Periodic Trends Review n The elements of the















- Slides: 15

Periodic Trends

Review n The elements of the P. T. are placed into: rows by the number of energy levels they have. n groups by the number of valence electrons they have. n blocks by what kind of sublevel they are filling. n n Atoms can gain or lose electrons. Metallic atoms lose e- and form cations. n Nonmetallic atoms gain e- and form anions. n

Metallicity Less Metallic More Metallic

The Big Questions n What is atomic radius? How is ionic radius related to atomic radius? n What is the trend concerning atomic radius? n What is ionization energy, and what trend is associated with it? n What is electronegativity, and what trend is associated with it? n

Atomic Radius n Atomic radius – distance from center of atom to outer electrons. Decreases across a period. n Increases down a group. n

Atomic Radii in Period 2 Li Be B C N Increasing atomic number means greater attraction for electrons! O F Ne

Increasing number of energy levels means a larger atom. Atomic Radii of the Halogens F Cl Br I At

Atomic Radii General Trend Radius Increases Radius Decreases

Ionic Radius Cations are smaller than their atoms. n Anions are larger than their atoms. n

Ionic Radius

Ionization Energy n Ionization Energy – energy required to remove 1 electron from an atom.

Ionization Energy +

Ionization Energy Increases across a period. n Decreases down a group. n

Electronegativity n Electronegativity (EN) - Tendency of an atom to attract electrons in a chemical bond. Increases across a period. n Decreases down a group. n Excludes noble gases. n

Electronegativity
 Patterns in the periodic table
Patterns in the periodic table Alien periodic table periodic trends answers
Alien periodic table periodic trends answers Periodic trends in properties of elements
Periodic trends in properties of elements Ion size trend
Ion size trend Trends on periodic table
Trends on periodic table Chemistry periodic table cheat sheet
Chemistry periodic table cheat sheet Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity Graphing periodic trends
Graphing periodic trends Oxidation trends periodic table
Oxidation trends periodic table Electronegativity meaning
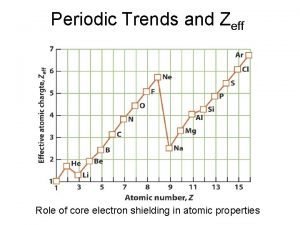
Electronegativity meaning Zeff periodic table trend
Zeff periodic table trend Periodic trends activity worksheet
Periodic trends activity worksheet Periodic trends practice quiz
Periodic trends practice quiz Reactivity trend periodic table
Reactivity trend periodic table Summary of periodic trends
Summary of periodic trends Electron practice problems
Electron practice problems