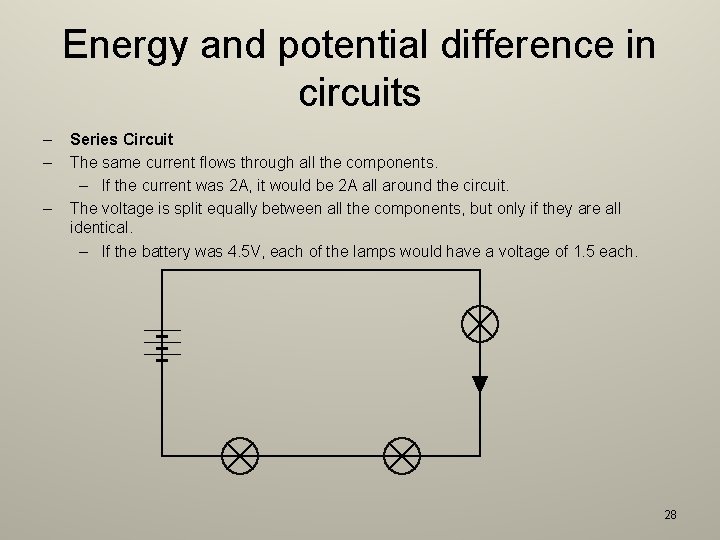
Energy and potential difference in circuits 2. 8 explain why a series or parallel circuit is more appropriate for particular applications, including domestic lighting – Parallel circuits are appropriate for domestic lighting because if one outlet is disconnected, the others will continue working. – Series circuits are appropriate for decorative lighting because the voltage in each lamp is lower. However, the lights all go out if one bulb fails. 27

Energy and potential difference in circuits – – – Series Circuit The same current flows through all the components. – If the current was 2 A, it would be 2 A all around the circuit. The voltage is split equally between all the components, but only if they are all identical. – If the battery was 4. 5 V, each of the lamps would have a voltage of 1. 5 each. 28

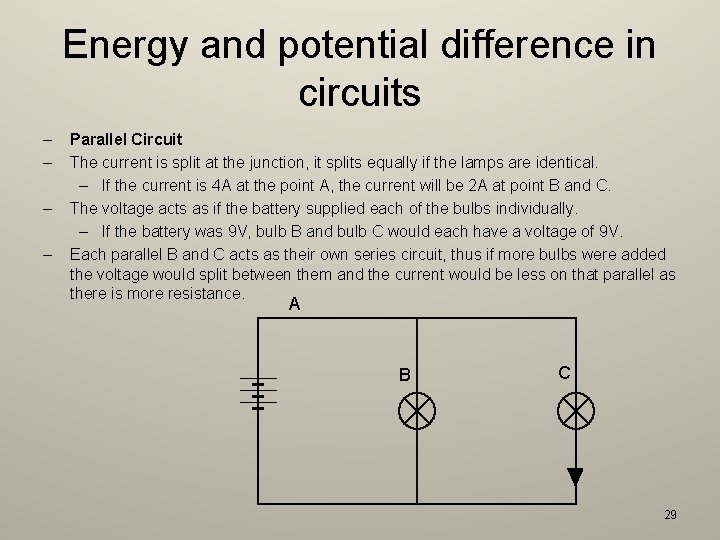
Energy and potential difference in circuits – – Parallel Circuit The current is split at the junction, it splits equally if the lamps are identical. – If the current is 4 A at the point A, the current will be 2 A at point B and C. The voltage acts as if the battery supplied each of the bulbs individually. – If the battery was 9 V, bulb B and bulb C would each have a voltage of 9 V. Each parallel B and C acts as their own series circuit, thus if more bulbs were added the voltage would split between them and the current would be less on that parallel as there is more resistance. A B C 29

Energy and potential difference in circuits 2. 9 understand that the current in a series circuit depends on the applied voltage and the number and nature of other components – If the number of components is constant, an increase in voltage would induce an increase in current. – If the voltage is constant, an increase in the number of components would induce a decrease in current. – This is because each component has its own resistance. The more resistance there is, the lower the current. – Ohm’s law is that current is proportional to voltage if the resistance is constant. 30

Energy and potential difference in circuits 2. 10 describe how current varies with voltage in wires, resistors, metal filament lamps and diodes, and how this can be investigated experimentally – This variable can be investigated experimentally by using a variable resistor, ammeter, and voltmeter connected like this. – The variable resistor is so that the voltage can be changed. – Remember that the voltmeter is connected in parallel. – Resistors and wires obey Ohm’s law, which means the resistance is constant and the voltage and current is proportional. This is a straight line on the current-voltage graph. – Filament lamps get very hot and the resistance of a metal increases with temperature. The resistance increases as the voltage increases. This is a straight line then plateauing line on the current-voltage graph. – Diodes have a very large resistance when the voltage is applied in the wrong direction. This is shown by the horizontal line when the voltage is negative (showing the wrong direction). 31

Energy and potential difference in circuits 2. 11 describe the qualitative effect of changing resistance on the current in a circuit – Increasing the resistance would lower the current. – Decreasing the resistance would increase the current. 2. 12 describe the qualitative variation of resistance of LDRs with illumination and of thermistors with temperature – Light Dependent Resistor: – More light Less resistance – Less light More resistance – Thermistor: – More heat Less resistance – Less heat More resistance 32

Energy and potential difference in circuits 2. 13 know that lamps and LEDs can be used to indicate the presence of a current in a circuit – When a lamp is on, it shows that the flow of electricity is present. 2. 14 know and use the relationship between voltage, current and resistance: – Voltage = current × resistance 2. 15 understand that current is the rate of flow of charge – Current is how fast the electricity flows. 2. 16 know and use the relationship between charge, current and time: – Charge = current X time 33

Energy and potential difference in circuits 2. 17 know that electric current in solid metallic conductors is a flow of negatively charged electrons – The electrons are negatively charged therefore they are repelled from the negative terminal and attracted towards the positive terminal. Thus they flow from negative positive. This is the electron flow. – The conventional current is from positive negative. 2. 18 understand that: – voltage is the energy transferred per unit charge passed – the volt is a joule per coulomb. – The equation linking energy transferred, current, voltage and time is energy transferred = current X voltage X time. – Current X time = charge – Therefore, energy transferred = charge X voltage (J = CV) – Therefore, V = J / C 34

Electrical charge 2. 19 identify common materials which are electrical conductors or insulators, including metals and plastics – Materials that conduct electricity are conductors. – Usually metallic: copper, silver, gold. – Materials that cannot conduct electricity are insulators – Usually non-metallic: rubber, glass, plastics 2. 20 describe experiments to investigate how insulating materials can be charged by friction – When you rub two different insulators together they become electrically charged. – This is because electrons in one of the insulators is ripped off its surface, and thus becoming positively charged. The other insulator gains the electrons, and thus becoming negatively charged. – Charged insulators can attract small uncharged objects such as pieces of paper. – Polythene and clear acetate rods can be charged on a dry day by rubbing them with a dry cloth. 35

Electrical charge 2. 21 explain that positive and negative electrostatic charges are produced on materials by the loss and gain of electrons – Electrons have a negative charge. – The gain of electrons would induce a negative electrostatic charge. – The loss of electrons would induce a positive electrostatic charge. 2. 22 understand that there are forces of attraction between unlike charges and forces of repulsion between like charges – Positive and negative electrostatic charges attract to each other. – Like charges repel each other. 2. 23 explain electrostatic phenomena in terms of the movement of electrons – This is because electrons in one of the insulators is ripped off its surface, and thus becoming positively charged. The other insulator gains the electrons, and thus becoming negatively charged. 36

Electrical charge 2. 24 explain the potential dangers of electrostatic charges, e. g. when fuelling aircraft and tankers – Fuelling aircraft and tankers: When fuelling aircraft and tanker, a static charge may be built up. It is important to earth the plane or tanker to discharge the static charge, as a spark could cause a fire or explosion. – Handling computer components: It is important that there is no static charge on the worker as being static can easily destroy these components. – Electric shocks: Cars become charge with static electricity, and can give a shock when someone touches it. 37

Electric charge 2. 25 explain some uses of electrostatic charges, e. g. in photocopiers and inkjet printers – Inkjet printers: Charging parts of the paper negatively using a laser, then the oppositely charged toner is attracted to the negatively charged parts and thus forms an image which is pressed by a fuser. – Photocopier: A statically charged drum is exposed to light, reflected from the document. The light discharges all the parts except for where the dark print does not reflect. The charge parts of the drum attract the toner which is then transferred to printer paper. – Paint spraying: The tiny droplets of paint are given a static charge and the object to be painted is connected to a supply of opposite charge. This causes the paint droplets to be attracted, and the amount of wasted paint is drastically reduced. – Electrostatic precipitators: Small particles of soot and other dust produced in combustion are given a small static charge and are then passed through a highly charged grid which attracts dust particles stopping them from escaping. 38

Waves – – Units Properties of waves The electromagnetic spectrum Light and sound 39

Units 3. 1 use the following units: degree (o), hertz (Hz), meter (m), meter/second (m/s), second (s) – Degree (o) is for angle – Hertz (Hz) is for frequency – Meter (m) is for distance or length – Meter / second (m/s) is for speed or velocity – Second (s) is for time 40

Properties of waves 3. 2 understand the difference between longitudinal and transverse waves and describe experiments to show longitudinal and transverse waves in, for example, ropes, springs and water – Mechanical waves need a material medium to travel through. – Transverse : The medium moves perpendicular to the direction of the motion of the wave. – Ripples in a pond – Longitudinal: The medium moves parallel to the direction of the motion of the wave. – Sound wave – To show the properties of each wave, a slinky (spring) can be used: – Moving the slinky quickly vertically induces a transverse wave. – Moving the slinky quickly forwards and backwards induces a longitudinal wave. 41

Properties of waves 3. 3 define amplitude, frequency, wavelength and period of a wave – Amplitude: is the maximum displacement of a part of the medium from its rest position. – Wavelength is the distance between corresponding points in the wave – one crest to the next crest in a transverse wave. – Period of is the time for one complete cycle of the waveform. – Frequency is the number of cycles of the waveform per second. 3. 4 understand that waves transfer energy and information without transferring matter – Waves transfer energy without transferring matter. 3. 5 know and use the relationship between the speed, frequency and wavelength of a wave: – Wave speed = frequency X wavelength – The symbol of wavelength is λ. 42

Properties of waves 3. 6 use the relationship between frequency and time period: – Frequency = 1 / time period 3. 7 use the above relationships in different contexts including sound waves and electromagnetic waves – All electromagnetic waves travel at the same speed of 300, 000 m/s in a vacuum. – Radio station A transmits radio waves with wavelength 200 m. – Radio station B transmits radio waves with wavelength 300 m. – Use the wave equation to calculate the frequencies of the radio waves. – Wave speed = frequency X wavelength or frequency = wave speed / wavelength – Radio station A: 1, 500, 000 Hz – Radio station B: 1, 000 Hz 43

Properties of waves 3. 8 understand that waves can be diffracted when they pass an edge – Diffraction is the spreading of waves as they pass by edges of obstacles. – It stems from theory that one can hear a sound around the corner. 3. 9 understand that waves can be diffracted through gaps, and that the extent of diffraction depends on the wavelength and the physical dimension of the gap. – The closer the width of the gap and the wavelength, the greater the effect. 44

The electromagnetic spectrum 3. 10 understand that light is part of a continuous electromagnetic spectrum which includes radio, microwave, infrared, visible, ultraviolet, x-ray and gamma ray radiations and that all these waves travel at the same speed in free space – Electromagnetic waves are transverse. – They do not use a material medium. – All travel at the 300, 000 m/s through a vacuum 3. 11 identify the order of the electromagnetic spectrum in terms of decreasing wavelength and increasing frequency, including the colors of the visible spectrum – Radio waves Microwave Infrared Visible Ultraviolet X-Ray Gamma Ray – Red Orange Yellow Green Blue Indigo Violet – Increasing frequency Increasing wavelength – Since speed is constant, frequency and wavelength must be inversely proportional. 45

The electromagnetic spectrum 3. 12 explain some of the uses of electromagnetic radiations, including: – Radio waves: broadcasting and communications – Microwaves: cooking and satellite transmissions – Infrared: heaters and night vision equipment – Visible light: optical fibers and photography – Ultraviolet: fluorescent lamps – X-rays: observing the internal structure of objects and materials and medical applications – Gamma rays: sterilizing food and medical equipment 46

The electromagnetic spectrum 3. 13 understand the detrimental effects of excessive exposure of the human body to electromagnetic waves, including: – Microwaves: internal heating of body tissue – Infrared: skin burns – Ultraviolet: damage to surface cells and blindness – Gamma rays: cancer, mutation 47

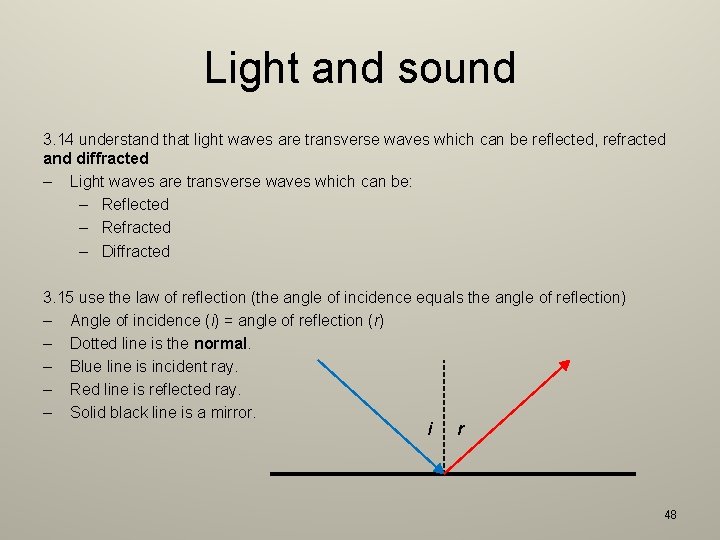
Light and sound 3. 14 understand that light waves are transverse waves which can be reflected, refracted and diffracted – Light waves are transverse waves which can be: – Reflected – Refracted – Diffracted 3. 15 use the law of reflection (the angle of incidence equals the angle of reflection) – Angle of incidence (i) = angle of reflection (r) – Dotted line is the normal. – Blue line is incident ray. – Red line is reflected ray. – Solid black line is a mirror. i r 48

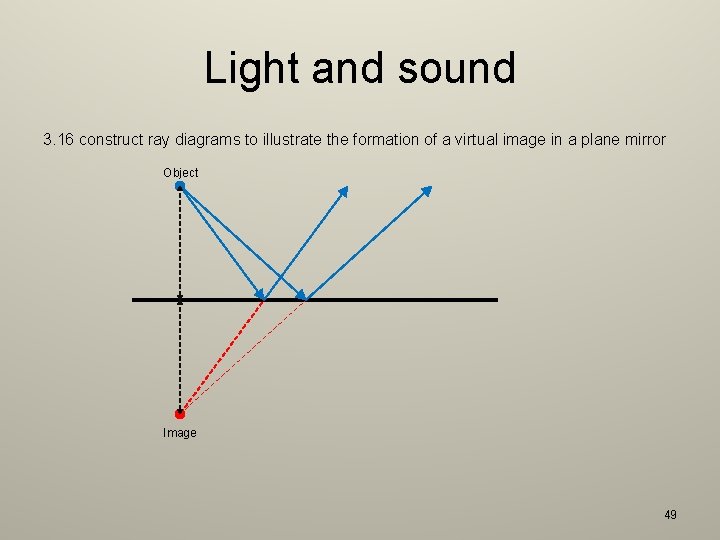
Light and sound 3. 16 construct ray diagrams to illustrate the formation of a virtual image in a plane mirror Object Image 49

Light and sound 3. 17 describe experiments to investigate the refraction of light, using rectangular blocks, semicircular blocks and triangular prisms – The solid is glass. – The refracted ray bends towards the normal because glass is denser than air. – The emergent ray bends away from the normal because air is less dense than glass. – How much it bends depends on the refractive index. 50

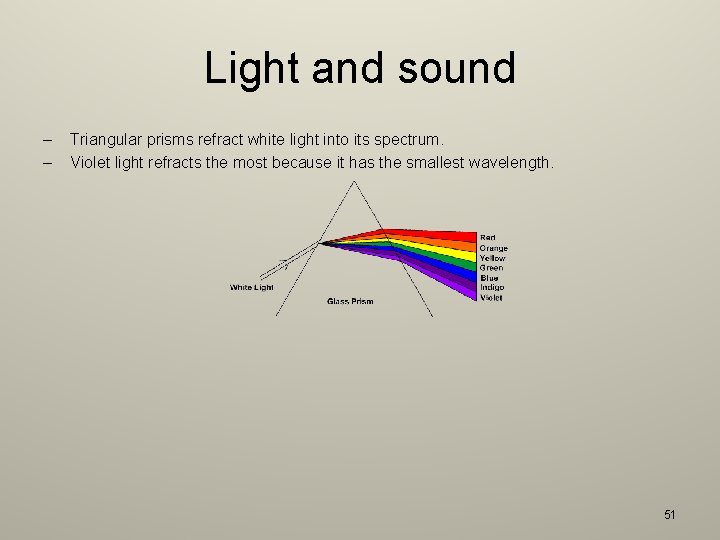
Light and sound – – Triangular prisms refract white light into its spectrum. Violet light refracts the most because it has the smallest wavelength. 51

Light and sound 3. 18 know and use the relationship between refractive index, angle of incidence and angle of refraction: – Refractive index = sin angle of incidence / sin angle of refraction – n = sini / sinr – This is known as Snell’s Law. 3. 19 describe an experiment to determine the refractive index of glass, using a glass block – The angle of incidence = 50 o – The angle of refraction = 31 o – n = sini / sinr – n = sin 50 / sin 31 – n = 1. 5 52

Light and sound 3. 20 describe the role of total internal reflection in transmitting information along optical fibers and in prisms – Optical fibers contain light signals without losing anything. It is used in communication. – The core of the optical fiber is layered with a material which has a lower refractive index. This is because total internal reflection only works if it travels from a medium where it travels more slowly towards a medium where it travels more quickly. 53

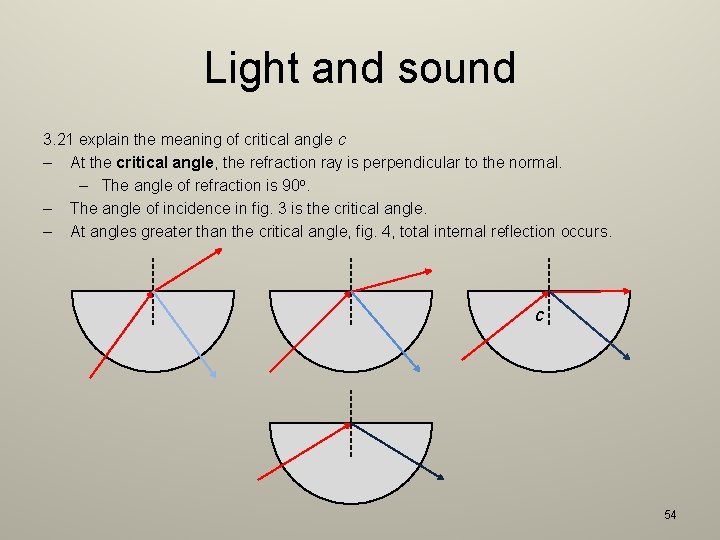
Light and sound 3. 21 explain the meaning of critical angle c – At the critical angle, the refraction ray is perpendicular to the normal. – The angle of refraction is 90 o. – The angle of incidence in fig. 3 is the critical angle. – At angles greater than the critical angle, fig. 4, total internal reflection occurs. c 54

Light and sound 3. 22 know and use the relationship between critical angle and refractive index: sin critical angle = 1 / refractive index sinc = 1 / n 3. 23 understand the difference between analogue and digital signals – Analogue electrical signals have continuously variable values (voltages). – Digital electrical signals have only two possible values. These are 0 and 1 in the binary number system and sometimes represent on and off. 3. 24 describe the advantages of using digital signals rather than analogue signals – Analogue signals transmitted over large distances lose energy and they pick up unwanted interference. They can be amplified to boost the energy of the signal but both the wanted signal and unwanted noise are amplified, so the quality is reduced. – Digital signals can be regenerated, both amplifying and restoring their two value shape electronically thus virtually eliminating the unwanted noise from the signal. 55

Light and sound 3. 25 describe how digital signals can carry more information – Optical fibers allow a much wider bandwidth. This means that many different signals can share the same optical fiber, so much more information can be transmitted. 3. 26 understand that sound waves are longitudinal waves and how they can be reflected, refracted and diffracted – Sound waves are longitudinal waves which can be: – Reflected – Refracted – Diffracted 3. 27 understand that the frequency range for human hearing is 20 Hz – 20, 000 Hz – 20 Hz to 20, 000 k. Hz is the range that the human ear can detect. – Above 20, 000 k. Hz is ultrasound. 56

Light and sound 3. 28 describe an experiment to measure the speed of sound in air – Find a location which is close to a large reflective wall and record the distance from the wall to a microphone (at least 50 m). – Create a large sound next to a microphone. – Find time between the large sound and the reflection. – Sound waves travel at 340 m/s in air. 3. 29 understand how an oscilloscope and microphone can be used to display a sound wave – A microphone converts sound waves into voltages, an oscilloscope amplifies these voltages and produces a graph which show the voltages are changing over a period of time. 57

Light and sound 3. 30 describe an experiment using an oscilloscope to determine the frequency of a sound wave – The higher the trace the louder the note is. – The more frequent the waves, (low time period) the higher the frequency. 3. 31 relate the pitch of a sound to the frequency of vibration of the source – The pitch of a note depends on the frequency of the vibration of the source of sound. – Since T = 1 / f the more waves on the screen the higher the note. 3. 32 relate the loudness of a sound to the amplitude of vibration. – The height of the oscilloscope trace depends on the loudness of the sound. – The louder the sound the greater the amplitude. 58

Energy resources and energy transfer – – Units Energy transfer Work and power Energy resources and electricity generation 59

Units 4. 1 use the following units: kilogram (kg), joule (J), meter (m), meter/second (m/s), meter/second 2 (m/s 2), newton (N), second (s), watt (W) – Kilogram (kg) is for mass – Joule (J) is for energy – Meter (m) is for distance – Meter/second (m/s) is for speed – Meter/second 2 (m/s 2) is for acceleration – Newton (N) is force – Second (s) is for time – Watt (W) is for power 60

Energy transfer 4. 2 describe energy transfers involving the following forms of energy: thermal (heat), light, electrical, sound, kinetic, chemical, nuclear and potential (elastic and gravitational) – Lamp: electrical light – Heater: electrical heat – Energy plant (coal): chemical heat kinetic – Rubber band: Elastic potential Heat 4. 3 understand that energy is conserved Energy is conserved, it is never lost, only converted from one form to another. 4. 4 know and use the relationship: – Efficiency = useful energy output / total energy output. 61

Energy transfer 4. 5 describe a variety of everyday and scientific devices and situations, explaining the fate of the input energy in terms of the above relationship, including their representation by Sankey diagrams – e. g. Lamp: – Useful: light – Wasted: heat – e. g. Motor: – Useful: kinetic – Wasted: heat, sound – The Sankey diagram is of a filament lamp. 10 J of energy out of 100 J became light energy, which is the useful one. – 10 J / 100 J = 0. 1 – 0. 1 X 100 = 10% – The filament lamp is 10% efficient. 62

Energy transfer 4. 6 describe how energy transfer may take place by conduction, convection and radiation – Thermal Conduction is the transfer of heat energy through a substance without the substance itself moving. When heated, the particles vibrate faster, causing the particles in its vicinity to also vibrate. – Thermal Convection is the transfer of heat energy through fluids by the upward movement of warmer, less dense, regions in the fluid. When a fluid is heated, it expands meaning it is less dense, thus floating upwards. It is replaced by colder, denser fluid and that is heated up and floats upwards. – Thermal Radiation is the transfer of heat energy in the form of infrared (IR) waves. It is the only method of heat transfer through a vacuum. Infrared waves are part of the electromagnetic spectrum, they travel at the speed of light in a vacuum. 4. 7 explain the role of convection in everyday phenomena – The land warms quickly, the sea warms slowly during the day, there is an onshore breeze. – This land cools quickly, the sea cools slowly during the night, there is an offshore breeze. 63

Energy transfer 4. 8 explain how insulation is used to reduce energy transfers from buildings and the human body. – Reduce heat transfer by conduction: – Vacuum: Conduction needs matter. Used in vacuum flasks, types of double glazing. – Air: Air is a poor conductor of heat, materials like wool, feathers trap air so it cannot circulate. – Water: Wetsuits trap a layer of water around the body because water is a poor conductor of heat. – Reduce heat transfer by convection: – Vacuum: Convection needs fluids. Used in vacuum flasks, types of double glazing. – Trapped gas or liquid: Restricted circulation. Circulation is necessary for convection to occur. – Reduce heat transfer by radiation: – Use shiny surfaces: It reflects infrared radiation. 64

Work and power 4. 9 know and use the relationship between work, force and distance moved in the direction of the force: – Work done = force X distance moved – Work done is measured in Joules. 4. 10 understand that work done is equal to energy transferred – Work done is equal to energy transferred: – e. g. Lifting a box – Using force to lift the box upwards. – Transferring chemical energy kinetic energy gravitational potential energy 4. 11 know and use the relationship: gravitational potential energy = mass × g × height – GPE = m X g X h – g is gravitational field strength. 65

Work and power 4. 12 know and use the relationship: kinetic energy = 1/2 × mass × speed 2 – KE = 1/2 X m X v 2 – KE and GPE is measured in Joules. 4. 13 understand how conservation of energy produces a link between gravitational potential energy, kinetic energy and work – A roller coaster cart is dragged to the highest point in a slope. The cart has a mass of 800 kg and the point is 12 m high. – The GPE at this point is: – GPE = 800 X 12 = 96, 000 Joules – Assuming that no energy is lost, KE = 96, 000 Joules. – 96, 000 = 1/2 mv 2 – 96, 000 X 2 / 800 = v 2 – v 2 = 240 – v = 15. 491 m/s 66

Work and power 4. 14 describe power as the rate of transfer of energy or the rate of doing work – Power is the rate of transfer of energy. 4. 15 use the relationship between power, work done (energy transferred) and time taken: – Power = work done / time taken 67

Energy resources and electricity generation 4. 16 describe the energy transfers involved in generating electricity using: – Wind – Rotational kinetic energy electrical energy – Water – Gravitational potential energy kinetic energy of moving water rotational kinetic energy electrical energy – Solar cells – Light energy electrical energy – Geothermal resources, solar heating systems, fossil fuels, nuclear power – Heat energy steam kinetic energy rotational kinetic energy electrical energy 68

Energy resources and electricity generation 4. 17 describe the advantages and disadvantages of methods of large scale electricity production from various renewable and nonrenewable resources. – Cost – Renewable / non-renewable – Greenhouse gas emissions – Supply and demand – Environmental impact – Location 69

Solids, liquids and gases – – Units Density and pressure Change of state Ideal gas molecules 70

Units 5. 1 use the following units: degrees Celsius (o. C), kelvin (K), joule (J), kilogram (kg), kilogram/metre 3 (kg/m 3), meter (m), metre 2 (m 2 ), metre 3 (m 3), meter/second (m/s), meter/second 2 (m/s 2 ), newton (N), pascal (Pa) – Degrees Celsius (o. C) is for temperature – Kelvin (K) is for temperature – Joule (J) is for energy – Kilogram (kg) is for mass – Kilogram/meter 3 (kg/m 3) is for density – Meter is for distance – Meter 2 is for area – Meter 3 is for volume – Meter/second (m/s) is for velocity or speed – Meter/second 2 (m/s 2) is for acceleration – Newton (N) is force – Pascal (Pa) is for pressure 71

Density and pressure 5. 2 know and use the relationship between density, mass and volume: – Density = mass / volume 5. 3 describe experiments to determine density using direct measurements of mass and volume – A block of wood that measures 20 cm X 5 cm x 10 cm has a mass of 528 g. Calculate the density of the wood. – Density = 528 g / (20 x 5 x 10)cm 3 – Density = 0. 528 g/cm 3 or 528 kg/m 3 5. 4 know and use the relationship between pressure, force and area: – Pressure = force / area – Force / area is N / m 2 – 1 Pascal (Pa) = 1 N/m 2 72

Density and pressure 5. 5 understand that the pressure at a point in a gas or liquid which is at rest acts equally in all directions – Air pressure exerts almost 100 kilopascals. However, a thin can stays in shape. This is because the air inside exerts the equal amount of pressure. 5. 6 know and use the relationship for pressure difference: – Pressure difference = height X density X gravitational field strength – Calculate the pressure difference between point X at the top surface of the water in a tank and at point Y at the bottom of the tank. The density of the water is 1000 kg/m 3 and the height is 5 m. – Pressure difference = 5 m X 1000 kg/m 3 X 10 N/kg = 50, 000 Pa 73

Change of state 5. 7 understand the changes that occur when a solid melts to form a liquid, and when a liquid evaporates or boils to form a gas – Melting: The particles gain kinetic energy as they are heated and vibrate faster and faster. This allows the particles to overcome the forces of attraction between them. This allows the particles to break the regular pattern and to flow over one another. – Freezing: The particles lose kinetic energy as they are cooled and this allows the forces of attraction to hold them together. The particles arrange themselves into a regular pattern and are no longer able to flow over one another. – Evaporation: The particles gain kinetic energy as they are heated and move further apart. Eventually the forces of attraction between the particles are completely broken and they are able to escape. – Condensation: The particles lose kinetic energy as they are cooled and this allows the forces of attraction to bring them closer together. The particles gradually clump together to form a liquid. 74

Change of state 5. 8 describe the arrangement and motion of particles in solids, liquids and gases – Solid: Particles in fixed positions, regular repeating pattern, strong inter-particle forces. – Liquid: Particles not in fixed positions, can flow, irregular pattern, weaker inter-particle forces. – Gas: Particles not in fixed positions, can flow, irregular pattern, non-existent interparticle forces. 75

Ideal gas molecules 5. 9 understand the significance of Brownian motion, as supporting evidence for particle theory – The particles in mater are extremely small and, in liquids and gases, they are in a continuous state of rapid random motion. 5. 10 understand that molecules in a gas have a random motion and that they exert a force and hence a pressure on the walls of the container – A gas exerts a pressure on objects as a result of the continuous collisions between the gas molecules and the container. o. C 5. 11 understand why there is an absolute zero of temperature which is – 273� – Absolute zero which is -273 o. C or 0 K is when the gas no longer exerts pressure. 76

Ideal gas molecules 5. 12 describe the Kelvin scale of temperature and be able to convert between the Kelvin and Celsius scales – To convert Kelvin Celsius: Kelvin - 273 = Celsius – To convert Celsius Kelvin: Celsius + 273 = Kelvin 5. 13 understand that an increase in temperature results in an increase in the average speed of gas molecules – The speed of molecules increases with temperature, so the collisions with walls are more energetic and occur more frequently. 5. 14 understand that the Kelvin temperature of the gas is proportional to the average kinetic energy of its molecules – The temperature of a gas in kelvin is proportional to the average kinetic energy of the gas molecules. 77

Ideal gas molecules 5. 15 describe the qualitative relationship between pressure and Kelvin temperature for a gas in a sealed container – As the temperature increase, the speed of the particles increase. Therefore, the collisions with the walls of the container will be more frequent and more energetic. This increases the pressure exerted by the gas. 5. 16 use the relationship between the pressure and Kelvin temperature of a fixed mass of gas at constant volume: – P 1 / T 1 = P 2 / T 2 – Keep in mind that the temperature is in kelvin. – Pressure is proportional to temperature in kelvin. – If you double the kelvin temperature of a gas in a rigid container the pressure of the gas doubles. 78

Ideal gas molecules 5. 17 use the relationship between the pressure and volume of a fixed mass of gas at constant temperature: – P 1 X V 1 = P 2 X V 2 – Pressure is inversely proportional to volume. – If you double the pressure on a gas, its volume halves. 79

Magnetism and electromagnetism – – Units Magnetism Electromagnetic induction 80

Units 6. 1 use the following units: ampere (A), volt (V), watt (W). – Ampere (A) is for current – Volt (V) is for voltage – Watt (W) is for power 81

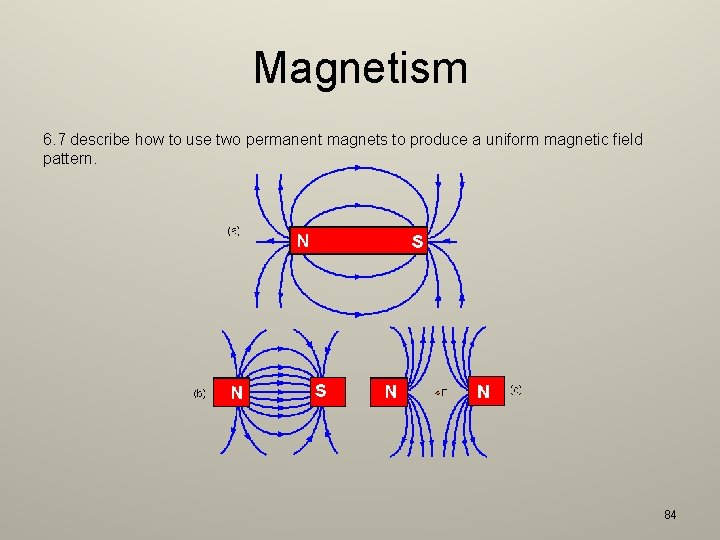
Magnetism 6. 2 understand that magnets repel and attract other magnets and attract magnetic substances – A magnet has a field around it and has two poles (North / South). – Unlike poles attract (N and S). – Like poles repel (N and N) / (S and S). – Will attract magnetic materials by inducing magnetism in them. – Temporary: Iron – Permanent: Steel – Will exert no force on non-magnetic materials. 6. 3 describe the properties of magnetically hard and soft materials – Hard magnetic materials are difficult to magnetize but do not readily lose their magnetism. These are normally alloys. – Soft magnetic materials are easy to magnetize but only temporary. Once out of a magnet’s field, it loses its induced magnetism. These are nickel, cobalt, iron. 82

Magnetism 6. 4 understand the term ‘magnetic field line’ – Magnetic field lines run from north to south of a magnet. – Magnetic field lines do not cross. – The field lines closest together are where the magnetic field is strongest. 6. 5 understand that magnetism is induced in some materials when they are placed in a magnetic field – Materials that are placed in a magnetic field is magnetized because a magnet induces them. This is done by aligning all the domains in one direction. The induced pole nearest to the magnet is opposite of the pole at the end of the magnet. 6. 6 describe experiments to investigate the magnetic field pattern for a permanent bar magnet and that between two bar magnets – Sprinkling iron filings onto a piece of paper with a magnet underneath it. The iron filings fall to form magnetic field lines. 83

Magnetism 6. 7 describe how to use two permanent magnets to produce a uniform magnetic field pattern. 84

Electromagnetism 6. 8 understand that an electric current in a conductor produces a magnetic field round it – When a current flows in a wire, a magnetic field is produced. 6. 9 describe the construction of electromagnets – By winding a coil around a magnetic material, the field is made stronger. – A soft magnetic material is used to do this, as it is magnetized easily. 6. 10 sketch and recognize magnetic field patterns for a straight wire, a flat circular coil and a solenoid when each is carrying a current – The right-hand grip rule: – Point your thumb in the direction of the current. – The curl of your fingers indicate the field direction. – In a solenoid: Curl your fingers in the direction of the current. The thumb shows the direction of North. 85

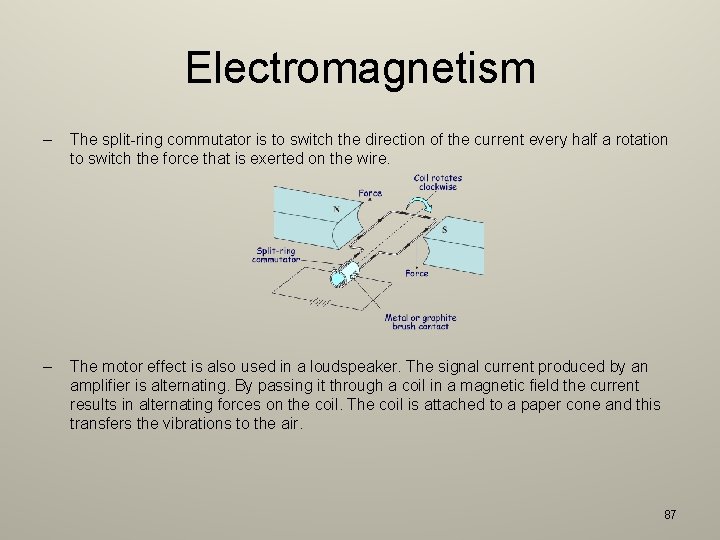
Electromagnetism 6. 11 understand that there is a force on a charged particle when it moves in a magnetic field as long as its motion is not parallel to the field – When a charge particle is in a magnetic field a force is exerted on it. 6. 12 understand that a force is exerted on a current-carrying wire in a magnetic field, and how this effect is applied in simple D. C. electric motors and loudspeakers – When a current is passed through a wire placed in a magnetic field a force is produced which acts on the wire. – This is used in the electric motor: The DC motor consists of a single turn of wire that is free to rotate in a magnetic field about an axle. Carbon brushes make contact with the ends of the coils that are connected to a split-ring commutator so that the coil spins in the same direction. 86

Electromagnetism – The split-ring commutator is to switch the direction of the current every half a rotation to switch the force that is exerted on the wire. – The motor effect is also used in a loudspeaker. The signal current produced by an amplifier is alternating. By passing it through a coil in a magnetic field the current results in alternating forces on the coil. The coil is attached to a paper cone and this transfers the vibrations to the air. 87

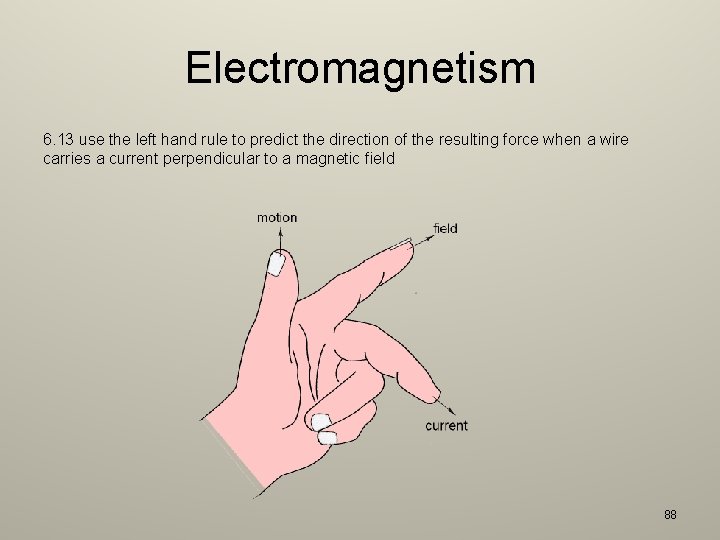
Electromagnetism 6. 13 use the left hand rule to predict the direction of the resulting force when a wire carries a current perpendicular to a magnetic field 88

Electromagnetism 6. 14 describe how the force on a current-carrying conductor in a magnetic field increases with the strength of the field and with the current. – Size of force: – Strength of magnetic field – Current in the wire – More turns on the coil. 89

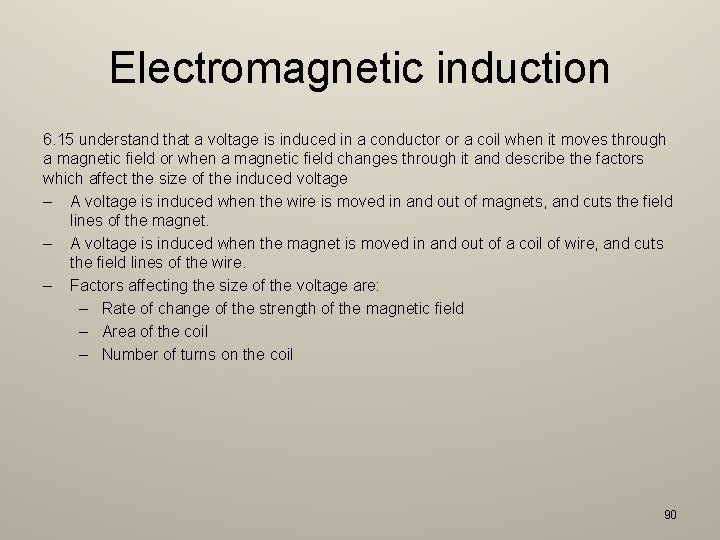
Electromagnetic induction 6. 15 understand that a voltage is induced in a conductor or a coil when it moves through a magnetic field or when a magnetic field changes through it and describe the factors which affect the size of the induced voltage – A voltage is induced when the wire is moved in and out of magnets, and cuts the field lines of the magnet. – A voltage is induced when the magnet is moved in and out of a coil of wire, and cuts the field lines of the wire. – Factors affecting the size of the voltage are: – Rate of change of the strength of the magnetic field – Area of the coil – Number of turns on the coil 90

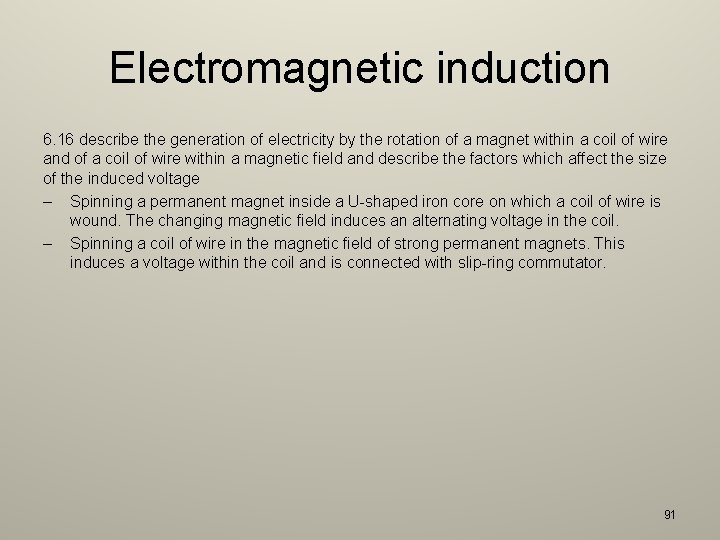
Electromagnetic induction 6. 16 describe the generation of electricity by the rotation of a magnet within a coil of wire and of a coil of wire within a magnetic field and describe the factors which affect the size of the induced voltage – Spinning a permanent magnet inside a U-shaped iron core on which a coil of wire is wound. The changing magnetic field induces an alternating voltage in the coil. – Spinning a coil of wire in the magnetic field of strong permanent magnets. This induces a voltage within the coil and is connected with slip-ring commutator. 91

Electromagnetic induction 6. 17 describe the structure of a transformer, and understand that a transformer changes the size of an alternating voltage by having different numbers of turns on the input and output sides – The voltage of an alternating current can be changed by number of turns of coils in the input and output in the sides. – The strength of the magnetic field depends on the number coils, therefore, more coils would induce a larger voltage. 92

Electromagnetic induction 6. 18 explain the use of step-up and step-down transformers in the large scale generation and transmission of electrical energy – Power loss due to resistance is given by the formula power loss = current 2 X resistance. Therefore, to reduce power loss, current should be minimalized. – Voltage and current is inversely proportionate and in a transformer power input = power output. – This means by maximizing voltage through a transformer, current is minimalized and power loss is reduced. 6. 19 know and use the relationship between input (primary) and output (secondary) voltages and the turns ratio for a transformer: – Input voltage / output voltage = primary turns / secondary turns – Vp / V s = n 1 / n 2 – Voltage and number of turns is proportional. – If n 2 is double of n 1, v 2 is double of v 1. 93

Electromagnetic induction 6. 20 know and use the relationship: – input power = output power – VP X I P = V S X I S – This only works in cases of 100% efficiency. 94

Radioactivity and particles – – – Units Radioactivity Particles 95

Units 7. 1 use the following units: Becquerel (Bq), centimeter (cm), hour (h), minute (min), second (s) – Becquerel (Bq) is for rate of decay (one decay per second) – Centimeter (cm) is for distance – Hour (h) is for time – Minute (min) is for time – Second (s) is for time 96

Radioactivity 7. 2 describe the structure of an atom in terms of protons, neutrons and electrons and use symbols such as 614 C to describe particular nuclei – Top number represents the atomic mass. – Bottom number represents the atomic number. – Neutron: An uncharged particle in the nucleus of an atom. – Proton: A positively charged particle in the nucleus of an atom. – Electron: A negatively charged particle which is not in the nucleus. 7. 3 understand the terms atomic (proton) number, mass (nucleon) number and isotope – Atomic number: the number of protons – Mass number: the number of protons + the number of neutrons – Isotope: the same element with a different number of neutrons 97

Radioactivity 7. 4 understand that alpha and beta particles and gamma rays are ionizing radiations emitted from unstable nuclei in a random process – Alpha and beta particles and gamma rays are types of ionizing radiation that may be emitted from unstable nuclei. 7. 5 describe the nature of alpha and beta particles and gamma rays and recall that they may be distinguished in terms of penetrating power – Alpha particles are helium nuclei. Heavily ionizing. Only 10 cm in air. Stopped by thin card. No electrons. +2 charge. – Beta particles are electrons. Less ionizing. Travel long distances. Stopped by 1 -2 mm of aluminum. -1 charge. – Gamma particles are photons of EM waves, extremely penetrating. Only stopped by tens of cm of lead. No charge. 98

Radioactivity 7. 6 describe the effects on the atomic and mass numbers of a nucleus of the emission of each of the three main types of radiation – Radioactive forms of some elements will decay randomly over time emitting ionizing radiation. The emission of gamma rays has no effect on the atomic mass or charge of the decaying atom. – Alpha Decay: – Atomic number decreases by 2 – Mass number decreases by 4 241 Am 237 Np + 4 He 95 93 2 – – Beta Decay – Atomic number increases by 1 – Mass number is unchanged 26 26 0 11 Am 12 Np + -1 e – 99

Radioactivity 7. 7 understand how to complete balanced nuclear equations – Strontium-90 undergoes beta decay and forms Yttrium-90. 90 90 0 38 Sr 39 Y + -1 e – 7. 8 understand that ionizing radiations can be detected using a photographic film or a Geiger-Muller detector – Photographic film: Photographic film gets fogged when exposed to ionizing radiation. – Layers of material are put between the photographic film to allow measurements of each radiation type. – Geiger-Muller tube: When ionizing radiation enters the GM tube it ionizes the gases (argon) within allowing a pulse of current to pass between the electrodes. 100

Radioactivity 7. 9 explain the sources of background radiation – Earth’s rocks: The slow decay of isotopes of uranium produces radon and thoron gases. – Radon is highly radioactive and is a problem in some parts of the UK. – Cosmic rays: When stars explode the very violent reactions produce cosmic rays. – Medical: Radioactive materials used in diagnosis and medical treatment contribute to background radiation. – Nuclear power and weapons: Testing of nuclear weapons and nuclear power station leaks contribute a small amount to background radiation. 7. 10 understand that the activity of a radioactive source decreases over a period of time and is measured in becquerels – The activity of a radioactive source randomly decays over a period of time. The rate of decay is measured in becquerels – one Bq is one decay per second. – The smaller the remaining unstable nuclei, the more slowly the decay process proceeds. This is an exponential decay. 101

Radioactivity 7. 11 understand the term ‘half-life’ and understand that it is different for different radioactive isotopes – The half life of a radioactive isotope is the time taken for half the original number of unstable nuclei to decay. – The half life of a isotope does not change, but different isotopes have different half lives. – Uranium-238: 4. 5 billion years – Iodine-131: 8 days 7. 12 use the concept of half-life to carry out simple calculations on activity – Initial sample X 1/2(time / half Life) = end sample – e. g. How much of a sample of 400 micrograms of Iodine-131 remain after 16 days? – 400 X 1/2(16/8) = end sample – 400 X 1/22 = 100 micrograms 102

Radioactivity 7. 13 describe the uses of radioactivity in medical and non-medical tracers, in radiotherapy, and in the radioactive dating of archaeological specimens and rocks – Gamma emitters can be swallowed or injected into the body to follow the transport of the tracer through the digestive system or the veins and arteries. – The beta emitter iodine-131 is taken orally to treat a thyroid gland condition. – Focused beams of gamma rays are used to kill off cancerous cells in some tumors. – Ionizing radiation is used to kill bacteria and microorganisms on surgical equipment. – The flow of liquids and gases in industrial processes can be mapped using radioactive tracers and detectors. – Organic materials absorb the carbon-14, which is a radioactive isotope. The time of which the material has been dead can be measured by the proportion of carbon-14 to carbon-12. – Inorganic materials can be measured with the amount of uranium nuclei. 103

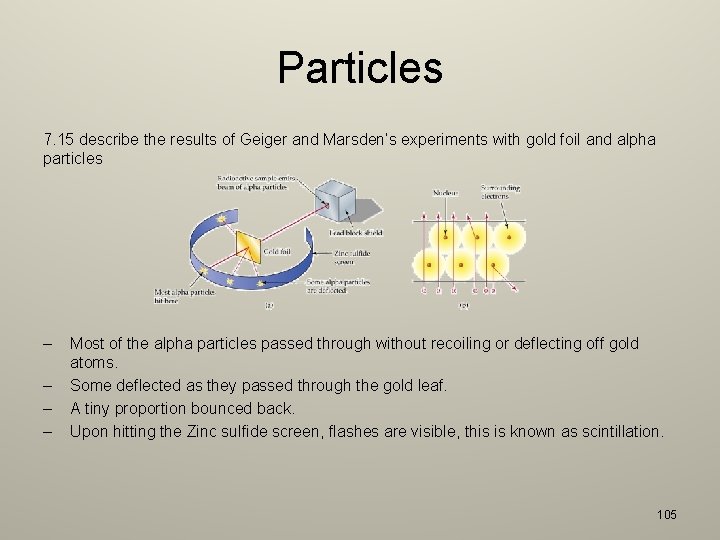
Radioactivity 7. 14 describe the dangers of ionizing radiations, including: – Radiation cause mutations in living organisms – This can result in organs malfunctioning or tumors exerting pressure on other organs. – Radiation can damage cells and tissue – Ionizing radiation can kill living cells. – The problems arising in the disposal of radioactive waste and describe how the associated risks can be reduced – Waste materials from fission reactors can be highly radioactive. – These materials have to be encapsulated and stored in places far away from human contact. The capsules have to be made of lead or concrete to ensure no radiation escapes. 104

Particles 7. 15 describe the results of Geiger and Marsden’s experiments with gold foil and alpha particles – – Most of the alpha particles passed through without recoiling or deflecting off gold atoms. Some deflected as they passed through the gold leaf. A tiny proportion bounced back. Upon hitting the Zinc sulfide screen, flashes are visible, this is known as scintillation. 105

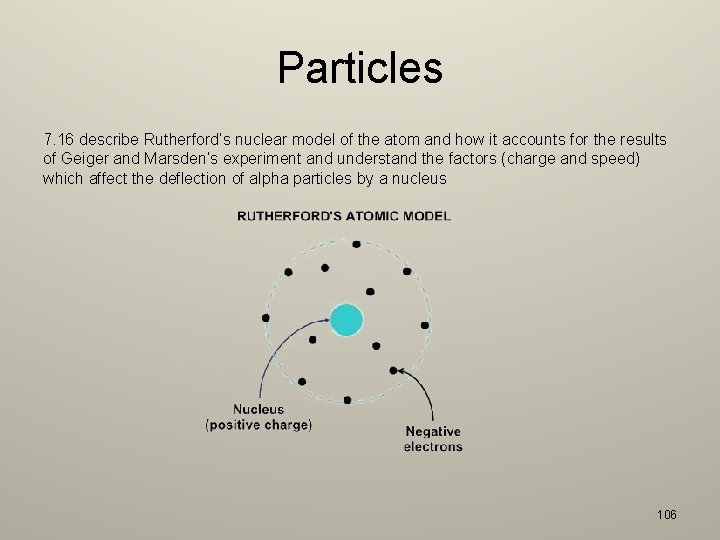
Particles 7. 16 describe Rutherford’s nuclear model of the atom and how it accounts for the results of Geiger and Marsden’s experiment and understand the factors (charge and speed) which affect the deflection of alpha particles by a nucleus 106

Particles – – The deflections were caused by the electric repulsion between the positive charges of the alpha particles and the positive charges in the gold nucleus. The amount of deflection depends on: – The nuclear charge – more highly charged nuclei produce greater deflections – Speed of the alpha particles – faster particles are deflected in smaller angles – Proximity of the alpha particle to the nucleus – the electric force diminishes Most particles did not deflect because a lot of the atom is empty space and the positive charge is concentrated in a very small central region of the atom. 107

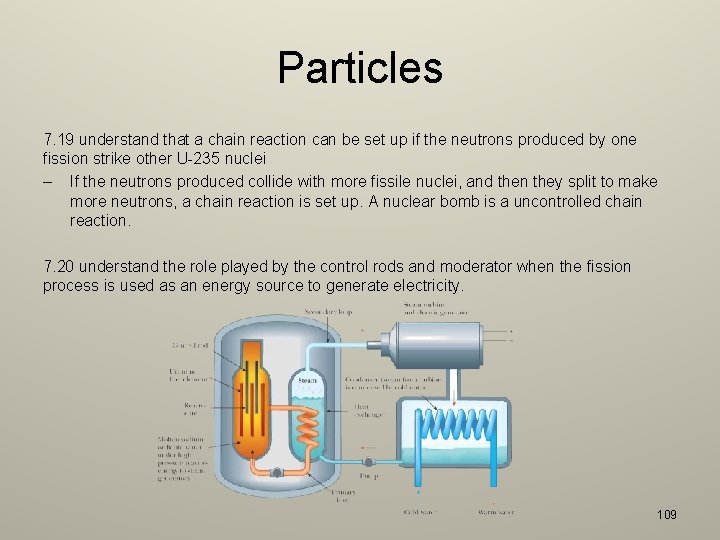
Particles 7. 17 understand that a nucleus of U-235 can be split (the process of fission) by collision with a neutron, and that this process releases energy in the form of kinetic energy of the fission products – The isotope uranium-235 is fissile. – Fissile means that it can be split into lighter elements easily. – When an atom of U-235 collides with a low energy neutron it forms the unstable U 236 then breaks apart. – This releases energy in the form of the kinetic energy of the decay fragments. 7. 18 understand that the fission of U-235 produces two daughter nuclei and a small number of neutrons – When U-235 splits, it forms: – Two lighter nuclei, namely krypton-89 and barium-144. – Three neutrons – Gamma radiation 108

Particles 7. 19 understand that a chain reaction can be set up if the neutrons produced by one fission strike other U-235 nuclei – If the neutrons produced collide with more fissile nuclei, and then they split to make more neutrons, a chain reaction is set up. A nuclear bomb is a uncontrolled chain reaction. 7. 20 understand the role played by the control rods and moderator when the fission process is used as an energy source to generate electricity. 109

Particles – – Graphite Moderators: Slow neutrons. Boron Control Rods: Absorb neutrons to stop reactions. 110