SEPARATION POWER The degree of separation which can
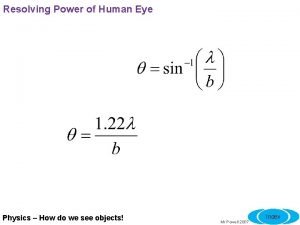
- Slides: 13

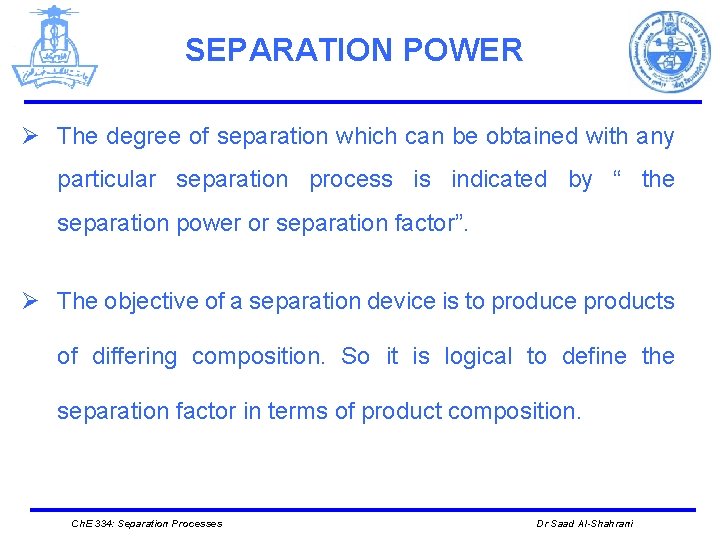
SEPARATION POWER Ø The degree of separation which can be obtained with any particular separation process is indicated by “ the separation power or separation factor”. Ø The objective of a separation device is to produce products of differing composition. So it is logical to define the separation factor in terms of product composition. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

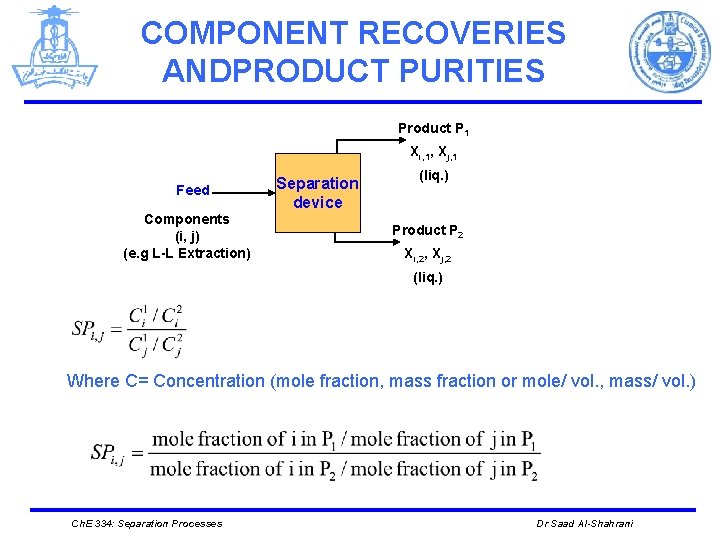
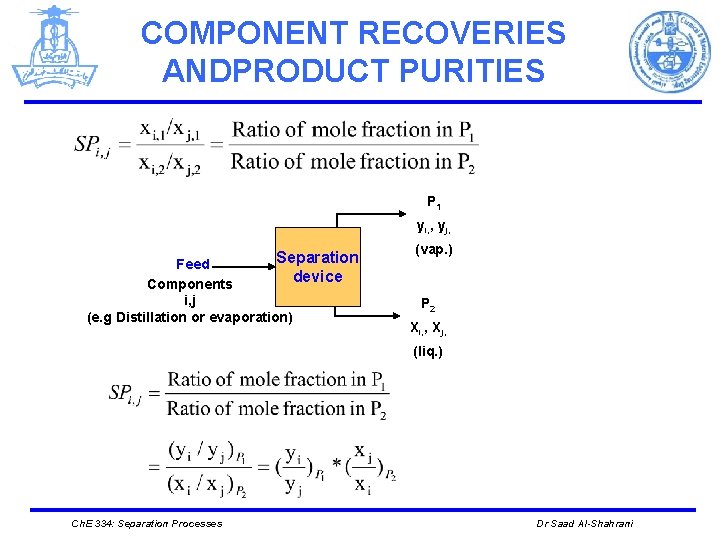
COMPONENT RECOVERIES ANDPRODUCT PURITIES Product P 1 Xi, 1, Xj, 1 Feed Components (i, j) (e. g L-L Extraction) Separation device (liq. ) Product P 2 Xi, 2, Xj, 2 (liq. ) Where C= Concentration (mole fraction, mass fraction or mole/ vol. , mass/ vol. ) Ch. E 334: Separation Processes Dr Saad Al-Shahrani

COMPONENT RECOVERIES ANDPRODUCT PURITIES P 1 yi, , yj, Separation device Feed Components i, j (e. g Distillation or evaporation) (vap. ) P 2 Xi, , Xj, (liq. ) Ch. E 334: Separation Processes Dr Saad Al-Shahrani

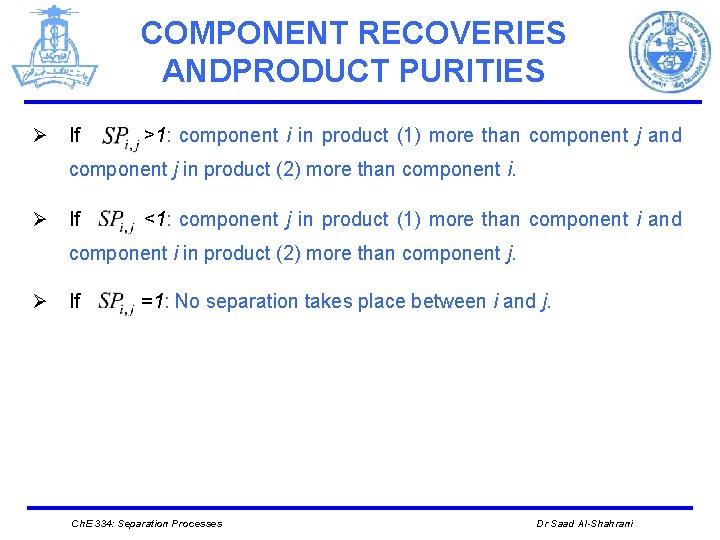
COMPONENT RECOVERIES ANDPRODUCT PURITIES Ø If >1: component i in product (1) more than component j and component j in product (2) more than component i. Ø If <1: component j in product (1) more than component i and component i in product (2) more than component j. Ø If =1: No separation takes place between i and j. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

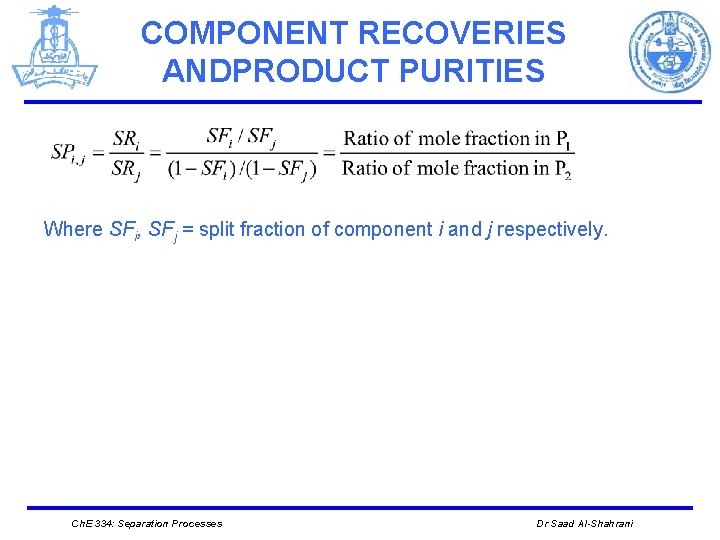
COMPONENT RECOVERIES ANDPRODUCT PURITIES Where SFi, SFj = split fraction of component i and j respectively. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

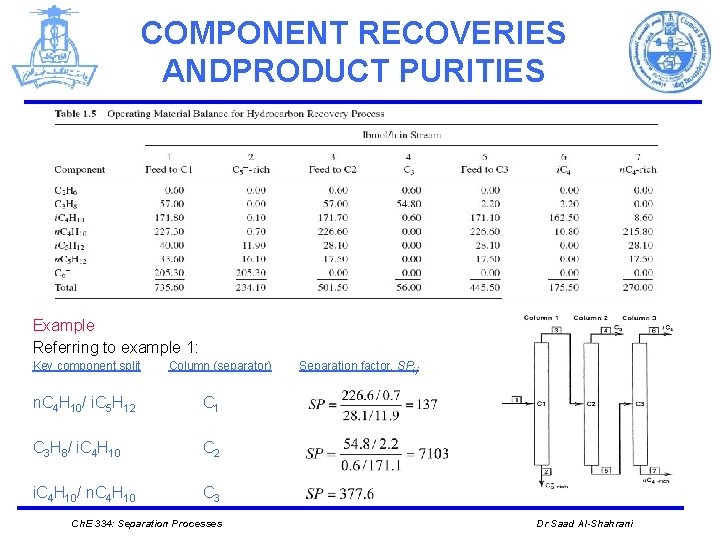
COMPONENT RECOVERIES ANDPRODUCT PURITIES Example Referring to example 1: Key component split Column (separator) n. C 4 H 10/ i. C 5 H 12 C 1 C 3 H 8/ i. C 4 H 10 C 2 i. C 4 H 10/ n. C 4 H 10 C 3 Ch. E 334: Separation Processes Separation factor, SPi, j Dr Saad Al-Shahrani

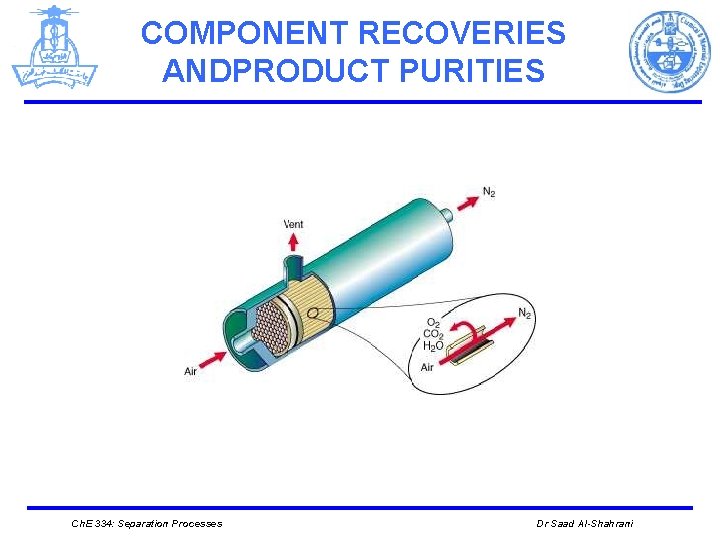
COMPONENT RECOVERIES ANDPRODUCT PURITIES Example: A feed, F, of 100 kmol/h of air containing 21 mol% O 2 and 79 mol% N 2 is to be partially separated by a membrane unit according to each of the following four sets of specifications. For each case, compute the amounts, in kmol/h, and compositions, in kmol%, of the two products (retentate, R, and permeate, P ). The membrane is more permeable to the oxygen. Case 1: 50% recovery of O 2 to the permeate and 87. 5% recovery of N 2 to the retentate. Case 2: 50% recovery of O 2 to the permeate and 50 mol% purity of O 2 in the permeate. Case 3: 85 mol% purity of N 2 in the retentate and 50 mol% purity of O 2 in the permeate. Case 4: 85 mol% purity of N 2 in the retentate and a split ratio of O 2 in the permeate to the retentate equal to 1. 1. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

COMPONENT RECOVERIES ANDPRODUCT PURITIES Ch. E 334: Separation Processes Dr Saad Al-Shahrani

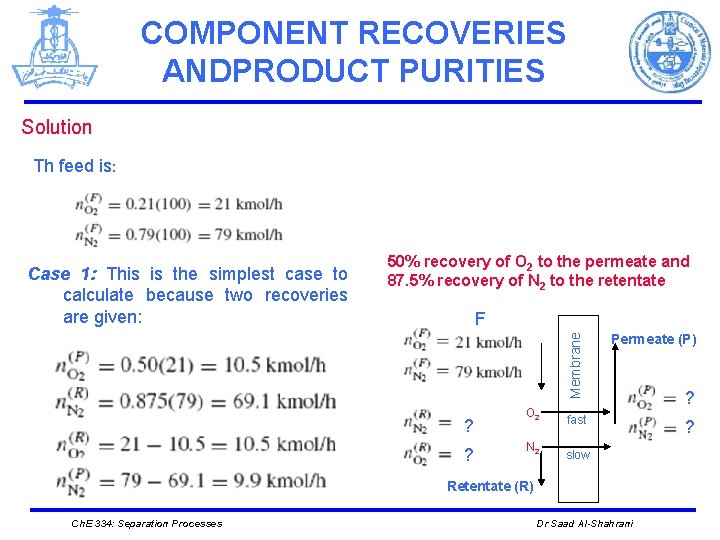
COMPONENT RECOVERIES ANDPRODUCT PURITIES Solution Th feed is: 50% recovery of O 2 to the permeate and 87. 5% recovery of N 2 to the retentate F ? ? O 2 N 2 Membrane Case 1: This is the simplest case to calculate because two recoveries are given: Permeate (P) fast ? slow Retentate (R) Ch. E 334: Separation Processes Dr Saad Al-Shahrani ?

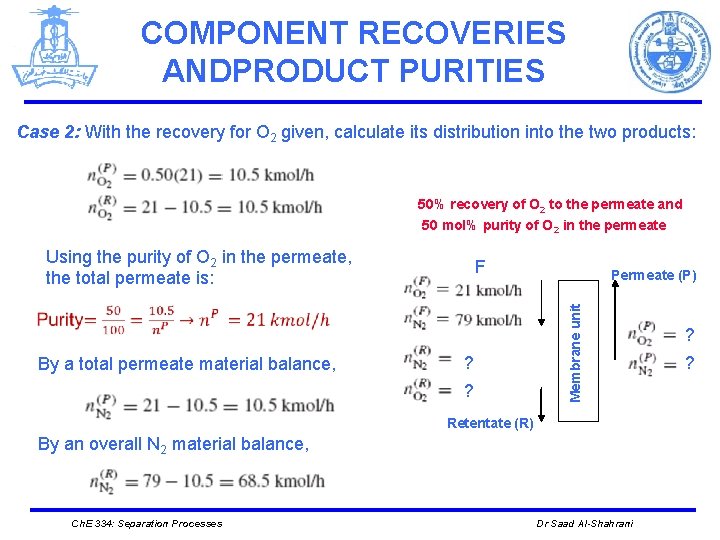
COMPONENT RECOVERIES ANDPRODUCT PURITIES Case 2: With the recovery for O 2 given, calculate its distribution into the two products: 50% recovery of O 2 to the permeate and 50 mol% purity of O 2 in the permeate Using the purity of O 2 in the permeate, the total permeate is: F By a total permeate material balance, ? ? Membrane unit Permeate (P) Retentate (R) By an overall N 2 material balance, Ch. E 334: Separation Processes Dr Saad Al-Shahrani ? ?

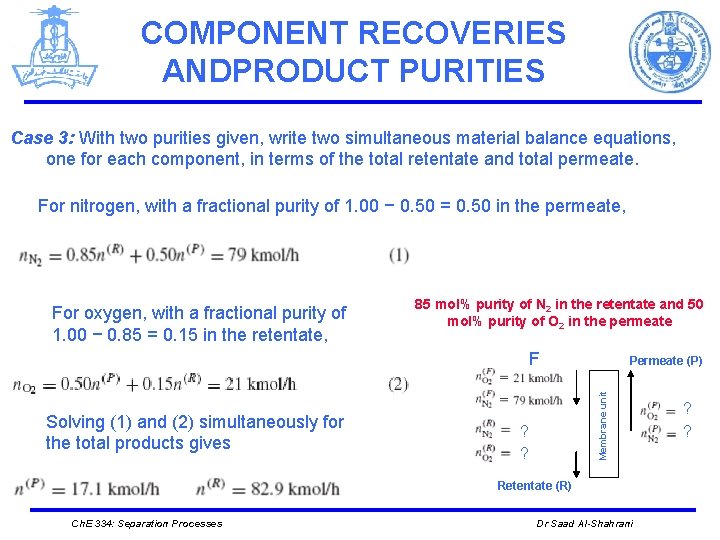
COMPONENT RECOVERIES ANDPRODUCT PURITIES Case 3: With two purities given, write two simultaneous material balance equations, one for each component, in terms of the total retentate and total permeate. For nitrogen, with a fractional purity of 1. 00 − 0. 50 = 0. 50 in the permeate, For oxygen, with a fractional purity of 1. 00 − 0. 85 = 0. 15 in the retentate, 85 mol% purity of N 2 in the retentate and 50 mol% purity of O 2 in the permeate F Membrane unit Solving (1) and (2) simultaneously for the total products gives Permeate (P) ? ? Retentate (R) Ch. E 334: Separation Processes Dr Saad Al-Shahrani ? ?

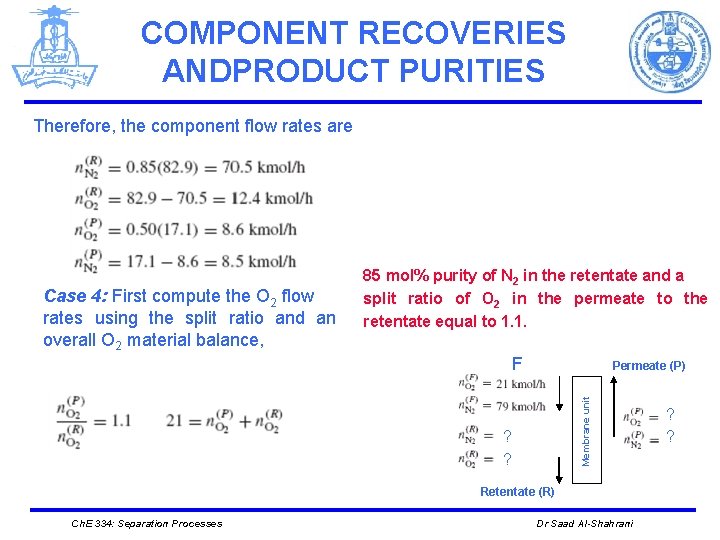
COMPONENT RECOVERIES ANDPRODUCT PURITIES Therefore, the component flow rates are Case 4: First compute the O 2 flow rates using the split ratio and an overall O 2 material balance, 85 mol% purity of N 2 in the retentate and a split ratio of O 2 in the permeate to the retentate equal to 1. 1. F Membrane unit Permeate (P) ? ? Retentate (R) Ch. E 334: Separation Processes Dr Saad Al-Shahrani ? ?

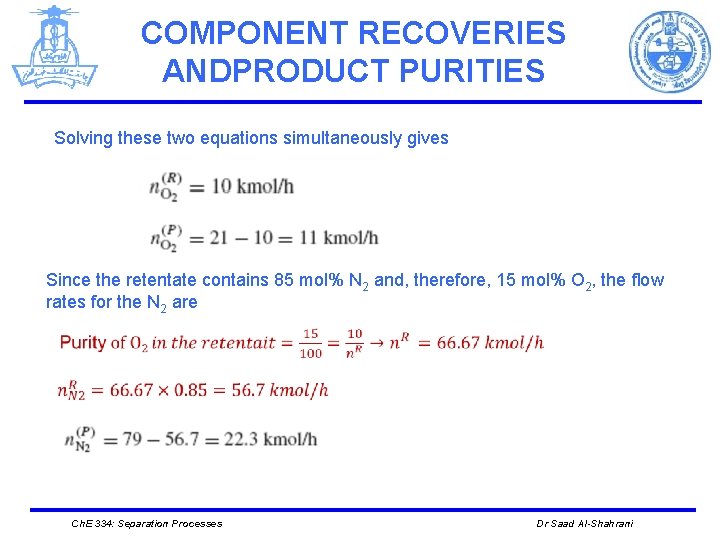
COMPONENT RECOVERIES ANDPRODUCT PURITIES Solving these two equations simultaneously gives Since the retentate contains 85 mol% N 2 and, therefore, 15 mol% O 2, the flow rates for the N 2 are Ch. E 334: Separation Processes Dr Saad Al-Shahrani