Percutaneous Treatment of Mitral Regurgitation Why do we




















- Slides: 37

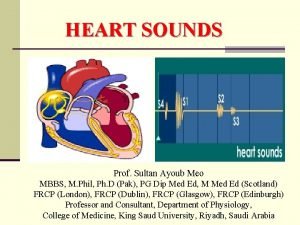
Percutaneous Treatment of Mitral Regurgitation: Why do we need it at the VA? Santiago Garcia, MD Associate Professor of Medicine, University of Minnesota Director, Structural Heart Program MVAHCS Garci 205@umn. edu

Disclosures <Type of Relationship>: <Company 1> <Company 2> Examples of relationships are: Advisory Board/Board Member, Consultant, Honoraria, Research Support, Speaker’s Bureau, Stockholder Please list full company name

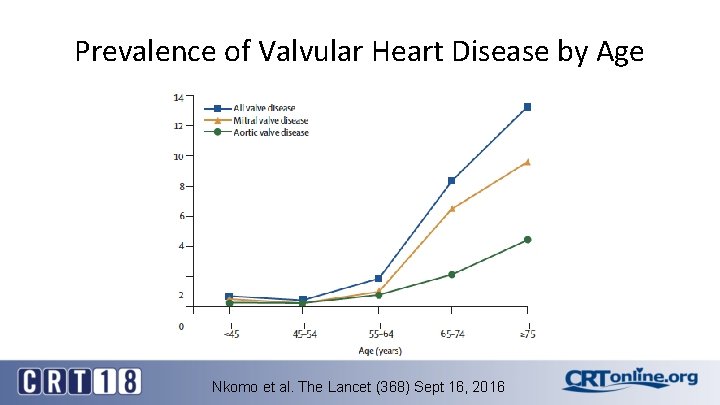
Prevalence of Valvular Heart Disease by Age Nkomo et al. The Lancet (368) Sept 16, 2016

The mitral valve

Primary MR: The problem is in the leaflets

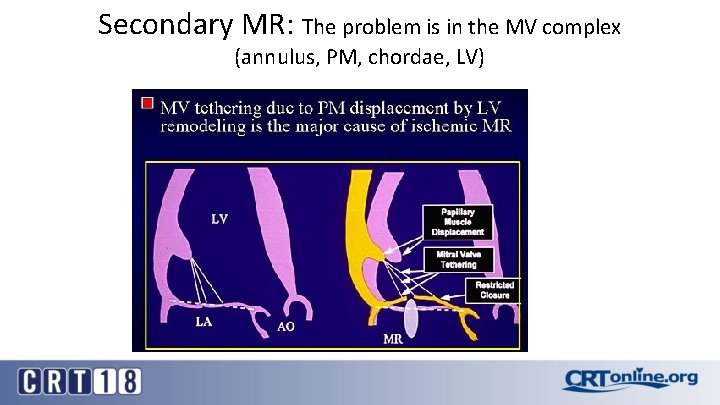
Secondary MR: The problem is in the MV complex (annulus, PM, chordae, LV)

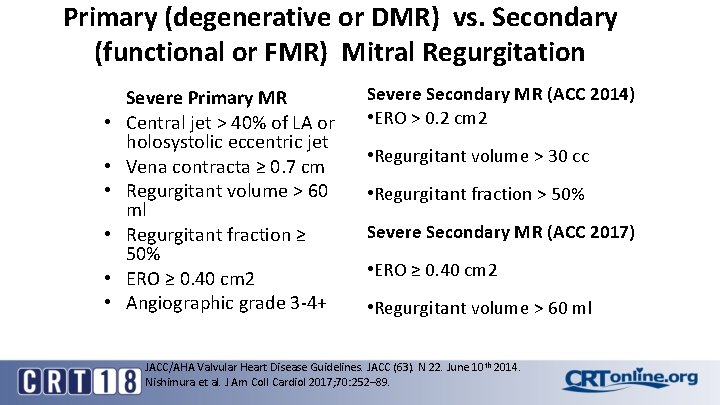
Primary (degenerative or DMR) vs. Secondary (functional or FMR) Mitral Regurgitation • • • Severe Primary MR Central jet > 40% of LA or holosystolic eccentric jet Vena contracta ≥ 0. 7 cm Regurgitant volume > 60 ml Regurgitant fraction ≥ 50% ERO ≥ 0. 40 cm 2 Angiographic grade 3 -4+ Severe Secondary MR (ACC 2014) • ERO > 0. 2 cm 2 • Regurgitant volume > 30 cc • Regurgitant fraction > 50% Severe Secondary MR (ACC 2017) • ERO ≥ 0. 40 cm 2 • Regurgitant volume > 60 ml JACC/AHA Valvular Heart Disease Guidelines. JACC (63). N 22. June 10 th 2014. Nishimura et al. J Am Coll Cardiol 2017; 70: 252– 89.

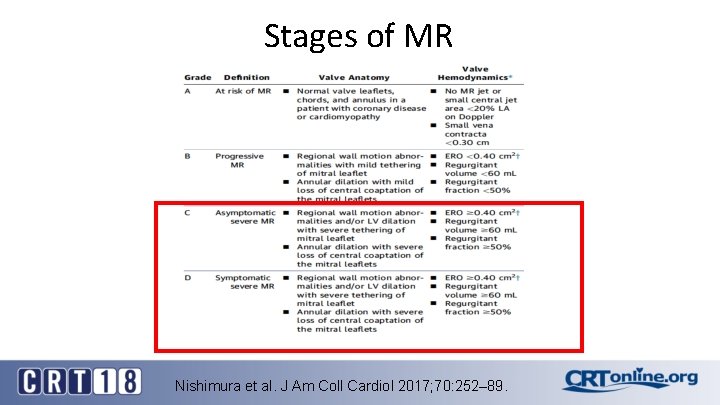
Stages of MR Nishimura et al. J Am Coll Cardiol 2017; 70: 252– 89.

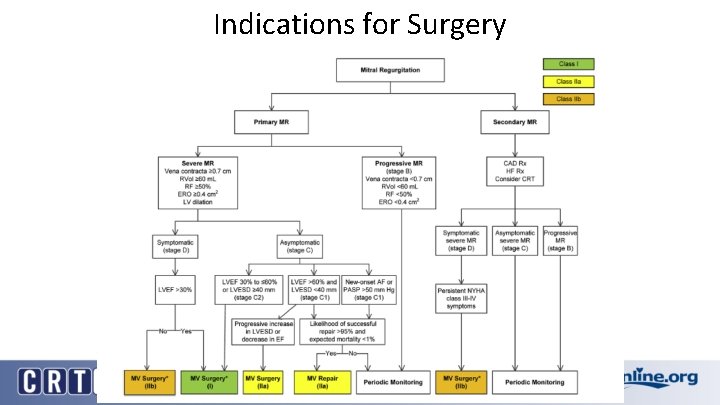
Indications for Surgery

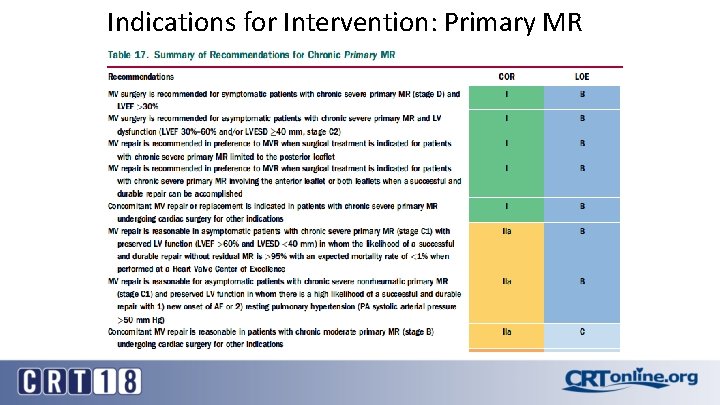
Indications for Intervention: Primary MR

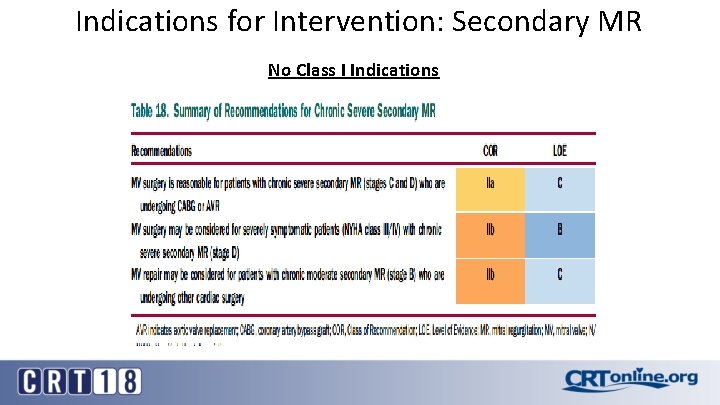
Indications for Intervention: Secondary MR No Class I Indications

Standard of care: MV Repair

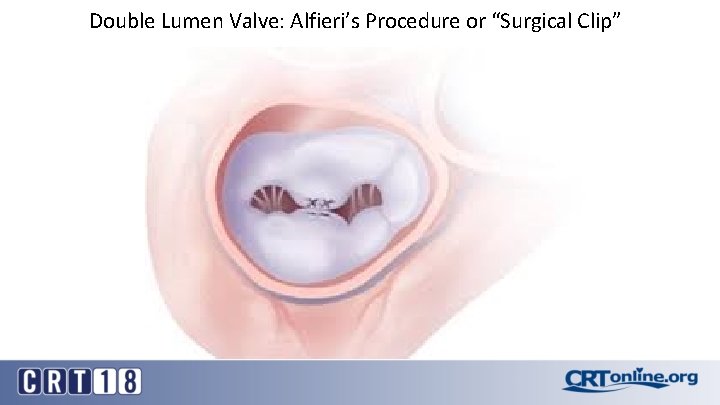
Double Lumen Valve: Alfieri’s Procedure or “Surgical Clip”


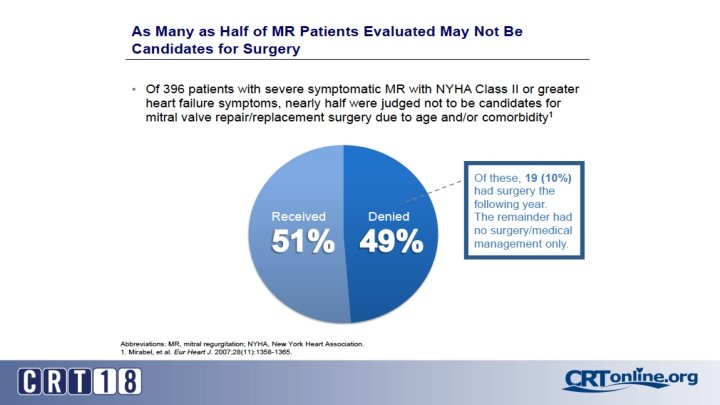
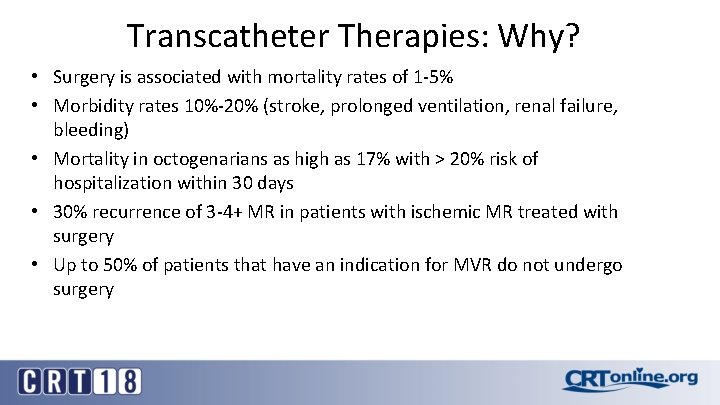
Transcatheter Therapies: Why? • Surgery is associated with mortality rates of 1 -5% • Morbidity rates 10%-20% (stroke, prolonged ventilation, renal failure, bleeding) • Mortality in octogenarians as high as 17% with > 20% risk of hospitalization within 30 days • 30% recurrence of 3 -4+ MR in patients with ischemic MR treated with surgery • Up to 50% of patients that have an indication for MVR do not undergo surgery

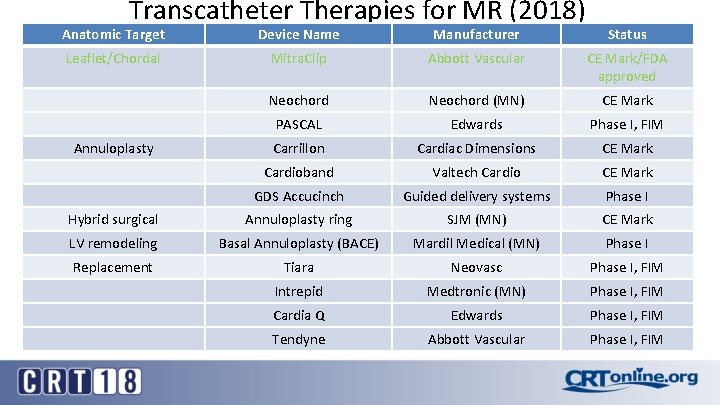
Transcatheter Therapies for MR (2018) Anatomic Target Device Name Manufacturer Status Leaflet/Chordal Mitra. Clip Abbott Vascular CE Mark/FDA approved Neochord (MN) CE Mark PASCAL Edwards Phase I, FIM Carrillon Cardiac Dimensions CE Mark Cardioband Valtech Cardio CE Mark GDS Accucinch Guided delivery systems Phase I Hybrid surgical Annuloplasty ring SJM (MN) CE Mark LV remodeling Basal Annuloplasty (BACE) Mardil Medical (MN) Phase I Replacement Tiara Neovasc Phase I, FIM Intrepid Medtronic (MN) Phase I, FIM Cardia Q Edwards Phase I, FIM Tendyne Abbott Vascular Phase I, FIM Annuloplasty

The Clinical Evidence: EVEREST II Trial




EVEREST II 1 -Year Results “percutaneous repair less effective… but associated with superior safety” Device Control (Surgery) Death 6% 6% Surgery for MV dysfunction 20% 2% Grade 3+ or 4+ MR 21% 20% Primary End-Point (freedom from death, MV surgery or MR grade 3 -4) MACE 55% 73% 15% 48% Feldman et al. NEJM 2011; 364: 1395 -406

FDA approved indications and ACC/AHA Guidelines: Mitra. Clip

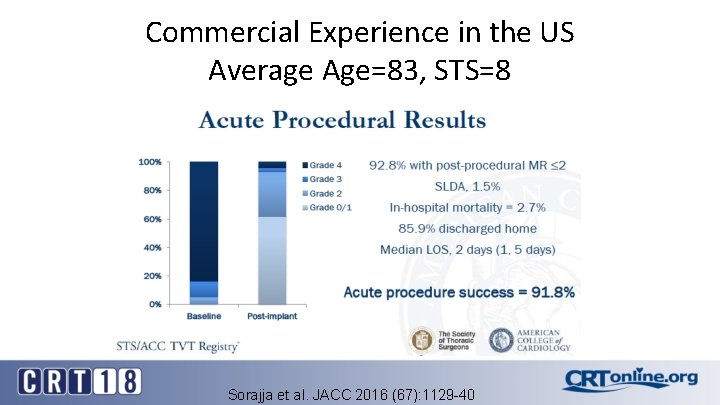
Commercial Experience in the US Average Age=83, STS=8 Sorajja et al. JACC 2016 (67): 1129 -40

Residual MR After Mitra. Clip 93 % with Residual MR Grade 1 or 2. 37% required > 1 Clip

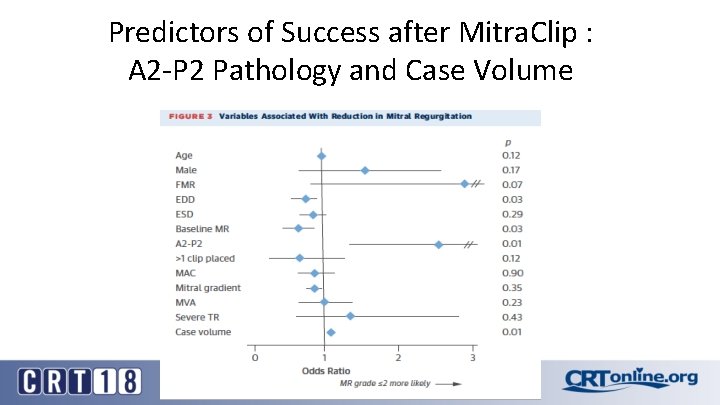
Predictors of Success after Mitra. Clip : A 2 -P 2 Pathology and Case Volume

Short-Term (30 -day) Outcomes Sorajja et al. JACC 2016 (67): 1129 -40

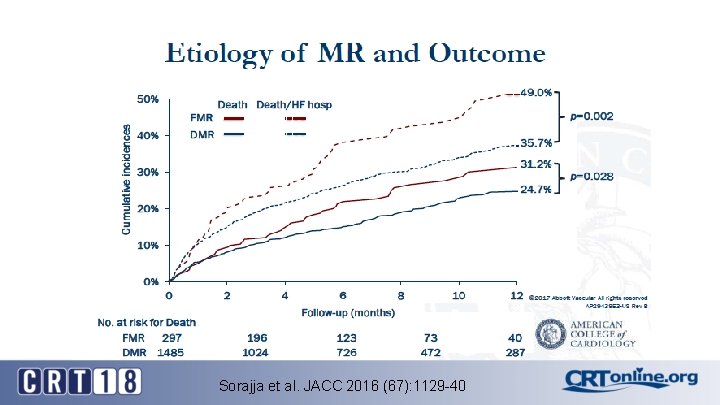
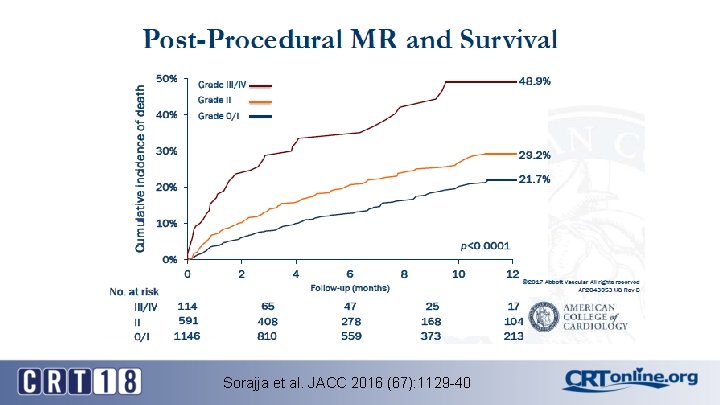
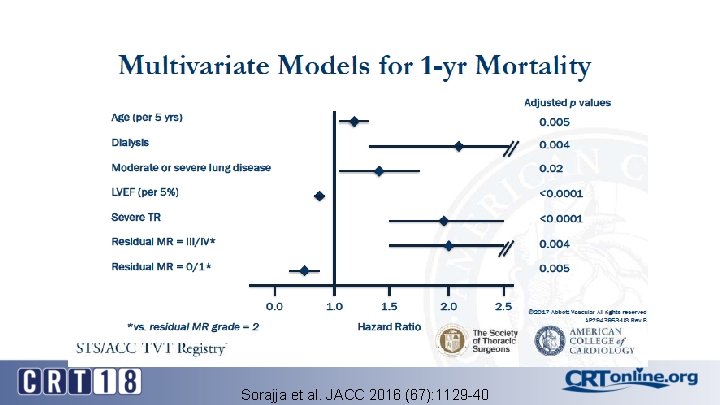
Sorajja et al. JACC 2016 (67): 1129 -40

Sorajja et al. JACC 2016 (67): 1129 -40


Sorajja et al. JACC 2016 (67): 1129 -40

Sorajja et al. JACC 2016 (67): 1129 -40

Ongoing Mitra. Clip Trials • COAPT: Clinical Outcomes Assessment of the Mitra. Clip Percutaneous Therapy for Extremely High-Surgical Risk Patients (NCT: 01626079)


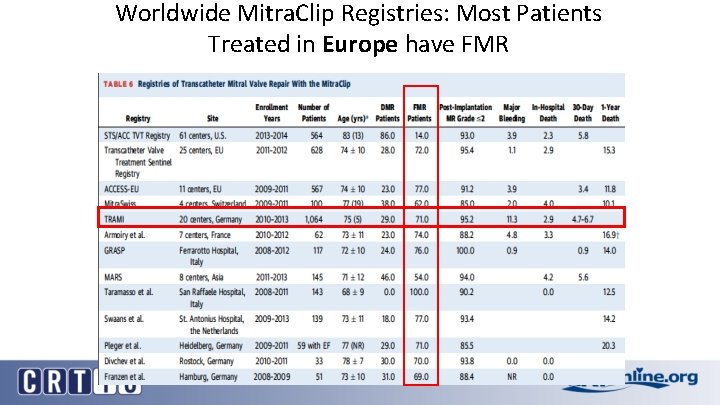
Worldwide Mitra. Clip Registries: Most Patients Treated in Europe have FMR


FDA approved indications and ACC/AHA Guidelines: Mitra. Clip (2017)

Percutaneous Treatment of Mitral Regurgitation Santiago Garcia, MD Associate Professor of Medicine, University of Minnesota Director, Structural Heart Program MVAHCS Garci 205@umn. edu
 Mitral regurgitation symptoms
Mitral regurgitation symptoms Ejection systolic murmur causes
Ejection systolic murmur causes Aortic regurgitation murmur
Aortic regurgitation murmur Pictures
Pictures Percutaneous image-guided lumbar decompression (pild)
Percutaneous image-guided lumbar decompression (pild) Percutaneous balloon pericardiotomy
Percutaneous balloon pericardiotomy Indication for cholecystectomy
Indication for cholecystectomy Percutaneous umbilical blood sampling
Percutaneous umbilical blood sampling Common bile duct diameter
Common bile duct diameter Ellis curve radiology
Ellis curve radiology Primary tricuspid regurgitation
Primary tricuspid regurgitation Jet fazil
Jet fazil Pannus
Pannus Tricuspid regurgitation echo assessment
Tricuspid regurgitation echo assessment Right sided vs left sided murmurs
Right sided vs left sided murmurs Peripheral signs of aortic regurgitation
Peripheral signs of aortic regurgitation Chemosis
Chemosis Lacrimal sac
Lacrimal sac Mitrial
Mitrial Tricuspid valve
Tricuspid valve Mitral facies
Mitral facies Professor richard schilling
Professor richard schilling Severe ms heart
Severe ms heart Pathophysiology of valvular heart disease
Pathophysiology of valvular heart disease Define mitral stenosis
Define mitral stenosis Mitral stenosis chest x ray
Mitral stenosis chest x ray Rhumatic fever criteria
Rhumatic fever criteria Atheromatous thoracic aorta
Atheromatous thoracic aorta Mitral stenosis measurements
Mitral stenosis measurements Apical pulse and mitral valve
Apical pulse and mitral valve Orttirilgan yurak nuqsonlari
Orttirilgan yurak nuqsonlari Presistolik şiddetlenme
Presistolik şiddetlenme Insuficiencia mitral
Insuficiencia mitral Pht mitral valve
Pht mitral valve Pressure half time formula
Pressure half time formula Valvuloplastia mitral percutánea
Valvuloplastia mitral percutánea Valvula mitral en paracaidas
Valvula mitral en paracaidas Mitral stenosis pulmonary hypertension
Mitral stenosis pulmonary hypertension