PHARMACOGNOSY I 2 nd stage 2 nd Tremester





















- Slides: 25

PHARMACOGNOSY I/ 2 nd stage / 2 nd Tremester A. Lecturer: Mohammed S. Nawrooz th 4 e r u t c e L

Deterioration of Crude Drugs • Factors : 1. Moisture content. 2. Temperature. 3. Light. 4. Presence of oxygen. These conditions are suitable , living oganisms like(bacteria, moulds, mites and insects) will rapidly multiply using a drug as a source of nutrient. These drugs are excluded by national pharmacopoeias

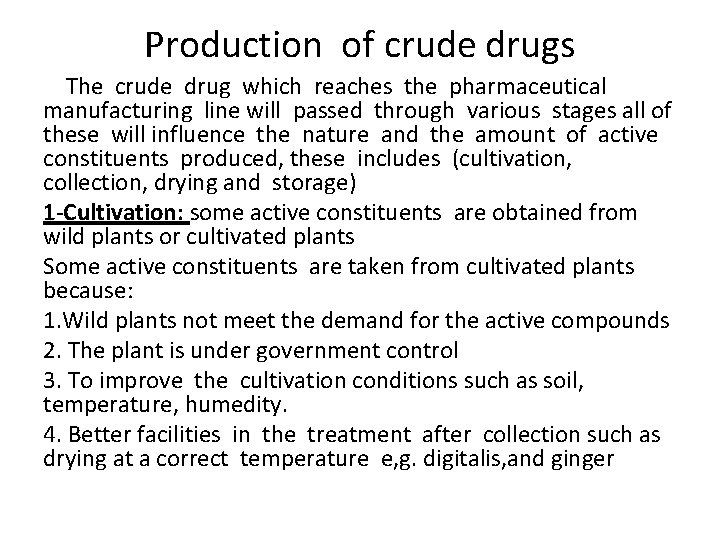
Production of crude drugs The crude drug which reaches the pharmaceutical manufacturing line will passed through various stages all of these will influence the nature and the amount of active constituents produced, these includes (cultivation, collection, drying and storage) 1 -Cultivation: some active constituents are obtained from wild plants or cultivated plants Some active constituents are taken from cultivated plants because: 1. Wild plants not meet the demand for the active compounds 2. The plant is under government control 3. To improve the cultivation conditions such as soil, temperature, humedity. 4. Better facilities in the treatment after collection such as drying at a correct temperature e, g. digitalis, and ginger

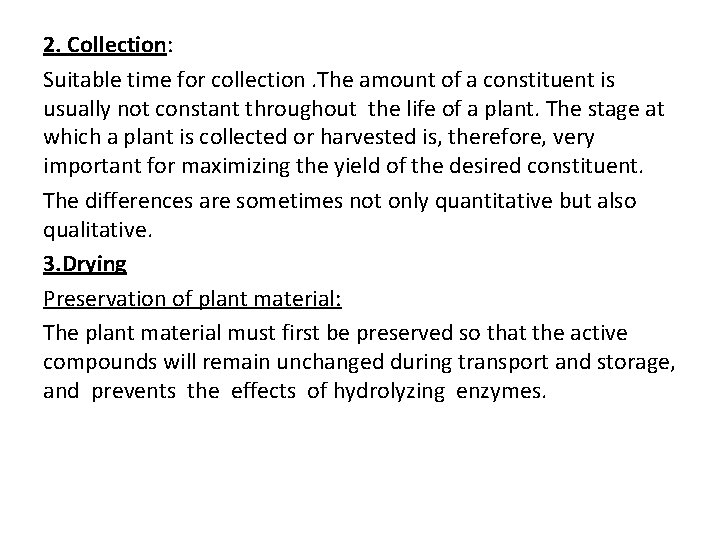
2. Collection: Suitable time for collection. The amount of a constituent is usually not constant throughout the life of a plant. The stage at which a plant is collected or harvested is, therefore, very important for maximizing the yield of the desired constituent. The differences are sometimes not only quantitative but also qualitative. 3. Drying Preservation of plant material: The plant material must first be preserved so that the active compounds will remain unchanged during transport and storage, and prevents the effects of hydrolyzing enzymes.

The most common method for preserving plant material is drying. Enzymic processes take place in aqueous solution. Rapid removal of the water from the cell will, therefore, largely prevent degradation of the cell constituents. Drying also decreases the risk of external attack, e. g. by moulds. Living plant material has a high water content: leaves may contain 60 -90% water, roots and rhizomes 70 -85%, wood 40 -50%. The lowest percentage, often no more than 5 -10%, is found in seeds.

To stop the enzymic processes, the water content must be brought down to about 10 %. Drying must be done quickly, in other words at raised temperatures and with rapid and efficient removal of the water vapor. The air temperature is kept at 20 -40 °C for thin materials such as leaves, but is often raised to 60 -70 °C for plant parts that are harder to dry, e. g. roots and barks. In a dried drug the enzymes are not destroyed but only rendered inactive due to the low water content. As soon as water is added, they become active again. Hence, dried drugs must be protected from moisture during storage.

Freeze-drying (lyophilization) is a very mild method. Frozen material is placed in an apparatus which has a cold surface maintained at -60 to -80 °C. The method requires a relatively complicated apparatus and is much more expensive than hot-air drying. For this reason, it is not used as a routine method, but it is very important for drying heat-sensitive substances, e. g. antibiotics and proteins.

4. Storage of crude drugs In order to keep crude drugs as long as possible: 1. It is essential to store them in a dry condition in carefully closed containers. 2. It is also advisable to exclude light, because - even if it does not affect the active constituents - it almost always causes changes in the appearance of the drug, especially loss of color. 3. It is also necessary to protect the drug against insect attack. The amount of taxol in Taxus. baccata leaves and extracts decreased upon storage at room temperature for one year by 30%-40% and 70%-80% respectively.

Grinding of crude drugs Regardless of whether the crude drug is to be used for isolation of a pure compound or for manufacture of a simple preparation, the first operation that must be performed is grinding of the plant material to a powder of suitable particle size. It is important that the particles are of as uniform a size as possible. Excessive dust can clog percolators and result in a turbid extract which is hard to clarify. Large particles take a longer time for complete extraction than small ones and large differences in particle size thus slow down the extraction process. Several types of machines are available for grinding crude drugs: 1. Hammer mill. 2. Knife mill.

Extraction of crude drugs • Extraction refers to processes for the isolation of the active ingredients from drug material. This may be by physical means or by dissolving in a suitable solvent. • The product obtained from extraction called (Extract) and it is relatively impure. • The choice of extraction procedure depends on the nature of the plant material and the components to be isolated. • Dried materials are usually powdered before extraction, whereas fresh plants (leaves. ) can be homogenized and macerated with a solvent such as alcohol.

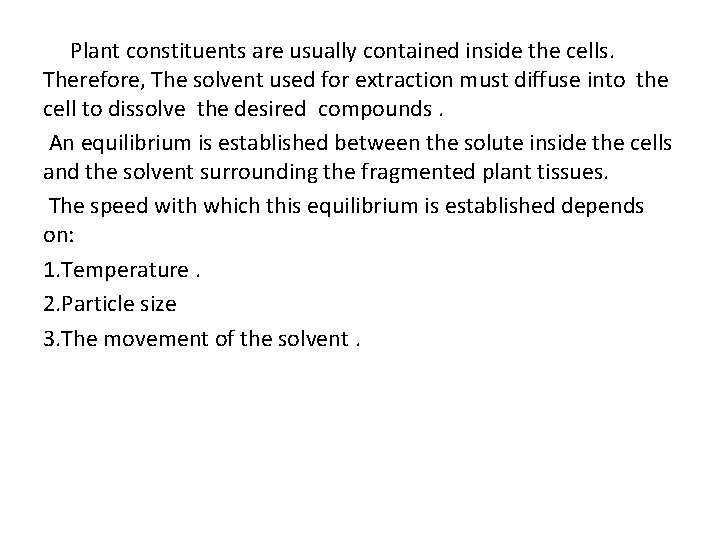
Plant constituents are usually contained inside the cells. Therefore, The solvent used for extraction must diffuse into the cell to dissolve the desired compounds. An equilibrium is established between the solute inside the cells and the solvent surrounding the fragmented plant tissues. The speed with which this equilibrium is established depends on: 1. Temperature. 2. Particle size 3. The movement of the solvent.

Choice of solvent The ideal solvent for a certain pharmacologically active constituent should: 1. Be highly selective for the compound to be extracted. 2. Have a high capacity for extraction in terms of coefficient of saturation of the compound in the medium. 3. Not react with the extracted compound or with other compounds in the plant material. 4. Have a low price. 5. Be harmless to man and to the environment. 6. Be completely volatile.

• Aliphatic alcohols with up to three carbon atoms, or mixtures of the alcohols with water, are the solvents with the greatest extractive power for almost all natural substances of low molecular weight like alkaloids, saponins and flavonoids. • According to the pharmacopoeias, ethyl alcohol is the solvent of choice for obtaining classic extracts such as tinctures and fluid, soft and dry extracts. • Generally extraction methods can be classified into: Hot and Cold Methods.

EXTRACTION METHODS • Cold Methods • 1. Maceration: • It is a process that includes soaking of plant meterial with a solvent until penetration of the cellular structures take place and the active constituents softened and dissolved in the solvent. • The procedure includes placing the plant material in a container and then adding the solvent, cover the container and leave it for time that differs from 2 -5 days. After that pouring off the solvent, expressing the plant material and filter the solvent. The procedure done at 25 C.

Advantages : 1. Small amounts of solvent used(economic). 2. Used for toxic reagents which cannot be used in open air system. 3. Continous cold extraction method. Disadvantage: Time consuming Due to difficulty in obtaining the active constituents in one maceration process, and need to repeat the procedure many times. Repeated maceration may be more efficient than a single maceration, since an appreciable amount of active principle may be left behind in the first pressing of the solid residue. Also, The repeated maceration is more efficient in cases where active constituents are more valuable.

General procedure for maceration 1. Plant Material(Crushed or cut small or Moderately coarse powder). 2. Placed in a closed vessels. 3. Whole of the selected solvent (Menstruum) added. 4. Allowed to stand for about 2 -5 days shaking occasionally. 5. Liquid strained off. 6. Solid residue pressed. 7. expressed liquids mixed. 8. Clarified by filtration. 9. Evaporation and Concentration.

2. percolation(Exhaustive Extraction) ﺗﺮﺷﻴﺢ ﺗﻘﻄﻴﺮ • In this process, an organized vegetable drug, in a suitably powdered form, is packed in a percolator and the solvent is allowed to percolate through it. • The procedure includes placing a powder plant material in the percolator and start adding the solvent from the upper end. While the solvent going down to extract as much as possible the active constituent passing through it • Although some materials (e. g. ginger) may be packed directly into the percolator in a dry state, this may cause difficulties with other drugs. With the addition of solvent, the dry material swells and this swelling increases with increasing aqueous nature of the solvent. This swelling reduces or blocks the flow of the solvent, thus seriously affecting the extraction process.

Process 1. Organized vegetable drug in a suitably powdered form. 2. Uniform moistening of the powdered vegetable drugs with menstruum for a period of 4 hours in a separable vessel. 3. Packed into the percolator. 4. A piece of filter paper is placed on surface. 5. Sufficient menstruum is poured over the drug slowly and evenly to saturate it maintain a small layer above the drug and allowed to stand for 24 hours. After that, the outlet is opened and solvent is percolated at a control rate with continuous addition of fresh volume. 6. Substance residue is pressed and expressed liquid is added to the percolate giving 80% to 90% of the final volume. 7. Evaporation and concentration to get finished products by applying suitable techniques and apparatus.

Advantages : 1. Different types of solvents can be used in the same process with different polarities for the same process. 2. Used for heat sensitive substances. 3. Cheap, easy to done. Disadvantages : 1. Huge amounts of solvent required. 2. time consuming. 3. small amount of active constituents. 4. If the active substances are thermolabile, evaporation of large volumes of dilute percolate may result in partial loss of the active constituents.

3 -Expression • Expression or Cold Pressing is confined for some active costituents like citrus volatile oils. It involves inducing physical damage to the essential oil glands on the surface of citrus fruits to release the oil. • It is simultaneously washed by the passing water and recovered using an oil-water separator.

Hot Extraction Methods • 1. Digestion: • In this method, the plant material placed together with the solvent and we apply a gentle heat so that the solvent will increase its power for extraction and this method used in cases when moderate temperature required for substances are heat stable. • This is a form in which gentle heat is used during the process of extraction. It is used when moderately elevated temperature is not objectionable. The solvent efficiency of the menstruum is thereby increased.

3. Decoction ﺍﺳﺘﺨﻼﺹ ﺑﺎﻻﻏﻼﺀ In this process, the crude drug is boiled in a specified volume of water (direct or indiect heat) for a defined time; it is then cooled and strained or filtered. This procedure is suitable for extracting water-soluble, heat -stable constituents, and hard substances like bark, stems , roots. • The starting ratio of crude drug to water is fixed, e. g. 1: 4 ; the volume is then brought down to one-fourth its original volume by boiling during the extraction procedure. Then, the concentrated extract is filtered and used as such or processed further. • Cannot use volatile and toxic solvents because of direct heat. •

3. Continuous Hot Extraction A. (Soxhlet) In this method, the finely ground crude drug is placed in a porous bag or “thimble” made of strong filter paper, which is placed in chamber of the Soxhlet apparatus. The advantage of this method, compared to previously described methods, is that large amounts of drug can be extracted with a much smaller quantity of solvent. This effects economy in terms of time, energy and consequently financial inputs.

Advantages : 1. economic (small amount of the solvent). 2. can use of toxic solvent(closed system). 3. can be used for substances affected by direct heat. Disadvantages: 1. In case of toxic solvents used , there is a dangerous of poisoning. 2. need efficient condensor. 3. Agitation is not possible in the Soxhlet device. 4. The possibility of thermal decomposition of the target compounds cannot be ignored as the extraction usually occurs at the boiling point of the solvent for a long time.

• B. Clevenger Apparatus. • Advantages? , Disadvantages? . • C. Ordinary Reflux: • Advantages ? • Disadvantages ?
 Tremester
Tremester Enumerate the history and scope of pharmacognosy
Enumerate the history and scope of pharmacognosy Stage right stage left
Stage right stage left Stage positions
Stage positions Disadvantages of two stage tendering
Disadvantages of two stage tendering Stage 1 denial stage 2 anger
Stage 1 denial stage 2 anger The word drama comes from
The word drama comes from Down stage definition
Down stage definition Couramin
Couramin Application of plant tissue culture
Application of plant tissue culture Pharmaceutical uses of volatile oils
Pharmaceutical uses of volatile oils Define pharmacognosy
Define pharmacognosy Collection of crude drugs
Collection of crude drugs Name the various methods of classification of crude drugs
Name the various methods of classification of crude drugs Drug adulteration
Drug adulteration Allopathy meaning
Allopathy meaning Edible vaccines in pharmacognosy
Edible vaccines in pharmacognosy Difference between organised and unorganised drug
Difference between organised and unorganised drug Sterculia gum pharmacognosy
Sterculia gum pharmacognosy Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Explant is
Explant is Alkaloids definition
Alkaloids definition Tannins pharmacognosy
Tannins pharmacognosy Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Volatile oil definition pharmacognosy
Volatile oil definition pharmacognosy Volatile oils - pharmacognosy
Volatile oils - pharmacognosy