Pharmacognosy I Third Lecture 4 Principles of Separation




















![Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Advantages[edit] Selectivity[edit] Table Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Advantages[edit] Selectivity[edit] Table](https://slidetodoc.com/presentation_image_h2/1d686c3a25cc938703dd075aaedfb842/image-39.jpg)
![Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Limitations[edit] Table Critical Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Limitations[edit] Table Critical](https://slidetodoc.com/presentation_image_h2/1d686c3a25cc938703dd075aaedfb842/image-40.jpg)
- Slides: 42

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques in Natural Products Isolation Mohammed N. Sabir October 2016 12/19/2021 1

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Introduction to isolation of natural products (NP)… What are NP? Where they exist? How to know their activities? Why they are important? 12/19/2021 2

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques The systematic method for isolation of NP… • Identification of the natural source (plant, M. O. etc. ). • If plants are involved, you need taxonomic identification of the genus, species and the family as well as the plant part used. 12/19/2021 3

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques The systematic method for isolation of NP… • Physicochemical investigation for NP is needed. • The plant crude drug will be subjected for extraction procedure. (you need literature review here) • There are different extraction procedures selected according to the physicochemical preferences of the NP. 12/19/2021 4

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques The systematic method for isolation of NP… • The solubility of the bioactive metabolite is crucial for selection of the proper solvent for extraction. • Here comes the concept of like-dissolve-like. • The dielectric constant for the solvent determines its polarity. • The functional groups of the target metabolite determines its polarity and solubility preferences. 12/19/2021 5

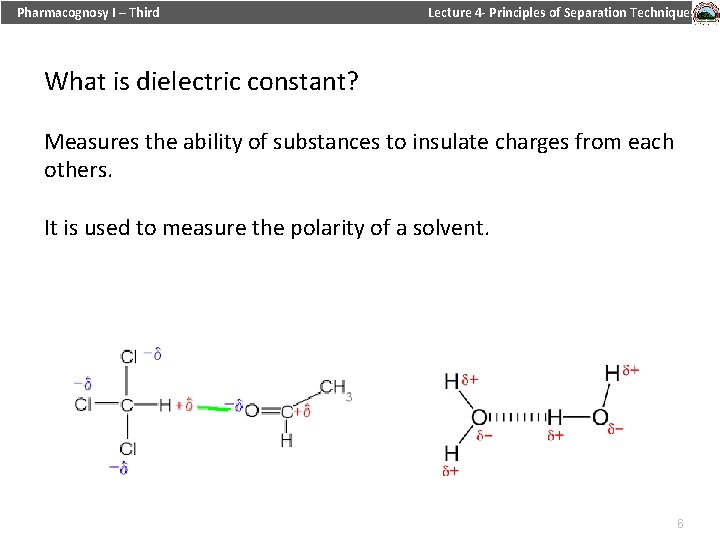
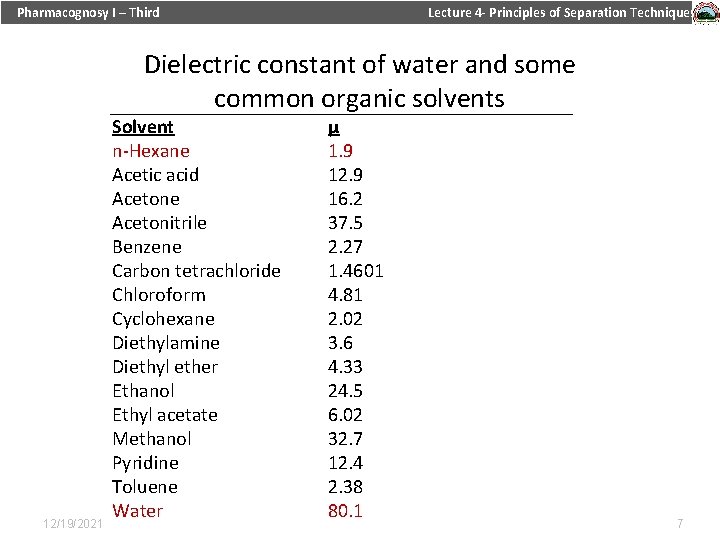
Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques What is dielectric constant? Measures the ability of substances to insulate charges from each others. It is used to measure the polarity of a solvent. 12/19/2021 6

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Dielectric constant of water and some common organic solvents 12/19/2021 Solvent n-Hexane Acetic acid Acetone Acetonitrile Benzene Carbon tetrachloride Chloroform Cyclohexane Diethylamine Diethyl ether Ethanol Ethyl acetate Methanol Pyridine Toluene Water μ 1. 9 12. 9 16. 2 37. 5 2. 27 1. 4601 4. 81 2. 02 3. 6 4. 33 24. 5 6. 02 32. 7 12. 4 2. 38 80. 1 7

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Why the polarity of a solvent is important? How the solvent polarity help in extraction of NP? 12/19/2021 8

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques The systematic method for isolation of NP… Extraction is a process through which a compound or closely related ones (probably isomers) are separated from a mixture. 12/19/2021 9

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques The systematic method for isolation of NP… Techniques for extraction of NP… • Maceration • Soxhelation • Distillation • Percolation or Infusion • Supercritical fluid extraction • Sonication • Decoction • Sublimation • Freeze drying 12/19/2021 10

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Important concepts about solvents… q Partition or distribution coefficient Is the distribution of solute between two immiscible phases. 12/19/2021 11

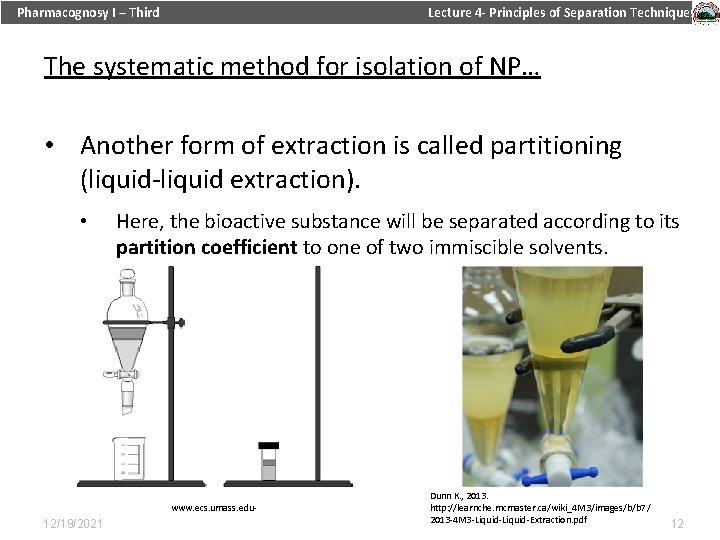
Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques The systematic method for isolation of NP… • Another form of extraction is called partitioning (liquid-liquid extraction). • Here, the bioactive substance will be separated according to its partition coefficient to one of two immiscible solvents. www. ecs. umass. edu- 12/19/2021 Dunn K. , 2013. http: //learnche. mcmaster. ca/wiki_4 M 3/images/b/b 7/ 2013 -4 M 3 -Liquid-Extraction. pdf 12

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques The key criteria for choosing proper solvent is the solubility of the solute in the a particular solvent. 12/19/2021 13

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques The systematic method for isolation of NP… • In addition to water, the most common organic solvents used for extraction of bioactive metabolites are: • Methanol • Ethanol • Chloroform • Diethyl ether • Petroleum ether • Ethyl acetate • n-hexane 12/19/2021 14

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques The systematic method for isolation of NP… • In some cases mixture of solvents are used as in water with alcohol. 12/19/2021 15

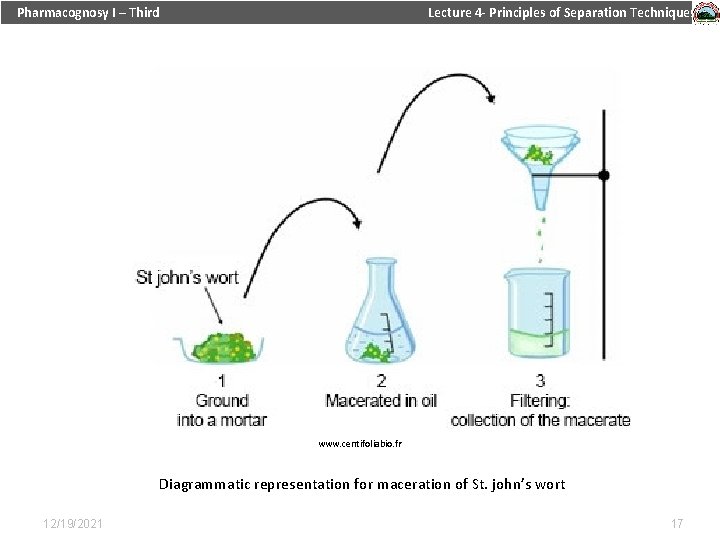
Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Method of extraction of NP… Maceration… When the crude plant material is displaced (soaked) in a solvent for a certain period of time to allow softening of the cellular material and dissolving its bioactive metabolites in the solvent. 12/19/2021 16

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques www. centifoliabio. fr Diagrammatic representation for maceration of St. john’s wort 12/19/2021 17

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Advantages… ü ü ü No heat or energy required. Easy. Simple. Applicable for a wide range of NP. No instrumentation required. Cheap. 12/19/2021 18

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Disadvantages… ü Longer time required. ü The amount of the extracted material is usually less when compared to other methods. ü Not applicable for crude materials with hard tissues. 12/19/2021 19

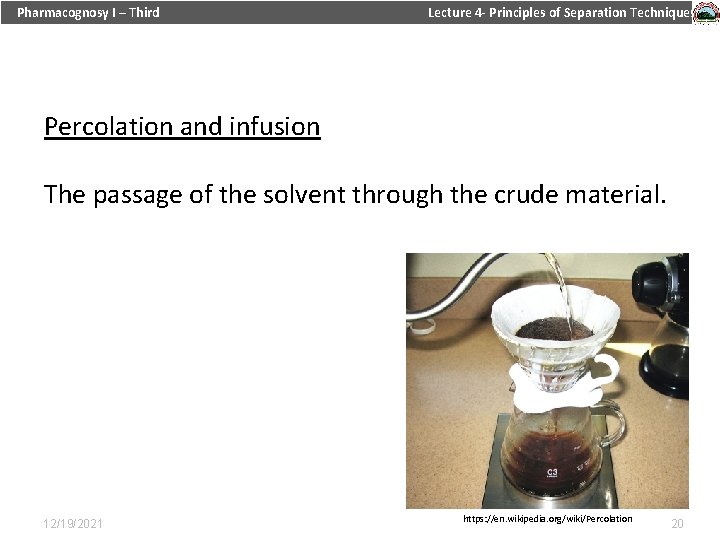
Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Percolation and infusion The passage of the solvent through the crude material. 12/19/2021 https: //en. wikipedia. org/wiki/Percolation 20

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Advantages… ü ü Easy and quick method. Cheap. Convenient for polar (water soluble) NP. Safe method. 12/19/2021 21

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Disadvantages… ü Minimum contact between the solute and the solvent. ü Not convenient for crude drugs that are rich with fibers. ü Not convenient for organic solvents. 12/19/2021 22

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Extraction by sonication… Is the use of Ultrasonic source as an aid for extraction. The U/S waves with cause cellular lysis (disruption) and softening which in turn facilitates the movement of the solvent through the crude plant material. 12/19/2021 23

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Extraction by sonication… A source of Ultrasound is needed. Ultrasonic extraction from herbs https: //www. hielscher. com/extraction_01. htm#48230 12/19/2021 24

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques When sonicating liquids at high intensities, the sound waves that propagate into the liquid media result in alternating highpressure (compression) and low-pressure (rarefaction) cycles, with rates depending on the frequency. During the low-pressure cycle, high-intensity ultrasonic waves create small vacuum bubbles or voids in the liquid. When the bubbles attain a volume at which they can no longer absorb energy, they collapse violently during a high-pressure cycle. This phenomenon is termed cavitation. 12/19/2021 25

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques During the implosion very high temperatures (approx. 5, 000 K) and pressures (approx. 2, 000 atm) are reached locally. The implosion of the cavitation bubble also results in liquid jets of up to 280 m/s velocity. The resulting shear forces break the cell envelope mechanically and improve material transfer. Ultrasound can have either destructive or constructive effects to cells depending on the sonication parameters employed. 12/19/2021 26

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques U/S can lead to a 1 permeabilization of cell membranes to ions, and it can 2 reduce the selectivity of the cell membranes significantly. 3 The mechanical activity of the ultrasound supports the diffusion of solvents into the tissue. 12/19/2021 27

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques As ultrasound breaks the cell wall mechanically by the cavitation shear forces, it facilitates the transfer from the cell into the solvent. The particle size reduction by the ultrasonic cavitation 4 increases the surface area in contact between the solid and the liquid phase. This process is also 5 economic and 6 environmental friendly. 12/19/2021 28

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Synergies of Ultrasound with Temperature and Pressure Ultrasonication is often more effective when combined with other anti-microbial methods, such as: thermo-sonication, i. e. heat and ultrasound mano-sonication, i. e. pressure and ultrasound mano-thermo-sonication, i. e. pressure, heat and ultrasound. 12/19/2021 29

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Disadvantages… ü Not convenient for glycosides. ü Not suitable for compounds that are affected by increase of energy of the medium (ex. Compounds with double bonds). ü High energy may cause inter- and intramolecular bonding. 12/19/2021 30

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Conclusion… In sonication, there should be a control over the intensity of the energy provided and the time of the extraction to get maximum amount of the intact solute. 12/19/2021 31

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Supercritical fluid extraction (SFE)… Supercritical Fluid Extraction (SFE) is the process of separating one component (the extractant) from another (the matrix) using supercritical fluids as the extracting solvent. Extraction is usually from a solid matrix, but can also be from liquids. SFE can be used as a sample preparation step for analytical purposes, or on a larger scale to either strip unwanted material from a product (e. g. decaffeination) or collect a desired product (e. g. essential oils). These essential oils can include limonene and other straight solvents. Carbon dioxide (CO 2) is the most used supercritical fluid, sometimes modified by co-solvents such as ethanol or methanol. Extraction conditions for supercritical carbon dioxide are above the critical temperature of 31 °C and critical pressure of 74 bar. Addition of modifiers may slightly alter this. The discussion below will mainly refer to extraction with CO 2, except where specified. 12/19/2021 32

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Supercritical fluid extraction (SFE)… A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid. In addition, close to the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be "fine-tuned". Supercritical fluids are suitable as a substitute for organic solvents in a range of industrial and laboratory processes. Carbon dioxide and water are the most commonly used supercritical fluids, being used for decaffeination and power generation, respectively. 12/19/2021 33

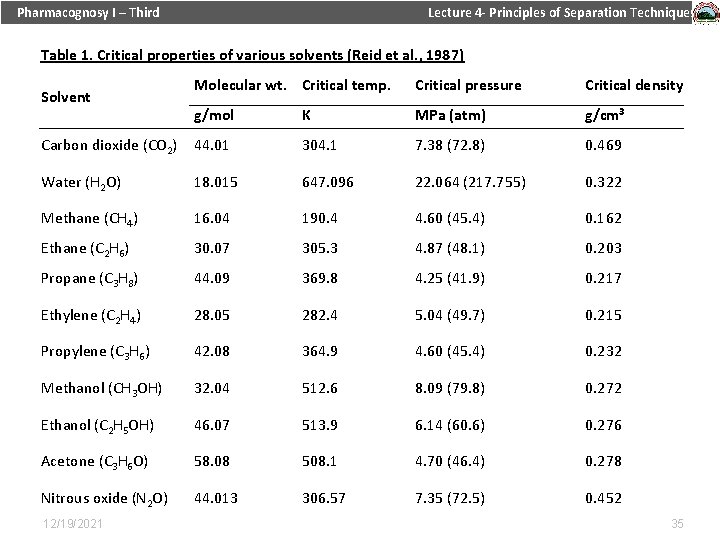
Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques In general terms, supercritical fluids have properties Table 1. Critical properties of various solvents (Reid et al. , 1987) between those of a gas and a liquid. In Table 1, the critical properties are shown for some components, which are commonly used as supercritical fluids. 12/19/2021 34

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Table 1. Critical properties of various solvents (Reid et al. , 1987) Molecular wt. Critical temp. Critical pressure Critical density g/mol K MPa (atm) g/cm 3 Carbon dioxide (CO 2) 44. 01 304. 1 7. 38 (72. 8) 0. 469 Water (H 2 O) 18. 015 647. 096 22. 064 (217. 755) 0. 322 Methane (CH 4) 16. 04 190. 4 4. 60 (45. 4) 0. 162 Ethane (C 2 H 6) 30. 07 305. 3 4. 87 (48. 1) 0. 203 Propane (C 3 H 8) 44. 09 369. 8 4. 25 (41. 9) 0. 217 Ethylene (C 2 H 4) 28. 05 282. 4 5. 04 (49. 7) 0. 215 Propylene (C 3 H 6) 42. 08 364. 9 4. 60 (45. 4) 0. 232 Methanol (CH 3 OH) 32. 04 512. 6 8. 09 (79. 8) 0. 272 Ethanol (C 2 H 5 OH) 46. 07 513. 9 6. 14 (60. 6) 0. 276 Acetone (C 3 H 6 O) 58. 08 508. 1 4. 70 (46. 4) 0. 278 Nitrous oxide (N 2 O) 44. 013 306. 57 7. 35 (72. 5) 0. 452 Solvent 12/19/2021 35

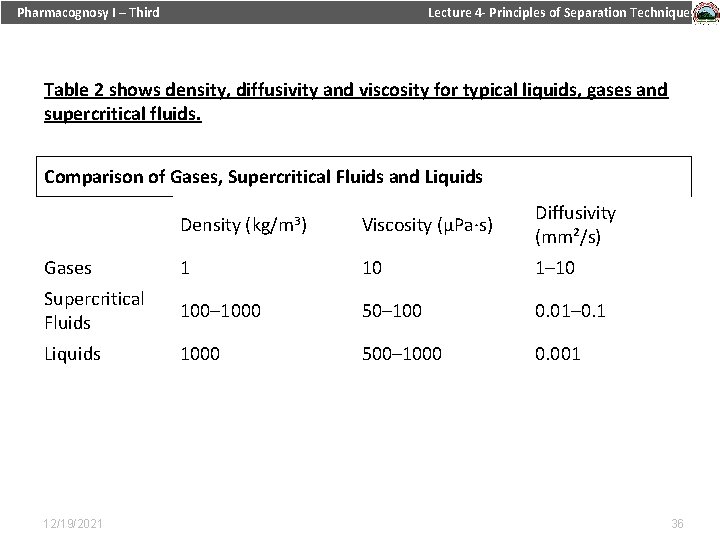
Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Table 2 shows density, diffusivity and viscosity for typical liquids, gases and supercritical fluids. Table 1. Critical properties of various solvents (Reid et al. , 1987) Comparison of Gases, Supercritical Fluids and Liquids Density (kg/m 3) Viscosity (µPa∙s) Diffusivity (mm²/s) Gases 1 10 1– 10 Supercritical Fluids 100– 1000 50– 100 0. 01– 0. 1 Liquids 1000 500– 1000 0. 001 12/19/2021 36

Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques In addition, there is no surface tension in a supercritical fluid, as there is no liquid/gas phase boundary. By changing the pressure and temperature of the fluid, the properties can be "tuned" to be more liquid- or more gas-like. One of the most important properties the solubility of material in the fluid. Table 1. Criticalisproperties of various solvents (Reid et al. , 1987) Solubility in a supercritical fluid tends to increase with density of the fluid (at constant temperature). Since density increases with pressure, solubility tends to increase with pressure. The relationship with temperature is a little more complicated. At constant density, solubility will increase with temperature. However, close to the critical point, the density can drop sharply with a slight increase in temperature. Therefore, close to the critical temperature, solubility often drops with increasing temperature, then rises again. [3] All supercritical fluids are completely miscible with each other so for a mixture a single phase can be guaranteed if the critical point of the mixture is exceeded 12/19/2021 37

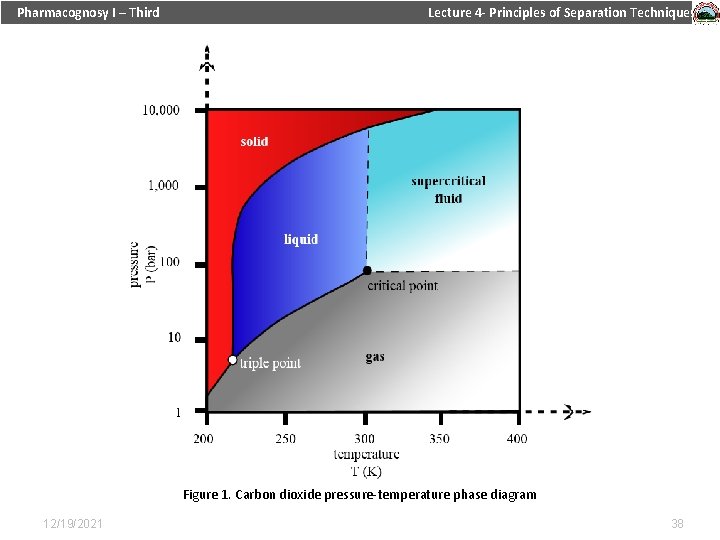
Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Table 1. Critical properties of various solvents (Reid et al. , 1987) Figure 1. Carbon dioxide pressure-temperature phase diagram 12/19/2021 38
![Pharmacognosy I Third Lecture 4 Principles of Separation Techniques Advantagesedit Selectivityedit Table Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Advantages[edit] Selectivity[edit] Table](https://slidetodoc.com/presentation_image_h2/1d686c3a25cc938703dd075aaedfb842/image-39.jpg)
Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Advantages[edit] Selectivity[edit] Table 1. Critical properties of various solvents (Reid et al. , 1987) The properties of a supercritical fluid can be altered by varying the pressure and temperature, allowing selective extraction. For example, volatile oils can be extracted from a plant with low pressures (100 bar), whereas liquid extraction would also remove lipids. Lipids can be removed using pure CO 2 at higher pressures, and then phospholipids can be removed by adding ethanol to the solvent. [1] The same principle can be used to extract polyphenols and unsaturated fatty acids separately from wine wastes. [2] Speed[edit] Extraction is a diffusion-based process, in which the solvent is required to diffuse into the matrix and the extracted material to diffuse out of the matrix into the solvent. Diffusivities are much faster in supercritical fluids than in liquids, and therefore extraction can occur faster. In addition, due to the lack of surface tension and negligible viscosities compared to liquids, the solvent can penetrate more into the matrix inaccessible to liquids. An extraction using an organic liquid may take several hours, whereas supercritical fluid extraction can be completed in 10 to 60 minutes. [3] 12/19/2021 39
![Pharmacognosy I Third Lecture 4 Principles of Separation Techniques Limitationsedit Table Critical Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Limitations[edit] Table Critical](https://slidetodoc.com/presentation_image_h2/1d686c3a25cc938703dd075aaedfb842/image-40.jpg)
Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Limitations[edit] Table Critical increases properties various solvents (Reid et al. , liquid 1987) The requirement for high 1. pressures theofcost compared to conventional extraction, so SFE will only be used where there are significant advantages. Carbon dioxide itself is non-polar, and has somewhat limited dissolving power, so cannot always be used as a solvent on its own, particularly for polar solutes. The use of modifiers increases the range of materials which can be extracted. Food grade modifiers such as ethanol can often be used, and can also help in the collection of the extracted material, but reduces some of the benefits of using a solvent which is gaseous at room temperature. 12/19/2021 40

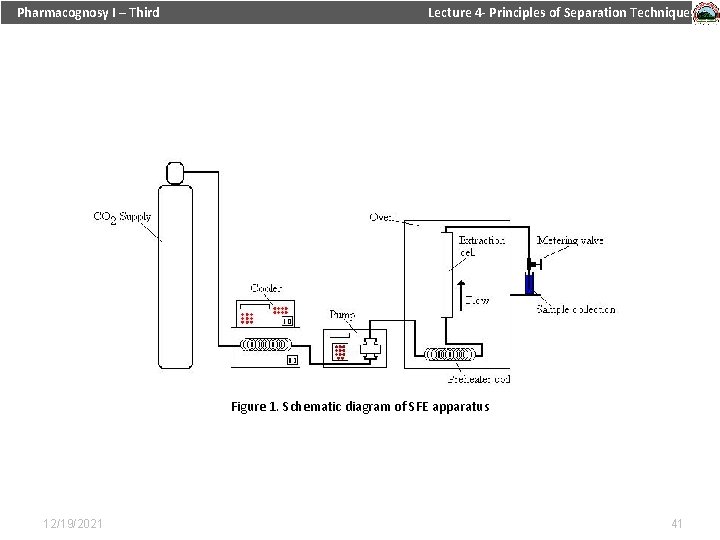
Pharmacognosy I – Third Lecture 4 - Principles of Separation Techniques Table 1. Critical properties of various solvents (Reid et al. , 1987) Figure 1. Schematic diagram of SFE apparatus 12/19/2021 41

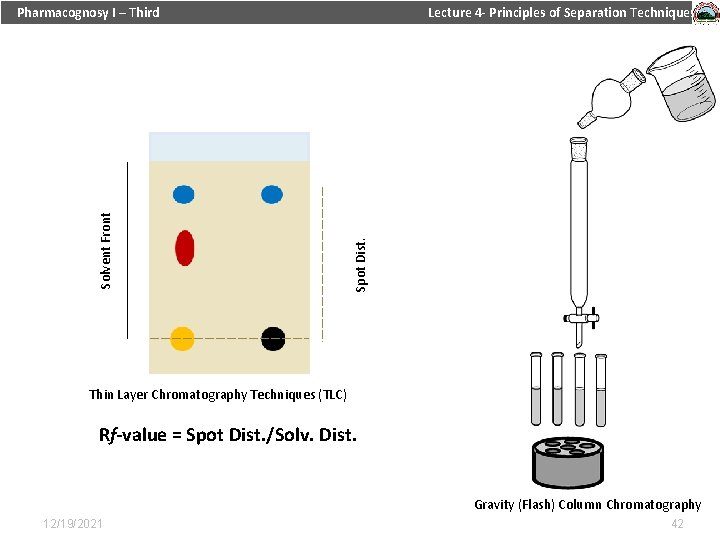
Lecture 4 - Principles of Separation Techniques Spot Dist. Solvent Front Pharmacognosy I – Third Thin Layer Chromatography Techniques (TLC) Rf-value = Spot Dist. /Solv. Dist. Gravity (Flash) Column Chromatography 12/19/2021 42
 Scope of pharmacognosy definition
Scope of pharmacognosy definition Application of pharmacognosy
Application of pharmacognosy 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Forward and backward caries
Forward and backward caries Odontoclasia definition
Odontoclasia definition Principles of economics powerpoint lecture slides
Principles of economics powerpoint lecture slides Principles of lecture method
Principles of lecture method Principles of economics third edition oxford pdf
Principles of economics third edition oxford pdf Unity meaning in art
Unity meaning in art Madar kabab chini tagar are example of
Madar kabab chini tagar are example of Aprophine
Aprophine Pharmacognosy alkaloids
Pharmacognosy alkaloids Differentiate between organised and unorganised drug
Differentiate between organised and unorganised drug Serpentiana
Serpentiana Couramin
Couramin Vein islet number example
Vein islet number example Advantages of alphabetical classification
Advantages of alphabetical classification Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Ketone volatile oils
Ketone volatile oils Uses of volatile oil in pharmacognosy
Uses of volatile oil in pharmacognosy Edible vaccines in pharmacognosy
Edible vaccines in pharmacognosy Tannins in pharmacognosy
Tannins in pharmacognosy What is ash value in pharmacognosy
What is ash value in pharmacognosy Menstruum pharmacognosy
Menstruum pharmacognosy Sterculia gum pharmacognosy
Sterculia gum pharmacognosy Isovaler synonym
Isovaler synonym Applications of plant tissue culture in pharmacognosy
Applications of plant tissue culture in pharmacognosy Types of adulteration in pharmacognosy
Types of adulteration in pharmacognosy Explant is
Explant is Applications of plant tissue culture
Applications of plant tissue culture What is the supreme ordeal in the odyssey
What is the supreme ordeal in the odyssey 12 degrees of kevin bacon
12 degrees of kevin bacon Software design separation of concerns
Software design separation of concerns Size separation define
Size separation define Separation techniques exam questions
Separation techniques exam questions 2021 involuntary separation pay chart
2021 involuntary separation pay chart Aristotle separation of powers
Aristotle separation of powers Separation of powers and checks and balances
Separation of powers and checks and balances Ternary mixture separation
Ternary mixture separation Evaporation mixtures examples
Evaporation mixtures examples The separation of some personality components
The separation of some personality components Pen lifts and separation
Pen lifts and separation Separation by phase creation
Separation by phase creation