Pharmacognosy II 3 rd stage3 rd lecture A















- Slides: 25

Pharmacognosy II 3 rd stage/3 rd lecture A. Lecturer Mohammed S. Nawrooz

Definition Organic natural compounds present in a lot of plants and some organisms , these compounds upon hydrolysis give one or more sugars (glycone) and non sugar (aglycone) or called (genin). • Glycosides, are found to exert a wide spectrum of therapeutic actions, both in modern medicines and in traditional medicaments, ranging from cardiotonic, analgesic, purgative, and anti-rheumatic, demulcent and other useful actions. •

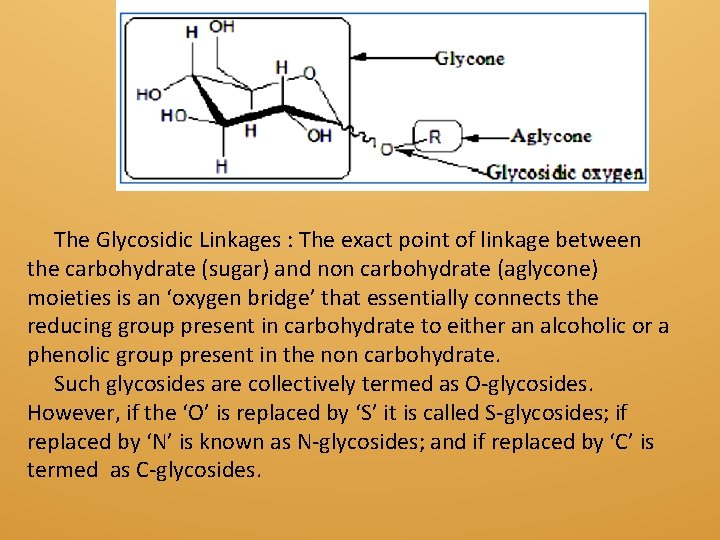
The Glycosidic Linkages : The exact point of linkage between the carbohydrate (sugar) and non carbohydrate (aglycone) moieties is an ‘oxygen bridge’ that essentially connects the reducing group present in carbohydrate to either an alcoholic or a phenolic group present in the non carbohydrate. Such glycosides are collectively termed as O-glycosides. However, if the ‘O’ is replaced by ‘S’ it is called S-glycosides; if replaced by ‘N’ is known as N-glycosides; and if replaced by ‘C’ is termed as C-glycosides.

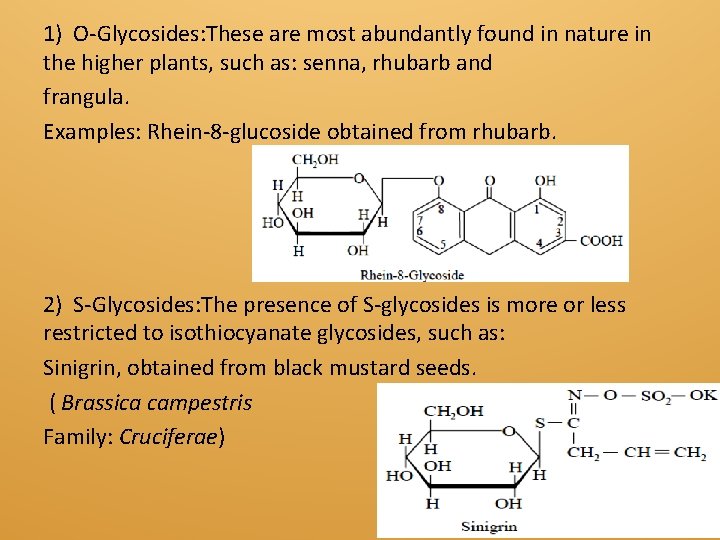
1) O-Glycosides: These are most abundantly found in nature in the higher plants, such as: senna, rhubarb and frangula. Examples: Rhein-8 -glucoside obtained from rhubarb. 2) S-Glycosides: The presence of S-glycosides is more or less restricted to isothiocyanate glycosides, such as: Sinigrin, obtained from black mustard seeds. ( Brassica campestris Family: Cruciferae)

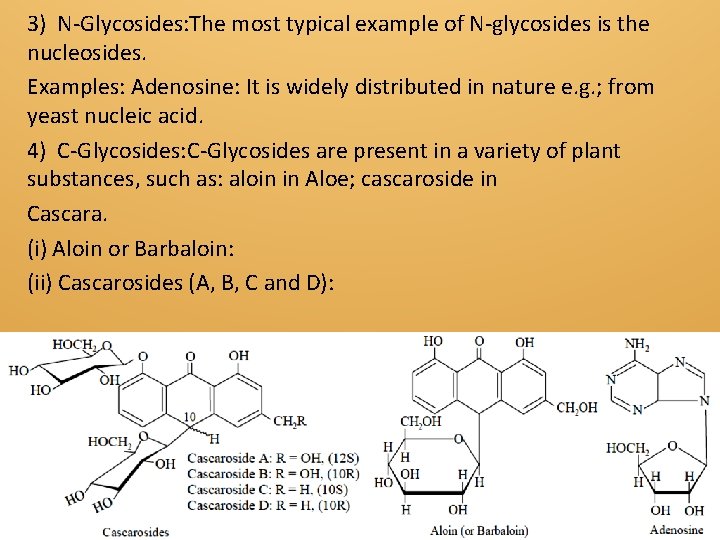
3) N-Glycosides: The most typical example of N-glycosides is the nucleosides. Examples: Adenosine: It is widely distributed in nature e. g. ; from yeast nucleic acid. 4) C-Glycosides: C-Glycosides are present in a variety of plant substances, such as: aloin in Aloe; cascaroside in Cascara. (i) Aloin or Barbaloin: (ii) Cascarosides (A, B, C and D):

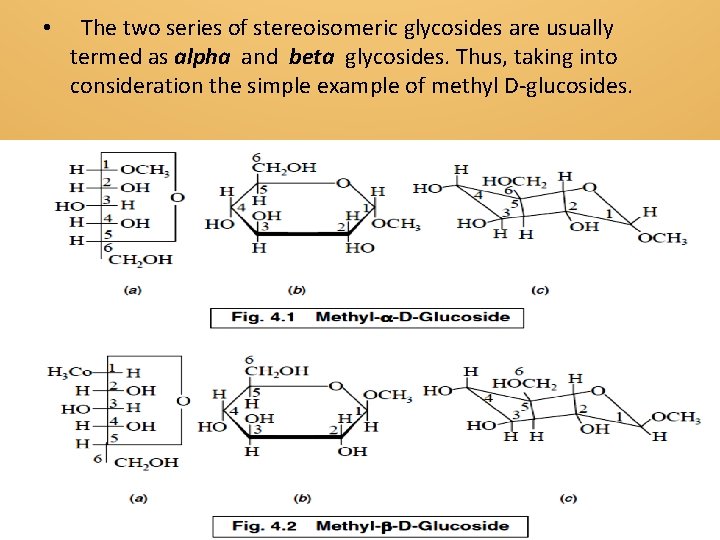
• The two series of stereoisomeric glycosides are usually termed as alpha and beta glycosides. Thus, taking into consideration the simple example of methyl D-glucosides.

The two Figures represent the open-chain structure, cyclic structure and boat configuration of methyl–alpha-D-glucoside and methyl–beta-D-glucoside respectively. In this particular instance the glycosidic linkage is established by dehydration involving a hydroxy group of the aglycone portion (i. e. , methyl alcohol) and the hydroxyl group on the hemiacetal carbon of the carbohydrate , thereby ultimately resulting into the formation of an acetal type of structure. Alpha -Configuration If the —OCH 3 moiety (generalized as OR′) is in opposite steric sense as the —CH 2 OH moiety on C-5 (for D-family sugars), the glycosidic structure is designated as α-configuration.

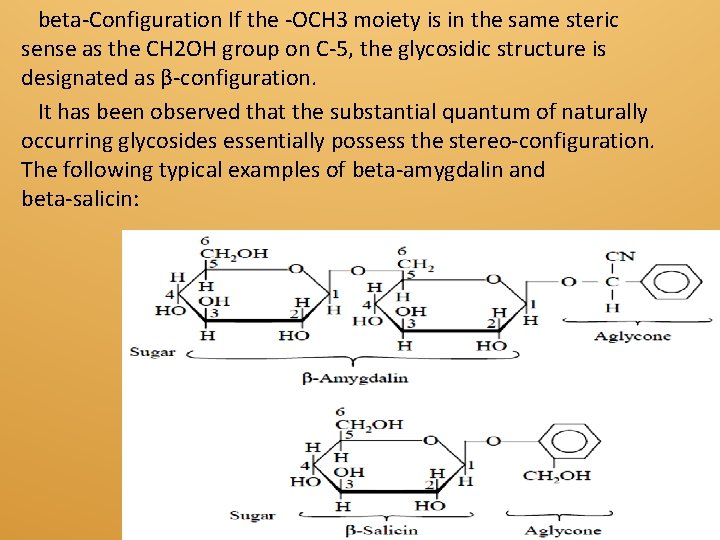
beta-Configuration If the -OCH 3 moiety is in the same steric sense as the CH 2 OH group on C-5, the glycosidic structure is designated as β-configuration. It has been observed that the substantial quantum of naturally occurring glycosides essentially possess the stereo-configuration. The following typical examples of beta-amygdalin and beta-salicin:

Features of (beta-Amygdalin): (i) Glycosidic linkage is beta and it is hydrolysed by emulsin (an enzyme). (ii) The linking oxygen is on the same side of the plane of the ring as the CH 2 OH moiety on C-5. (iii) It contains several asymmetric C atoms i. e. , chiral centres. (iv) It is optically active. Features of (b-Salicin): 1. Hydrolysed by emulsin, it has b-configuration. 2. The linking oxygen is on the same side of the plane of the ring as the CH 2 OH moiety on C-5, 3. It has several chiral centres. 4. It is optically active.

Almost all plants that contain glycosides also contain enzymes that bring about their hydrolysis (glycosidases). • Alpha and beta glycosides 1 -Sugars exist in isomeric α and β forms. Both α and β Glycosides are theoretically possible. 2 -All natural glycosides are of the β Type. 3 -Some α linkage exists in sucrose, glycogen and starch.

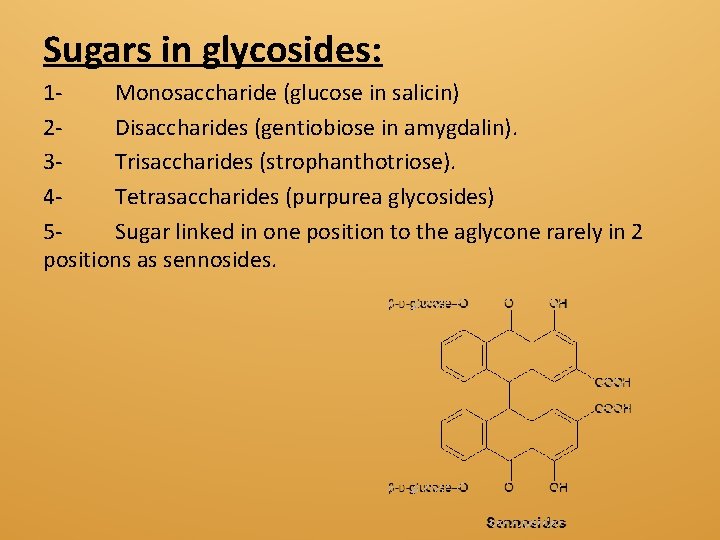
Sugars in glycosides: 1 Monosaccharide (glucose in salicin) 2 Disaccharides (gentiobiose in amygdalin). 3 Trisaccharides (strophanthotriose). 4 Tetrasaccharides (purpurea glycosides) 5 Sugar linked in one position to the aglycone rarely in 2 positions as sennosides.

Solubility and enzymatic effects • • Glycosides mostly are soluble in water or polar solvents , why? Aglycone soluble in organic solvents Glycones are water soluble Enzymes play a role in synthesis and hydrolysis of glycosides in the plants and this property may affect the extraction of these compounds. Notes: Glycosides are usually soluble in water and in polar organic solvents, whereas aglycones are normally insoluble or only slightly soluble in water. Although glycosides form a natural group in that they all contain a sugar unit, the aglycones are of such varied nature and complexity that glycosides vary very much in their physical and chemical properties and in their pharmacological action.

Physico-chemical Properties of Glycosides: 1. 2. 3. 4. Colorless , except, anthraquinone(red or orange). Amorphus, solid, non-volatile. Water soluble. Most of them are bitter except some of them like, glycyrrhizin. 5. Odorless except, saponin. 6. Hydrolyzed by acids , temperature or enzymes like, Myrosinase black mustard Emulsin bitter almond seeds.

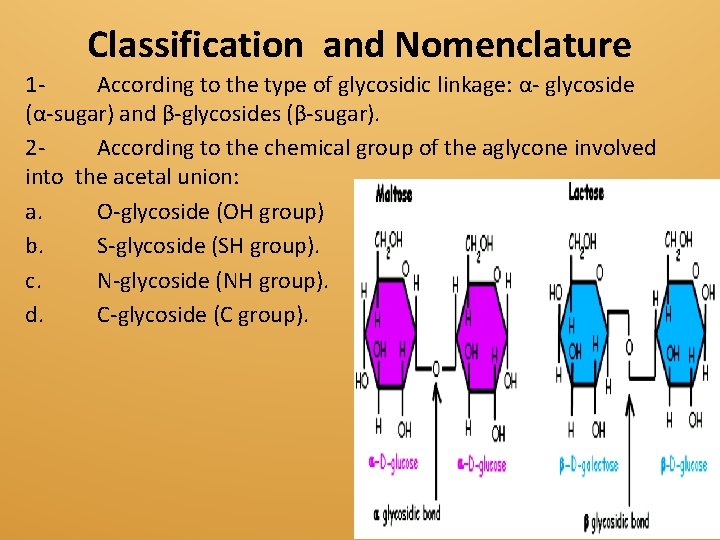
Classification and Nomenclature 1 According to the type of glycosidic linkage: α- glycoside (α-sugar) and β-glycosides (β-sugar). 2 According to the chemical group of the aglycone involved into the acetal union: a. O-glycoside (OH group) b. S-glycoside (SH group). c. N-glycoside (NH group). d. C-glycoside (C group).

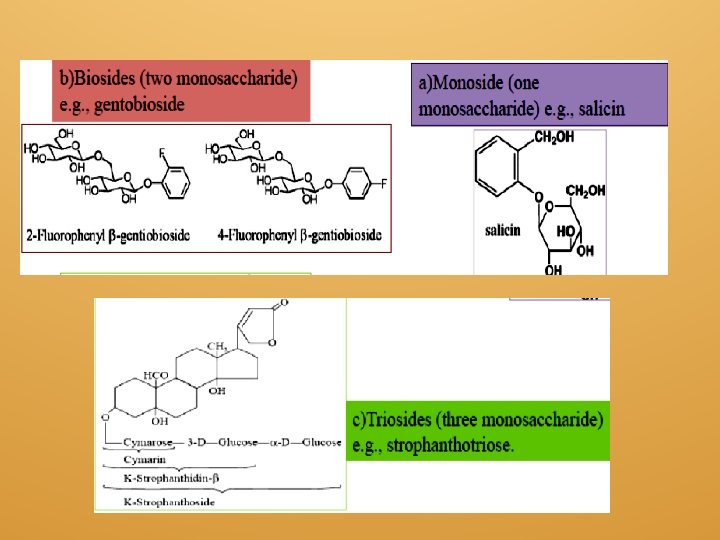
3 According to the nature of the simple sugar component of the glycoside: a. Glucosides (the glycone is glucose). b. Galactosides (the glycone is galactose). c. Mannosides (the glycone is mannose). d. Arabinosides (the glycone is arabinose). 4 According to the number of the monosaccharides in the sugar moiety: a. Monoside (one monosaccharide) e. g. , salicin. b. Biosides (two monosaccharide) e. g. , gentobioside. c. Triosides (three monosaccharide) e. g. , strophanthotriose.


5 According to the physiological or pharmacological activity ‘therapeutic classification) • a. Laxative glsycosides. • b. Cardiotonic glycosides. 6 according to the correlation to the parent natural glycoside: a. primary glycosides e. g. , amygdalin, purpurea glycoside A, b. Secondary glycosides e. g. , prunasin, digitoxin. 7 According to the plant families.

• 8 - According to the chemical nature of the aglycone: 1. Alcoholic and phenolic glycosides. . 2. Aldehydic glycoside. . 3. Cyanogenic glycosides. . 4. anthraquinone glycosides. . 5. Steroidal glycosides. . 6. Flavonoidal glycosides. . 7. Saponin glycosides. . 8. Coumarin and Furanocoumarin glycosides. . 9. Thioglycosides. . 10. Bitter glycosides. . 11. Miscellaneous glycosides. .

Stability • 1. Acid hydrolysis: a- Acetal linkage between the aglycon and glycone more unstable than that between two individual sugars within the molecule. b- all glycosides are hydrolysable by acids non specific (except Cglycosides). c- Glycosides containing 2 -deoxy sugars are more unstable towards acid hydrolysis even at room temperature. d- C-glycosides are very stable (need oxidative hydrolysis).

2. Enzyme hydrolysis: 1 - Enzymatic hydrolysis is specific for each glycoside, there is a specific enzyme that exerts a hydrolytic action on it. 2 - The same enzyme is capable to hydrolyze different glycosides, but α and β sterio-isomers of the same glycoside are usually not hydrolysed by the same enzyme. 3 - Emulsin is found to hydrolysed most β-glycoside linkages, those glycoside are attacked by emulsin are regarded as βglycosides. 4 - Maltase and invertase are α-glycosidases, capable of hydrolyzing α-glycosides only.

Medicinal importance of glycosides: 1 Cardiac drugs: cardiotonic glycosides e. g. , digitalis glycosides, strophanthus, squill. 2 Laxatives e. g. , anthraquinone glycosides of senna, aloes, rhubarb, cascara, frangula. 3 Counter irritants e. g. , thioglycosides and their hydrolytic products ‘allylisothiocyanate’ 4 Analgesics e. g. , methylsalicylate ‘a hydrolytic product of gaultherin. 5 Anti rheumatic e. g. , salicin. 6 Some glycosides are claimed to reduce the capillary fragility e. g. , flavonoidal glycosides, rutin, hisperidin. 7 Anti-inflammatory: e. g. , the glycoside glycyrrhizin has a demulcent, expectorant and antispasmodic action. 8 More recently as an anticancer agent e. g. , amygdalin.

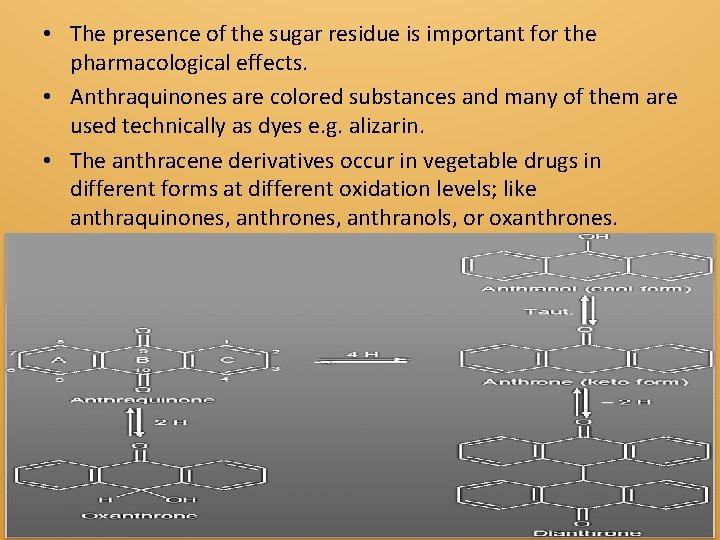
1 Anthraquinone Glycosides A number of glycosides in which the aglycones are anthracene derivatives occur as the pharmacologically active constituents of several cathartics of plant origin; e. g. cascara, rhubarb, aloe and senna. These anthraquinone glycosides are sometimes referred to as the anthracene glycosides or the anthraglycosides

• The presence of the sugar residue is important for the pharmacological effects. • Anthraquinones are colored substances and many of them are used technically as dyes e. g. alizarin. • The anthracene derivatives occur in vegetable drugs in different forms at different oxidation levels; like anthraquinones, anthranols, or oxanthrones.

• These anthracene compounds occur in these drugs or plant materials in some cases as the aglycones of O-glycosides (e. g. frangulin), and in other cases as the aglycones of C-glycosides (e. g. aloin). • Oxanthrones: An oxanthrone has been reported as a constituent of cascara bark. • Dianthrones: They are important aglycones in species of Cassia, Rheum and Rhamnus. • One of the best known is sennoside derived from two molecules of glucose and two molecules of anthrone. • Aloin-type or C-glycosides: • Aloin (Barbaloin) was obtained from species of Aloe.
