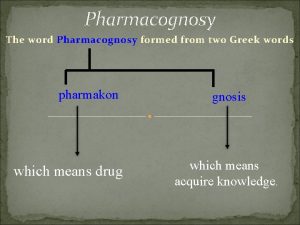
Silpa M Assistant professor Dept of Pharmacognosy Yenepoya











































- Slides: 77

Silpa M Assistant professor Dept. of Pharmacognosy Yenepoya pharmacy college & research centre

CONTENTS: Definition Historical development of plant tissue culture Types of cultures Nutrient’s requirement growth and their maintenance Applications of plant tissue culture in pharmacognosy. Edible vaccines 2

PLANT TISSUE CULTURE Definition Tissue culture is in vitro cultivation of plant cell or tissue under aseptic and controlled environmental conditions, in liquid or on semisolid well defined nutrient medium for the production of primary and secondary metabolites or to regenerate plant. Tissue culture relies on three fundamental abilities of plant there are: Totipotency Dedifferentiation competency 3

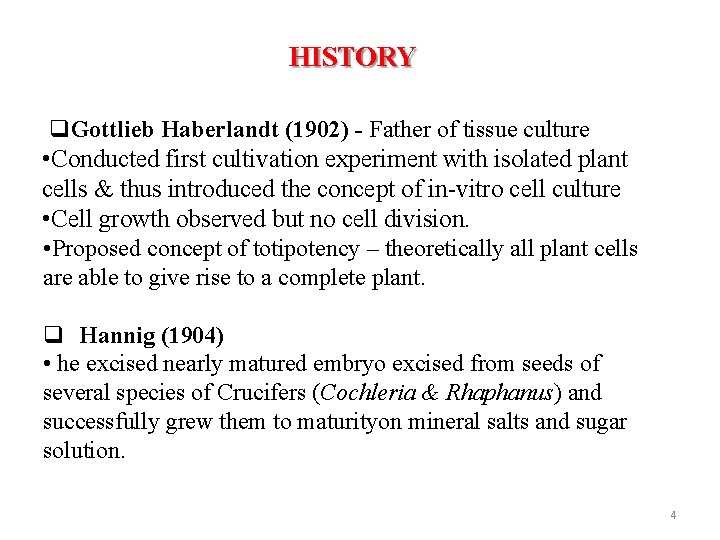
HISTORY Gottlieb Haberlandt (1902) - Father of tissue culture • Conducted first cultivation experiment with isolated plant cells & thus introduced the concept of in-vitro cell culture • Cell growth observed but no cell division. • Proposed concept of totipotency – theoretically all plant cells are able to give rise to a complete plant. Hannig (1904) • he excised nearly matured embryo excised from seeds of several species of Crucifers (Cochleria & Rhaphanus) and successfully grew them to maturityon mineral salts and sugar solution. 4

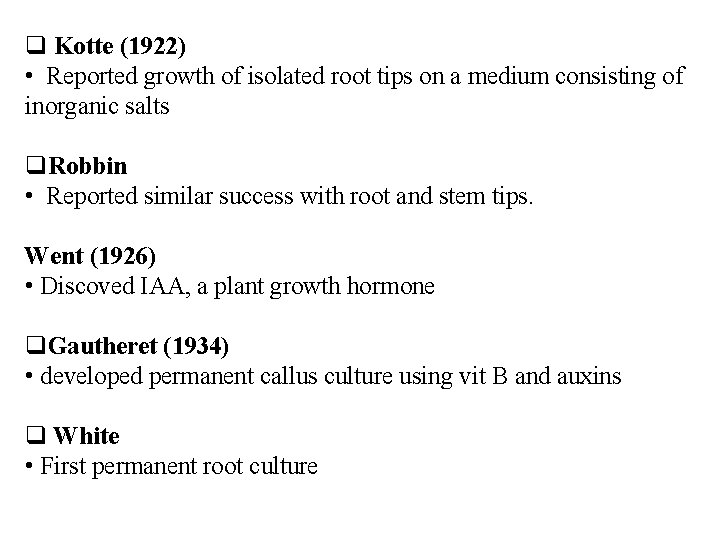
Kotte (1922) • Reported growth of isolated root tips on a medium consisting of inorganic salts Robbin • Reported similar success with root and stem tips. Went (1926) • Discoved IAA, a plant growth hormone Gautheret (1934) • developed permanent callus culture using vit B and auxins White • First permanent root culture

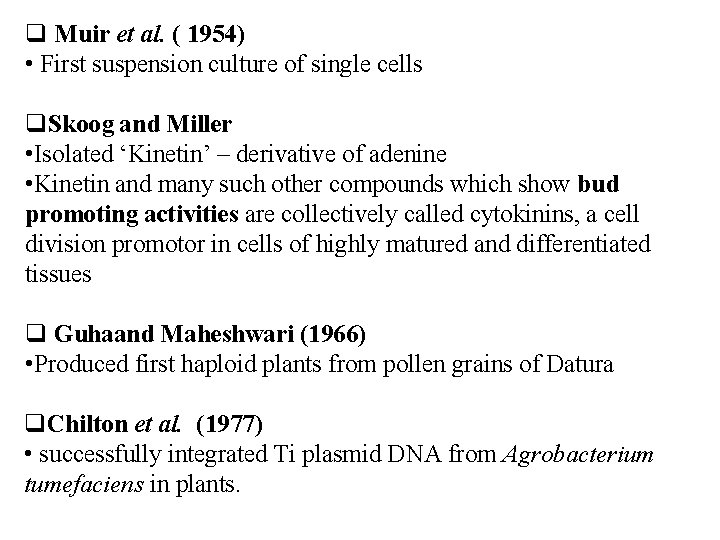
Muir et al. ( 1954) • First suspension culture of single cells Skoog and Miller • Isolated ‘Kinetin’ – derivative of adenine • Kinetin and many such other compounds which show bud promoting activities are collectively called cytokinins, a cell division promotor in cells of highly matured and differentiated tissues Guhaand Maheshwari (1966) • Produced first haploid plants from pollen grains of Datura Chilton et al. (1977) • successfully integrated Ti plasmid DNA from Agrobacterium tumefaciens in plants.

1984 • transformation of tobacco with Agrobacterium was accomplished to develop transgenic plants 1994 • Introduced genetically engineered Flavr. Savr tomato 1998 • About 30% of the US cotton and corn crops become products of genetic engineering 2002 • Researchers sequence the DNA of Rice is the first crop to have it genome decoded

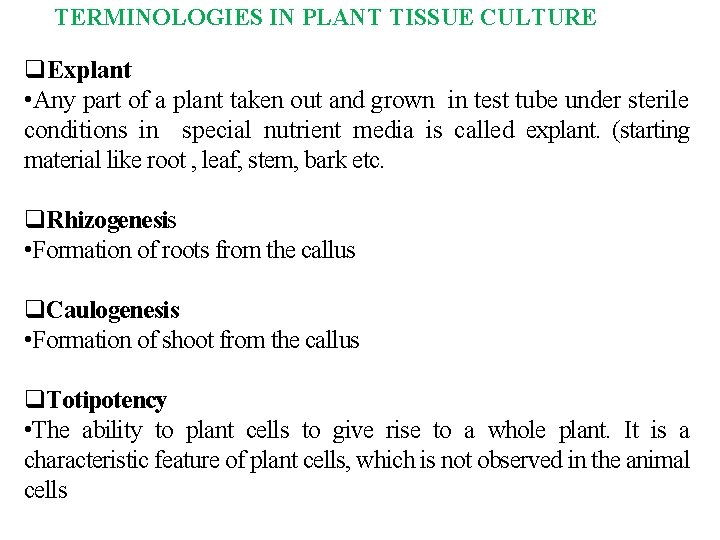
TERMINOLOGIES IN PLANT TISSUE CULTURE Explant • Any part of a plant taken out and grown in test tube under sterile conditions in special nutrient media is called explant. (starting material like root , leaf, stem, bark etc. Rhizogenesis • Formation of roots from the callus Caulogenesis • Formation of shoot from the callus Totipotency • The ability to plant cells to give rise to a whole plant. It is a characteristic feature of plant cells, which is not observed in the animal cells

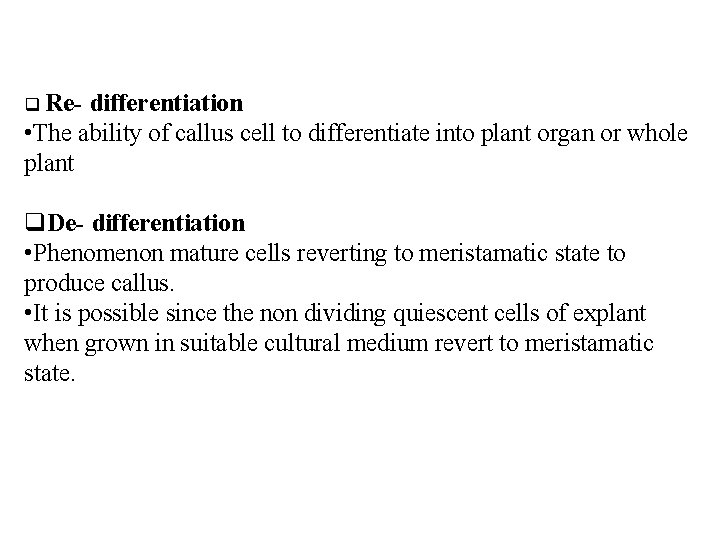
Re- differentiation • The ability of callus cell to differentiate into plant organ or whole plant De- differentiation • Phenomenon mature cells reverting to meristamatic state to produce callus. • It is possible since the non dividing quiescent cells of explant when grown in suitable cultural medium revert to meristamatic state.

EQUIPMENT & APPARATUS VESSELS & GLASS WARE : • All the glassware should be of Pyrex. • Large test tubes, flasks, graduated pipettes etc. . are used. EQUIPMENT : • • Scissors, scapels, foreceps are used for explants preparation. A spirit burner for flame sterilization. Hot air oven. A PH meter. A BOD incubator. Laminar air flow chamber. A balance to weigh nutrients. Data collection and recording room. 10

ESTABLISHMENT OF PLANT TISSUE CULTURE In vitro culturing of plant tissue culture involves the following steps. Collecting & sterilization of glassware tools/vessels. Preparation of explant. Surface sterilization of Explant. Production of callus from explant. Proliferation of culture. Sub culturing of callus. Suspension culture 11

NUTRIENT’S REQUIREMENT 1 2

Inorganic supplements : Sodium, potassium , ammonia Growth regulators : Auxins, cytokinins Other micro nutrients : Zinc, molybdenum , iron, boric acid carbon sources: glucose , sucrose others like liquid endosperm, protein hydrolyse, yeast extract Water : Demineralized or distilled water Solidifying agents : Agar, gelatin. p. H adjusters : 5 - 6 it is considered to be optimum. 1 3

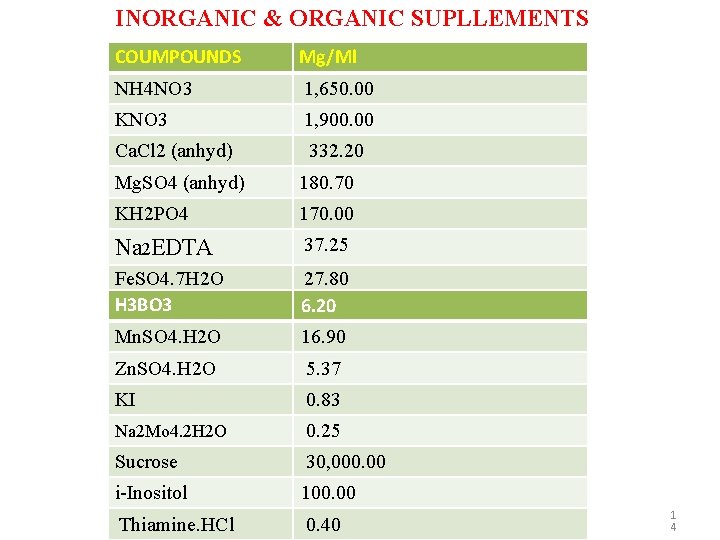
INORGANIC & ORGANIC SUPLLEMENTS COUMPOUNDS Mg/Ml NH 4 NO 3 1, 650. 00 KNO 3 1, 900. 00 Ca. Cl 2 (anhyd) 332. 20 Mg. SO 4 (anhyd) 180. 70 KH 2 PO 4 170. 00 Na 2 EDTA 37. 25 Fe. SO 4. 7 H 2 O H 3 BO 3 27. 80 6. 20 Mn. SO 4. H 2 O 16. 90 Zn. SO 4. H 2 O 5. 37 KI 0. 83 Na 2 Mo 4. 2 H 2 O 0. 25 Sucrose 30, 000. 00 i-Inositol 100. 00 Thiamine. HCl 0. 40 1 4

PREPARATION OF CULTURE MEDIA: Stock solution 1 : Mg. So 4, KH 2 Po 4, KNO 3, NH 4 No 3, Ca. Cl 2 Stock solution 2 : H 3 Bo 3, Mn. So 4, Zn. So 4, Cu. So 4, Cocl 2 Stock solution 3 : Fe. So 4, sodium EDTA Stock solution 4 : ionositol, thiamine, pyridamine, nicotinic acid, glycine To prepare 1 liter of medium: Take 50 ml of stock solution 1 + 5 ml of stock solution 2 & 4 in a beaker. The stock solution 3 prepared separately in a other 450 ml flask by adding double distilled water and heat with constant stirring. Mix two solutions and adjust PH to 5. 5. 1 5

BASIC REQUIREMENT FOR A TISSUE CULTURE LABORATORY For the successful achievement, the following general basic facilities are required: Equipment & apparatus Washing and storage facilities Media preparation room Sterilization room Aseptic chamber for culture Culture rooms or incubators fully equipped with temperature, light and humidity control devices Observation or recording area well equipped with computer for data processing 16

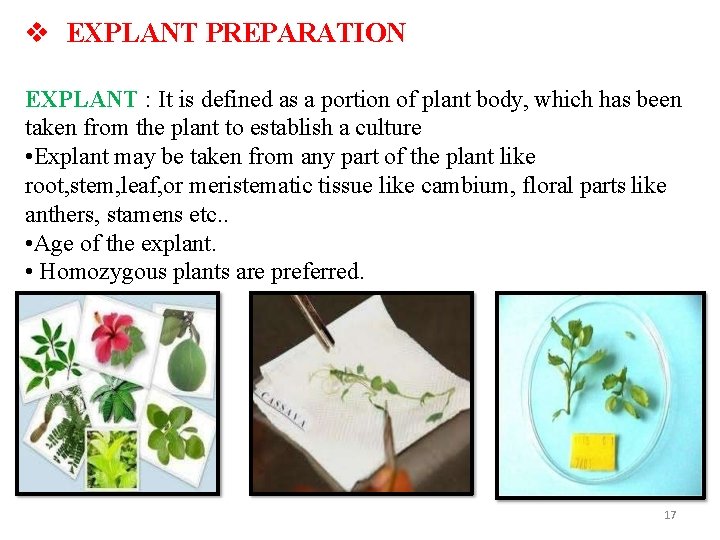
EXPLANT PREPARATION EXPLANT : It is defined as a portion of plant body, which has been taken from the plant to establish a culture • Explant may be taken from any part of the plant like root, stem, leaf, or meristematic tissue like cambium, floral parts like anthers, stamens etc. . • Age of the explant. • Homozygous plants are preferred. 17

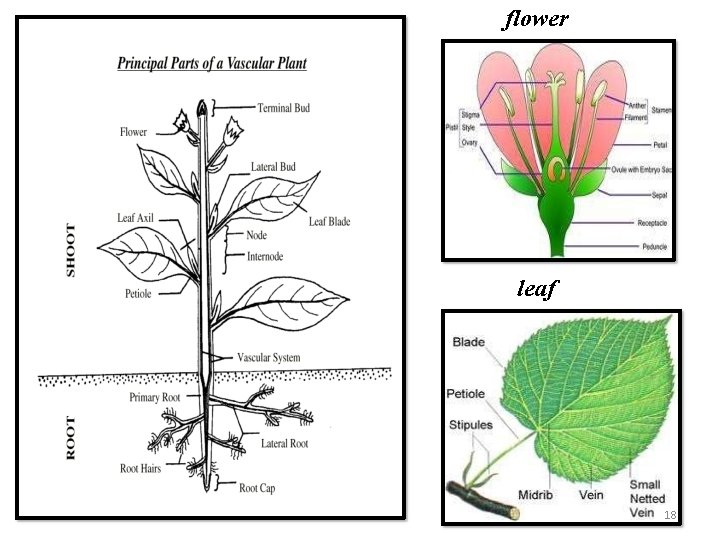
flower leaf 18

Aseptic Condition • Nutrient medium contains sugar which increases growth of microbes • These microbes compete with growing tissue and finally kill it. • It is important to maintain aseptic condition. • Sterilization is very important to stop the microbes. growth of • If sterile tissue are available, then exclusion of micro organisms is accomplished by using sterile instruments and culture media concurrently with standard bacteriological transfer procedures to avoid extraneous contamination

STERILISATION OF MEDIA • The prepared media should be sterilized by ISI mark Autoclave( for large amounts) at 121º Domestic pressure cookers( for small amounts) • For the sterilization of glassware and metallic equipments Hot air oven with adjustable tray is required. Incubator Hot air oven 9

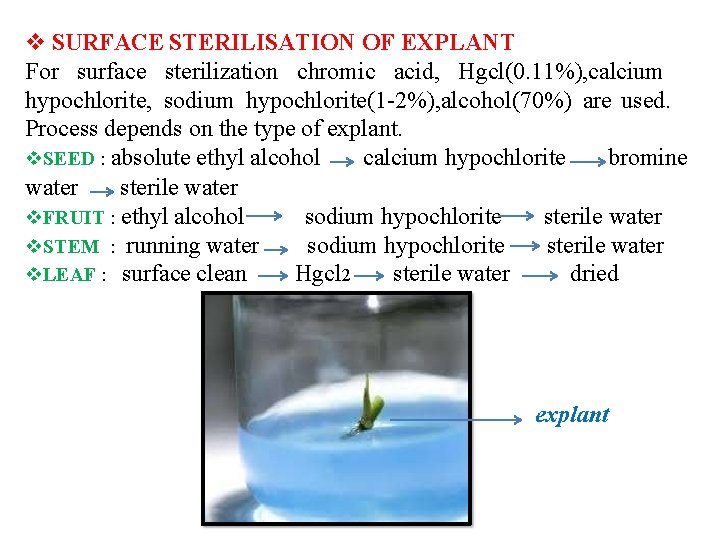
SURFACE STERILISATION OF EXPLANT For surface sterilization chromic acid, Hgcl(0. 11%), calcium hypochlorite, sodium hypochlorite(1 -2%), alcohol(70%) are used. Process depends on the type of explant. SEED : absolute ethyl alcohol calcium hypochlorite bromine water sterile water FRUIT : ethyl alcohol sodium hypochlorite sterile water STEM : running water sodium hypochlorite sterile water LEAF : surface clean Hgcl 2 sterile water dried explant

Plant material • Plant material used in plant tissue culture need to be healthy actively growing • Stressed plant especially water stressed plants, usually do not grow as tissue cultures. • Insects and diseases free are rendered aseptically more readily, so contamination rate is lowered when we used these types of plants. • Seeds which are easily surface sterilized usually produce contamination free plants that can be grown under greenhouse conditions for later experimental use.

Types of culture • Culture are generally initiated from sterile pieces of whole plant. These pieces are termed as ‘explants’, and may consist of pieces of organs, such as leaves or roots or may be specific cell type, such as pollen or endosperm. • Many features of the explants are known to affect the efficiency of culture initiation • Generally younger more rapidly growing tissues is more effective

TYPES OF CULTURE Callus culture • Cell – suspension culture Tissue or Organ Culture • Root tip culture • Leaf or leaf primordial culture • Shoot tip culture • Complete flower culture • Anther & pollen culture • Ovule & embryo culture • Microspore culture • Mass cell culture Protoplast culture 31

Callus Culture • In Callus culture, cell division in explant forms a callus. • Callus is irregular unorganized and undifferentiated mass of actively dividing cells. • Culture of differential tissue from an explant allowed to differentiate in vitro ; is known as a callus culture • Darkness & solid medium gelled by agar stimulates callus formation. • The medium contains the auxins and BAP (Benzyl amino purines). Both are growth regulators ( Hormones). • This stimulates cell division in explant.

PRODUCTION OF CALLUS FROM EXPLANT • • Sterilized explant is transferred aseptically onto defined medium. Transfer to BOD incubator. Temperature (25 2 ) and light is necessary for callus production. Callus produced with in 3 -8 days. 26

PROLIFERATION OF CULTURE • if callus is well developed, it should cut into small pieces & transferred to another fresh medium containing hormones, which supports growth. • The medium used for production of more amount of callus is called proliferation medium. 27

15 DAYS 30 DAYS CALLUS GROWTH 50 DAYS 80 DAYS 21

SUBCULTURING OF CALLUS • After sufficient growth of callus it should be periodically transferred to fresh medium to maintain viability of cells. • This subculture will be done at the interval of 4 -6 weeks. • After a maximum growth transfer into a pottling soil under required condition.

• During callus culture formation there is some degree of dedifferentiation , both in morphology and metabolism. • One major consequence of this dedifferentiation is that most plant culture is most plant cells lost to ability to photosynthesis • The metabolic profile will probably not match that of the donor plant • This necessitates the addition of other components – such as vitamin and most importantly a carbon source to the culture medium, in addition to the usual mineral nutrients

• Callus culture extremely important in plant biotechnology and they are in two categories 1. Compact callus – the cells are densely aggregated 2. Friable callus – the cells are only loosely associated with each other and callus become soft and break apart easily.

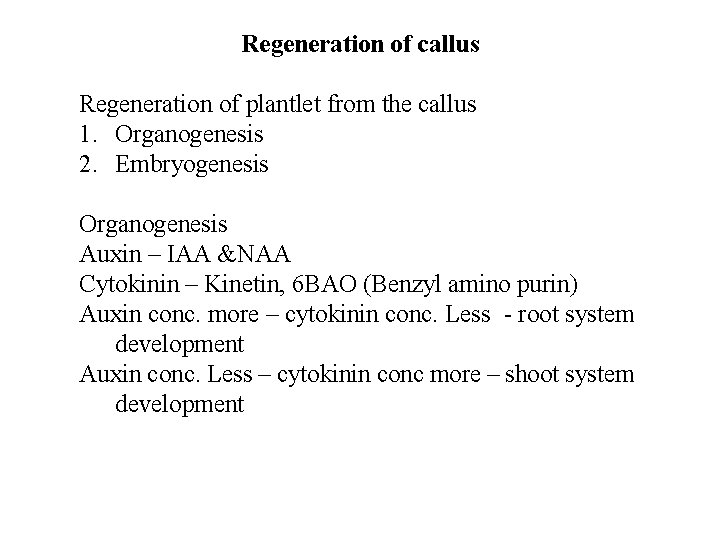
Regeneration of callus Regeneration of plantlet from the callus 1. Organogenesis 2. Embryogenesis Organogenesis Auxin – IAA &NAA Cytokinin – Kinetin, 6 BAO (Benzyl amino purin) Auxin conc. more – cytokinin conc. Less - root system development Auxin conc. Less – cytokinin conc more – shoot system development

Cell- suspension culture • The growth rate of the suspension cultured cells is generally higher than that of the solid culture.

SUSPENSION CULTURE • It contains a uniform suspension of separate cells in a liquid medium callus liquid medium agitated continuously finally cells separated sub-culture the cells • This can be achieved by rotary shaker attached within the incubator at a rate of 50 -150 rpm. • The time required to establish the cell suspension culture varies greatly and depends on the tissues of the plant species and medium 23 composition.

Different category of cell culture There 3 types of suspension culture 1. Batch culture : Here the suspension culture are maintained in a volume of agitated liquid with repeat sub culturing of a small aliquot of cell culture to fresh medium at regular intervals. Cell suspension are grown in flasks (100 -250 m. L) containing 20 -75 m. L of the medium incubated on orbital platform shakers at the speed of 8 -120 rpm

2. Continuous culture / mass culture The cell population is maintained in a steady state by regularly replacing a portion of the used or spent medium by fresh medium There are 2 type of continuous medium 1. Closed continuous medium 2. Open continuous medium Closed continuous medium • Cell separated from the used medium taken out for replacement and added back to the culture so that cell biomass keep on increasing.

Open continuous medium • Both the cells and used medium are taken out from the open continuous cultures and are replaced by equal volume of fresh medium. • Here the steady state of suspension culture is maintained

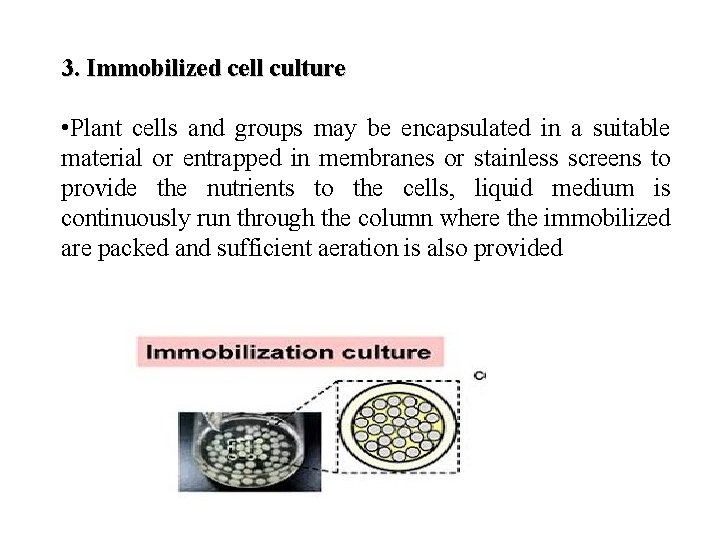
3. Immobilized cell culture • Plant cells and groups may be encapsulated in a suitable material or entrapped in membranes or stainless screens to provide the nutrients to the cells, liquid medium is continuously run through the column where the immobilized are packed and sufficient aeration is also provided

3. Protoplast culture • Culture of plant protoplasts i. e. cells devoid of their cell wall • Protoplasts are plant cells with the cell wall removed. • Protoplasts are most commonly isolated from either leaf mesophyll cell or suspensions, although other source can be used to advantage. • two general approaches to removing the cell wall ( a difficult task without the protoplast) can be taken – mechanical or enzymatic isolation. • Mechanical method: epidermis – plasmolysis – shrink away from cell wall - dissection

• Mechanical isolation also although possible, often results in low yields, poor quality and poor performance in culture due to substances released from damaged cells. • Enzymatic isolation is usually carried out in a simple salt solution with a high osmoticum, plus cell wall depredating enzymes. • It is usual to use a mix of both cellulose and pectinase enzymes, which must be high quality and purity. • Protoplast are fragile and easily damaged, and there for must be cultured carefully

• Liquid medium is not agitated and a high osmotic potential is maintained, at least in the initial stages. • The liquid medium must be shallow enough to allow aeration in the absence of agitation. • Protoplast can be plated out on to solid medium and callus produced • Whole plant can be regenerated by organogenesis or somatic embryogenesis from this callus.

Organ culture: • Culture of isolated plant organs such as root tips, shoot tips, embryo , meristem etc.

Root cultures • It can be established in – vitro from explants of the root tip of either primary or lateral roots and can be cultured on media. • The growth of root in vitro is potentially unlimited, as root are indeterminate organs. • This is the one of the first achievement of modern plant tissue culture. • They are not widely used in plant transformation studies.


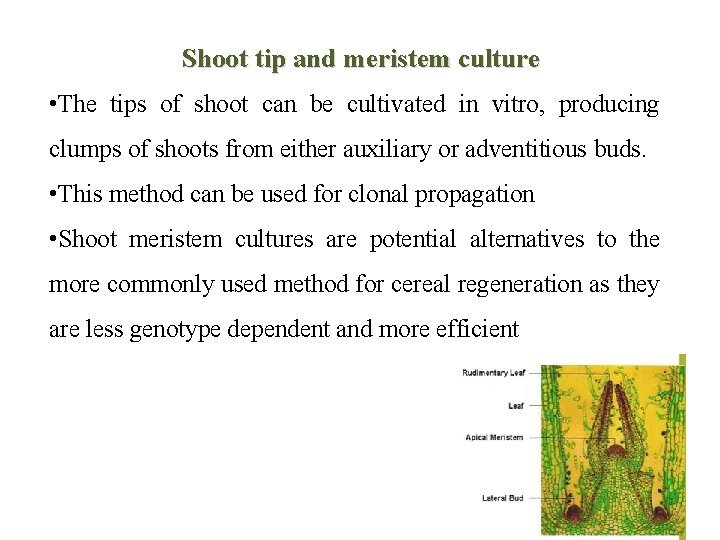
Shoot tip and meristem culture • The tips of shoot can be cultivated in vitro, producing clumps of shoots from either auxiliary or adventitious buds. • This method can be used for clonal propagation • Shoot meristem cultures are potential alternatives to the more commonly used method for cereal regeneration as they are less genotype dependent and more efficient

6. Embryo culture • Culture of excised mature or immature embryos from seeds, when cultivated in vitro is referred as embryo culture • Besides, roots, shoots and pollen, embryos can also be cultured to produce haploid plants. • Both mature and immature embryos can be used as explants • Immature, embryo- derived embryo genetic callus is the most popular method of monocot plant regeneration • It is useful in condition where embryo fails to develop due to degeneration of embryonic tissues. • It has been used as a routine technique in orchid propagation, in breeding of species showing dormancy.

7. Microspore culture • Haploid tissues can be cultivated in vitro by using pollen or anthers as an explants. Pollen contains the male gametophyte, which is termed the microspore • Both callus and embryo can be produced from pollen. • Two main approaches can be taken to produced in vitro culture from haploid tissues • The first method using anther as the explants • Anthers can be cultured on solid medium

• Anthers can also be cultured in liquid medium, and pollen released from the anthers can be induced to form embryos, although the efficiency of plant regeneration is often very low. • Immature of pollen can also be extracted from developing anthers and cultured directly, although this is a very time consuming process. • Both methods have advantages and disadvantages. • Some beneficial effect to the culture are observed when anthers are used as the explain material.

• There is, however, the danger that some of the embryos produced from anthers culture will originate from the somatic anthers tissue rather than the haploid microscope cells. • If isolated pollen is used there is no danger of mixed embryo formation, but the efficiency is low and the process is timeconsuming. • In microspore culture, the condition of the donor plant is of critical importance, as is the time of isolation. Pre-treatment’s, such as a cold treatment, are often found to increase the efficiency. These pre-treatments can be applied before culture or, in some species after placing anthers in culture.

• Plant species can be divided into two groups, depending on whether they require the addition of plant growth regulators to the medium for Pollen or anther culture; those that do also often require organic supplements eg: amino acids many of the cereals (rice, wheat, barley, and maize) require medium supplemented with plant growth regulators for Poland or anther culture.

• Regeneration from microspore explants can be obtained by direct embryogenesis, or via a callus stage and subsequent embryogenesis. • Haploid tissue cultures can also be initiated from the female gametophyte (the ovule). • In some cases this is a more efficient method than using pollen/anther culture. • The ploidy of the plant obtained from haploid cultures may not be haploid. • This can be a consequence of chromosome doubling during the culture period.

• Chromosome doubling (which often has to be induced by treatment with chemicals such as colchicines) may be an advantage as in many cases haploid plants are not the desired outcome of regeneration from haploid tissues. • Such plants are often referred to as ‘di-haploids’, because they containing two copies of the same haploid genome.

8. Mast cell culture • Plant cells are cultivated in specially designed ‘plant bioreactors’ which essentially do not have a stirrer as plant cells are sheared sensitive. • In place of stirrer, gas is gently bubbled which provide stirring as well as meet the demand of a higher oxygen supply

• Bergmann’s cell plating technique (culture of single phase) - In this technique, free cells are suspended in a liquid medium. • Equal volumes of liquid and agar media are mixed and rapidly spread in petri dish, which makes the cells evenly distributed in a thin layer after solidification. • After sealing the better dish with parafilm, they are examined under the inverted microscope to mark the single cells. • Plate are incubated in dark at 25ºC and cell colonies developing from marked single cells, are used to obtain single cell cultures.

9. Embryo rescue It has been observed that sometimes, despite successful pollination and fertilization, the embryos do not develop. The incompatibility between the embryo and endosperm or some inherent deficiency also result in the under development of embryo. These immature embryos can be dissected out from the seeds and can be grown artificially on culture medium. These embryos differentiate into shoot, root and plantlets under culture conditions.

• This technique of growing immature embryo is termed as ‘embryo rescue’ this technique is very useful in hybridization breaking dormancy of certain seeds, and to achieve complete growth of embryo into a plant.

• Anther and pollen culture (production of haploid plants) Haploid plants possess a single set of chromosomes (gametophytic no. of chromosome i. e, N) in the sporophyte in contrast to diploids which containing two sets of chromosomes (2 n). • The existence of haploid plants is reported by Bergner (1921) in Datura stramonium. Tulecke (1951) cultured the pollen grains of Ginkgo biloba (gymnosperm) and succeeded in inducing the development of haploid callus. .

• Guha and Maheshwari (1964) reported the direct development of haploid embryos and platelets plantlets from the score of Datura inoxia by the culture of excised anthers. • In 1967, Bourgin and Hitsch obtained the first full haploid plants from Nicotine tabacum

• At Present more than 247 plant species and hybrids belonging to 38 genera and 34 families of dicots and monocots have been regenerated using anther culture technique eg: rice, wheat, maize, coconut, rubber trees etc. • The Institute of Crop breeding and Cultivation (China) has developed the high yielding and blast resistant varieties of rice zhonghua No. 8 and zhonghua No. 9 through transfer of desired alien gene

10. The technique of anther culture The panthers with their filaments is removed from the flower buds after surface sterilization. Under the aseptic conditions, the anthers, are excised and crashed in 1% acetocarmine to test the stage of pollen development. The anthers in correct stage of development are separated and inoculated on a nutrient medium. The anther cultures are maintained at 28º C and alternating photo period of light (1218 h) and darkness (6 -12 h).

• The anthers proliferate and produce callus which forms an embryo and the embryo subsequently develop into a haploid plant.

11. The technique for Pollen culture – • The pollens is extracted by pressing and squeezing the anthers with a glass rod against the sides of beaker. • The anther tissue debris is removed by filtering the pollen suspension and large and healthy pollen are washed and collected. • The pollen is cultured on a solid or liquid medium and the callus or the embryo formed is transferred to a suitable medium to produce a haploid plant.

TISSUE CULTURE LABORATORY 63

Knife Laminar air flow chamber 64

DATA COLLECTION & RECORDING ROOM 65

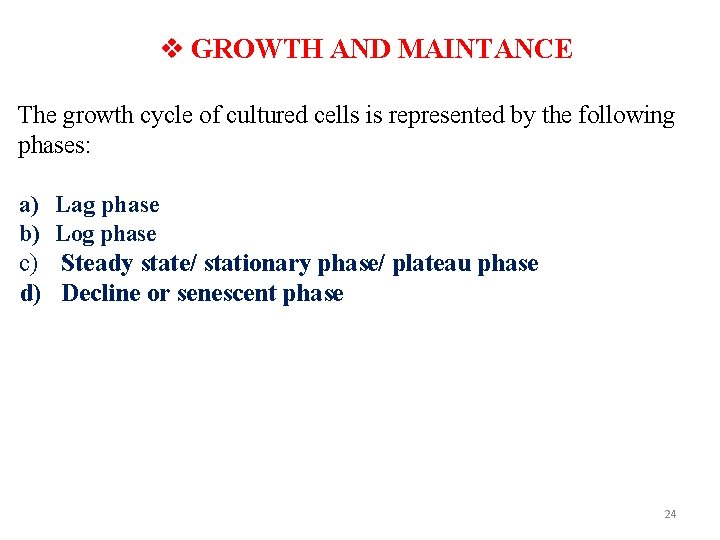
GROWTH AND MAINTANCE The growth cycle of cultured cells is represented by the following phases: a) b) c) d) Lag phase Log phase Steady state/ stationary phase/ plateau phase Decline or senescent phase 24

25

IN CALLUS CULTURE a) Lag phase: In this phase cell trying to adjust the new environment condition. b) Exponential phase : By utilizing nutrients rapid multiplication occurs. c) Decline phase : Due to starvation some cells leads to decline in the callus culture. d) Stationary phase : No growth is evident, requires sub culturin g. 26

Callus culture Ovule culture Protoplast culture Suspension culture Root tip culture Leaf primordial culture Pollen culture Shoot tip culture Flower culture 32

ADVANTAGES : Availability of raw materials. Fluctuation in supplies & quantity. Patent rights. Political reasons. Easy purification of the compound. 70

Modifications of chemical structure. Disease free & desired propagule. Crop improvement. Bio-synthetic pathway. Immobilization of cell. 71

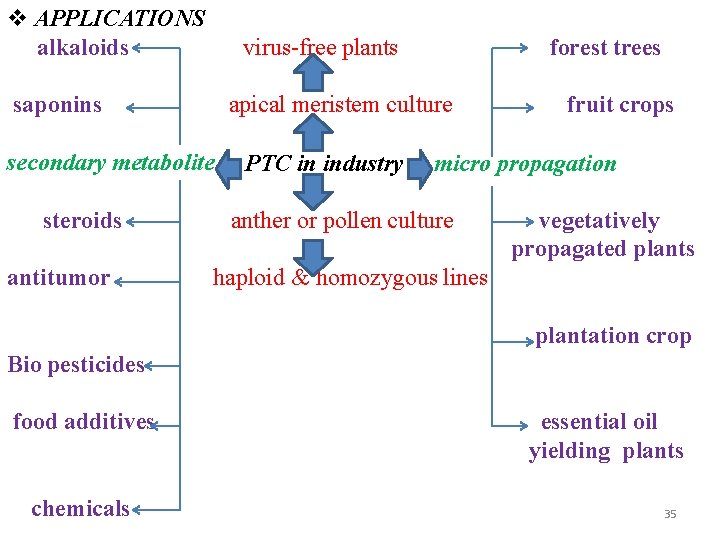
APPLICATIONS alkaloids virus-free plants saponins apical meristem culture secondary metabolite steroids antitumor forest trees PTC in industry fruit crops micro propagation anther or pollen culture vegetatively propagated plants haploid & homozygous lines plantation crop Bio pesticides food additives chemicals essential oil yielding plants 35

REFERENCES : • Trease and evans pharmacognosy. W. C evans-page num : 72, 73, 74. • A textbook of industrial pharmacognosy by A. N Kalia. Page num-105 to 114. • A textbook of pharmacognosy by M. K Gupta & P. K Sharma. Page num-171 to 185. • Pharmacognosy and photochemistry-part 2 by Vinod D Rangari. Page num-51 to 56. • Internet source. 36

37

Who is the father of tissue culture ? Haberlandt The production of secondary metabolites requires the use of ? Cell suspension Synthetic seed is produced by encapsulating somatic embryo with Sodium alginate Hormone pair required for a callus to differentiate are ? Auxin & cytokine DMSO (dimethyl sulfoxide) is used as ? Cryoprotectant The most widely used chemical for protoplast fusion, as fusogen is Poly ethylene glycol (PEG) 75

Callus is ? Unorganized actively dividing mass of cell, maintained in culture Which of the plant cell will show totipotency ? Meristem To obtain haploid plant, we culture ? Entire anther Growth hormone produce apical dominance ? Auxin The vector mostly used in crop improvement ? Agro bacterium A media which contains chemically defined compound is called ? Synthetic medium 76

77
 Amnuay silpa tuition fee
Amnuay silpa tuition fee Parent portal amnuay silpa
Parent portal amnuay silpa Pharmacognosy definition
Pharmacognosy definition Ilias yengage
Ilias yengage Yenepoya physiotherapy college
Yenepoya physiotherapy college Dr. santhosh thomas
Dr. santhosh thomas Promotion from associate professor to professor
Promotion from associate professor to professor Cuhk salary scale 2020
Cuhk salary scale 2020 Hoe dept
Hoe dept Employment first ohio
Employment first ohio La revenue dept
La revenue dept Dept nmr spectroscopy
Dept nmr spectroscopy Florida dept of agriculture and consumer services
Florida dept of agriculture and consumer services Central islip fire dept
Central islip fire dept Gome dept
Gome dept Dept ind onegov
Dept ind onegov Pt dept logistik
Pt dept logistik Dept. name of organization
Dept. name of organization Fire dept interview questions
Fire dept interview questions Hjdkdkd
Hjdkdkd Rewley house continuing education library
Rewley house continuing education library Florida dept of agriculture and consumer services
Florida dept of agriculture and consumer services Florida dept of agriculture and consumer services
Florida dept of agriculture and consumer services Rowan county medicaid transportation
Rowan county medicaid transportation Gome dept
Gome dept Albany county dss
Albany county dss Mn dept of education
Mn dept of education Nys dept of homeland security
Nys dept of homeland security Oviposition
Oviposition Vaginal dept
Vaginal dept Nebraska dept of agriculture
Nebraska dept of agriculture Dept of education
Dept of education Finance departments
Finance departments Dept a
Dept a Gome dept
Gome dept Dept. name of organization
Dept. name of organization Ms department of finance and administration
Ms department of finance and administration La dept of revenue
La dept of revenue Dept of education
Dept of education Gome dept
Gome dept Ee dept iitb
Ee dept iitb Bromocicloesano
Bromocicloesano Worcester ma building department
Worcester ma building department What are the advantages of biofertilizers
What are the advantages of biofertilizers Uses of volatile oil in pharmacognosy
Uses of volatile oil in pharmacognosy Difference between organised and unorganised crude drug
Difference between organised and unorganised crude drug Pharmacogosy
Pharmacogosy Significance of callus culture
Significance of callus culture Storage of crude drugs
Storage of crude drugs Isapagol
Isapagol Edible vaccines in pharmacognosy
Edible vaccines in pharmacognosy Serpentiana
Serpentiana Types of adulteration in pharmacognosy
Types of adulteration in pharmacognosy Sterculia gum pharmacognosy
Sterculia gum pharmacognosy Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Norlupinane
Norlupinane Tannins in pharmacognosy
Tannins in pharmacognosy Glycosides in pharmacognosy
Glycosides in pharmacognosy Applications of plant tissue culture in pharmacognosy
Applications of plant tissue culture in pharmacognosy Ketone volatile oils
Ketone volatile oils Madar kabab chini tagar are the examples of
Madar kabab chini tagar are the examples of Pharmacognosy scope
Pharmacognosy scope What is ash value
What is ash value Volatile oil definition pharmacognosy
Volatile oil definition pharmacognosy Vein islet number example
Vein islet number example D-kefs scoring assistant
D-kefs scoring assistant Comenius assistant
Comenius assistant Dental surfaces chart
Dental surfaces chart Hacheur assistant
Hacheur assistant Assistant computer control
Assistant computer control Cisco unified cm assistant console
Cisco unified cm assistant console Assistant
Assistant Manifesto for school head girl
Manifesto for school head girl Varicocele grading radiology assistant
Varicocele grading radiology assistant Scorer and assistant scorer
Scorer and assistant scorer Veternary science
Veternary science The assistant chapter 24
The assistant chapter 24 Fglair alexa
Fglair alexa