Percutaneous Treatment for Functional Mitral Regurgitation Using the











- Slides: 31

Percutaneous Treatment for Functional Mitral Regurgitation Using the Carillon® Mitral Contour System™ CRT 2012 February 5 -8, 2012 Steven L. Goldberg, MD Director, Cardiac Cath Lab Clinical Associate Professor of Medicine University of Washington Medical Center Chief Clinical Officer Cardiac Dimensions, Inc.

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial Interest /arrangement or affiliation with the organization(s) listed below Affiliation/Financial Relationship Grant/ Research Support: Company Consulting Fees/Honoraria: Cardiac Dimensions, Inc Major Stock Shareholder/Equity Interest: Cardiac Dimensions, Inc Royalty Income: Ownership/Founder: Salary: Intellectual Property Rights: Other Financial Benefit:

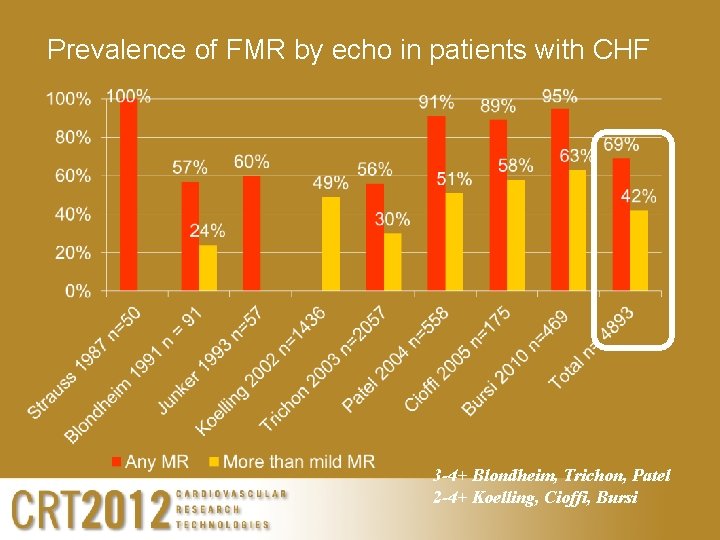
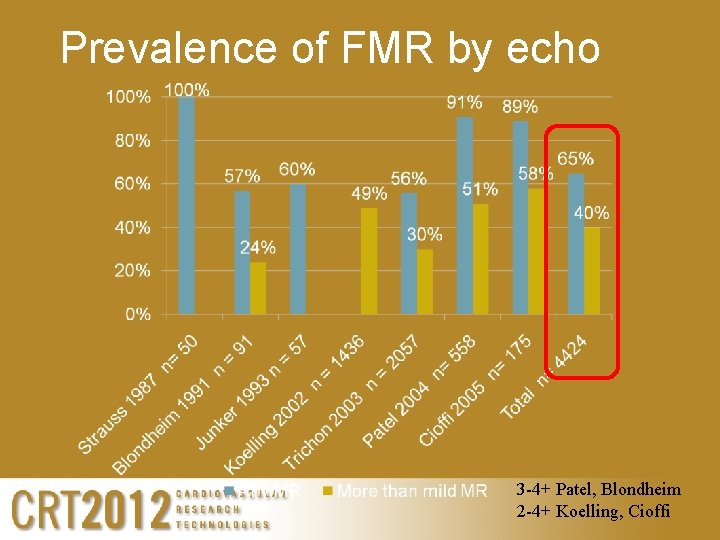
Prevalence of FMR by echo in patients with CHF 3 -4+ Blondheim, Trichon, Patel 2 -4+ Koelling, Cioffi, Bursi

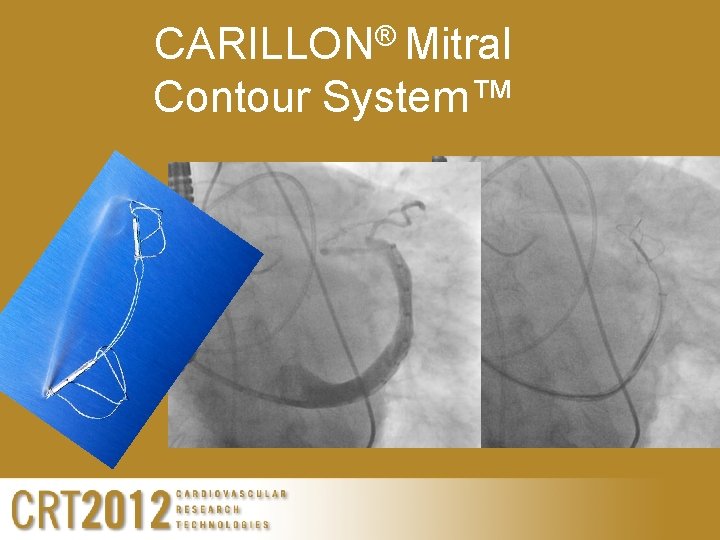
CARILLON® Mitral Contour System™

Removable • Compromise of coronary artery (resolve with device removal) • Insufficient acute reduction of mitral regurgitation

AMADEUS study

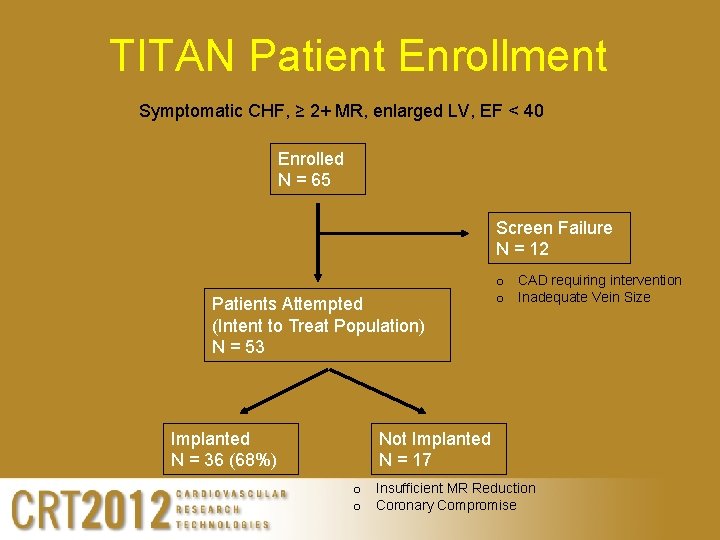
TITAN Patient Enrollment Symptomatic CHF, ≥ 2+ MR, enlarged LV, EF < 40 Enrolled N = 65 Screen Failure N = 12 Patients Attempted (Intent to Treat Population) N = 53 Implanted N = 36 (68%) o CAD requiring intervention o Inadequate Vein Size Not Implanted N = 17 o Insufficient MR Reduction o Coronary Compromise

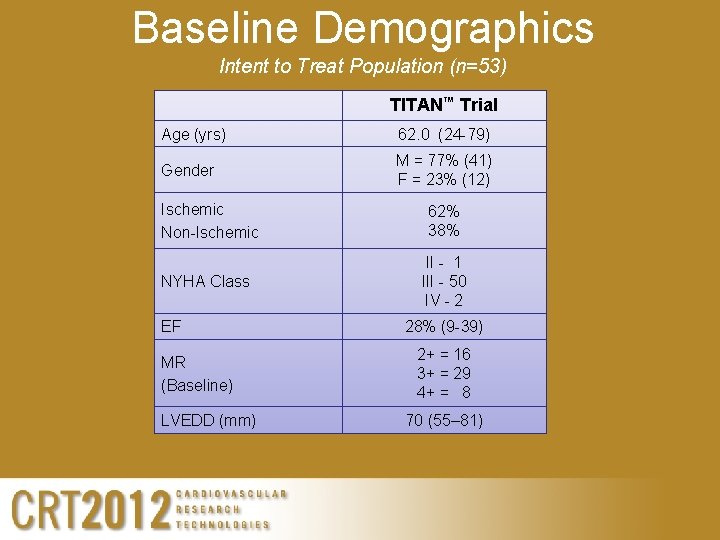
Baseline Demographics Intent to Treat Population (n=53) TITAN™ Trial Age (yrs) 62. 0 (24 -79) Gender M = 77% (41) F = 23% (12) Ischemic Non-Ischemic 62% 38% NYHA Class II - 1 III - 50 IV - 2 EF MR (Baseline) LVEDD (mm) 28% (9 -39) 2+ = 16 3+ = 29 4+ = 8 70 (55– 81)

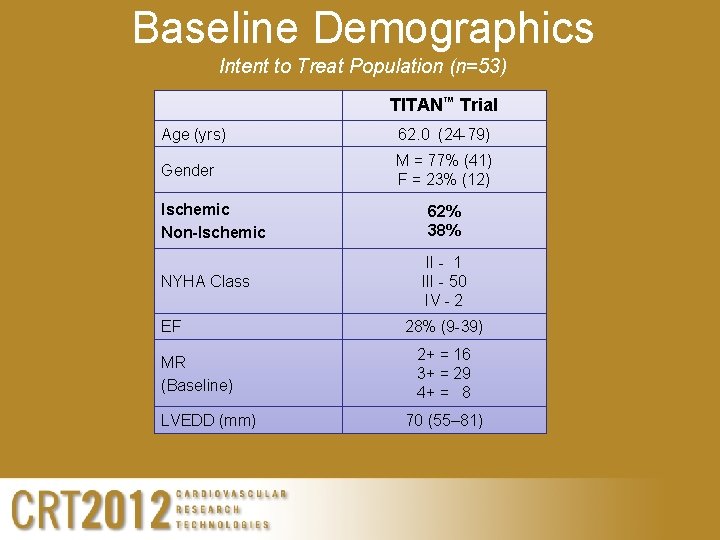
Baseline Demographics Intent to Treat Population (n=53) TITAN™ Trial Age (yrs) 62. 0 (24 -79) Gender M = 77% (41) F = 23% (12) Ischemic Non-Ischemic NYHA Class EF MR (Baseline) LVEDD (mm) 62% 38% II - 1 III - 50 IV - 2 28% (9 -39) 2+ = 16 3+ = 29 4+ = 8 70 (55– 81)

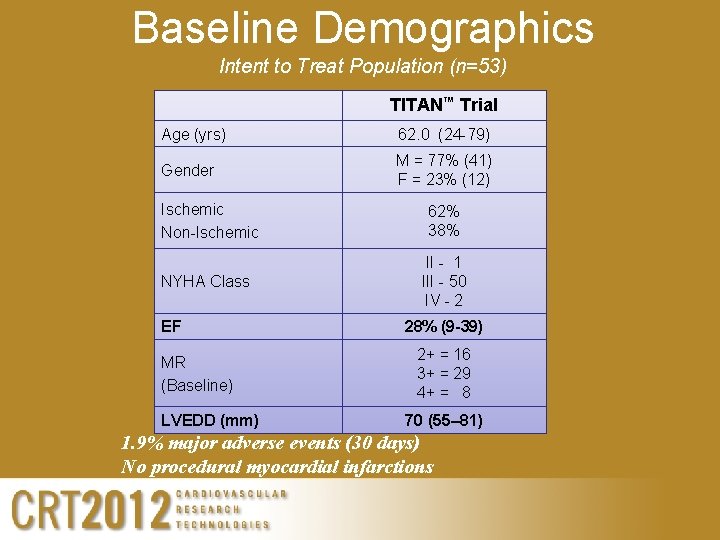
Baseline Demographics Intent to Treat Population (n=53) TITAN™ Trial Age (yrs) 62. 0 (24 -79) Gender M = 77% (41) F = 23% (12) Ischemic Non-Ischemic 62% 38% NYHA Class II - 1 III - 50 IV - 2 EF MR (Baseline) LVEDD (mm) 28% (9 -39) 2+ = 16 3+ = 29 4+ = 8 70 (55– 81) 1. 9% major adverse events (30 days) No procedural myocardial infarctions

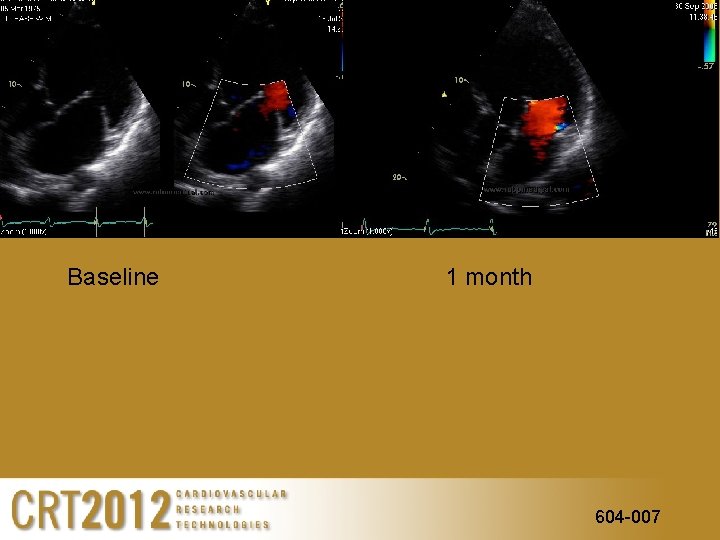
Baseline 1 month 604 -007

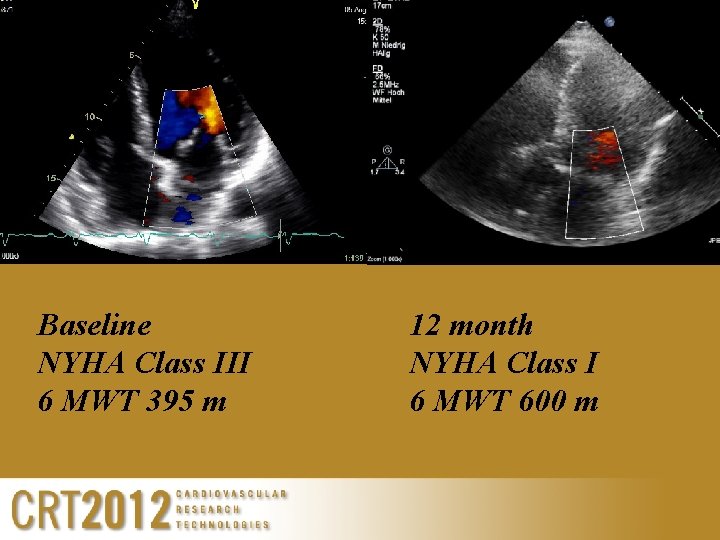
Baseline NYHA Class III 6 MWT 395 m 12 month NYHA Class I 6 MWT 600 m

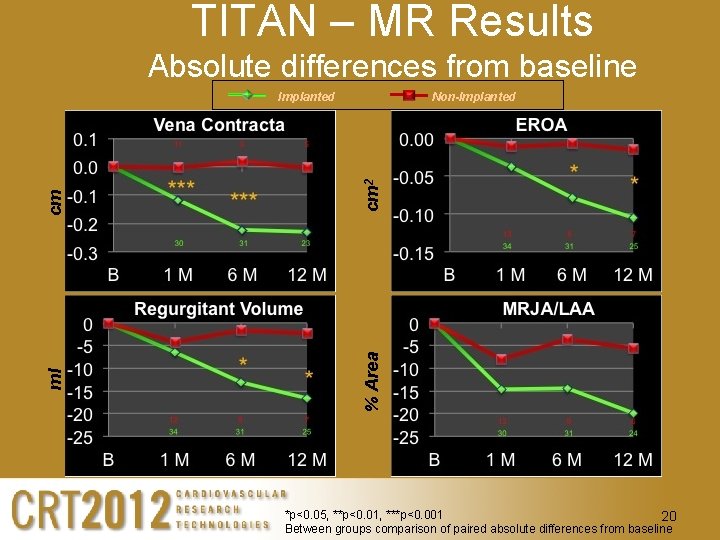
TITAN – MR Results Absolute differences from baseline cm 2 Non-Implanted % Area ml cm Implanted *p<0. 05, **p<0. 01, ***p<0. 001 20 Between groups comparison of paired absolute differences from baseline

How important is a dramatic reduction in MR at the time of implant with the CARILLON® Mitral Contour System™? • An argument that “conventional wisdom” may not hold up

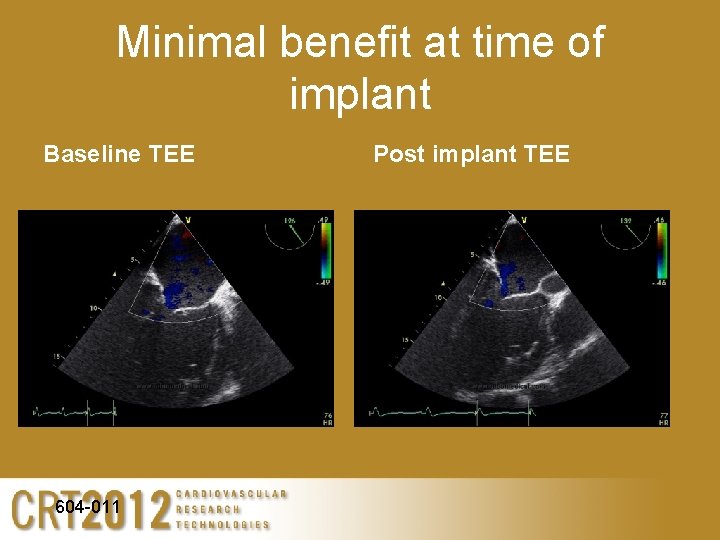
Minimal benefit at time of implant Baseline TEE 604 -011 Post implant TEE

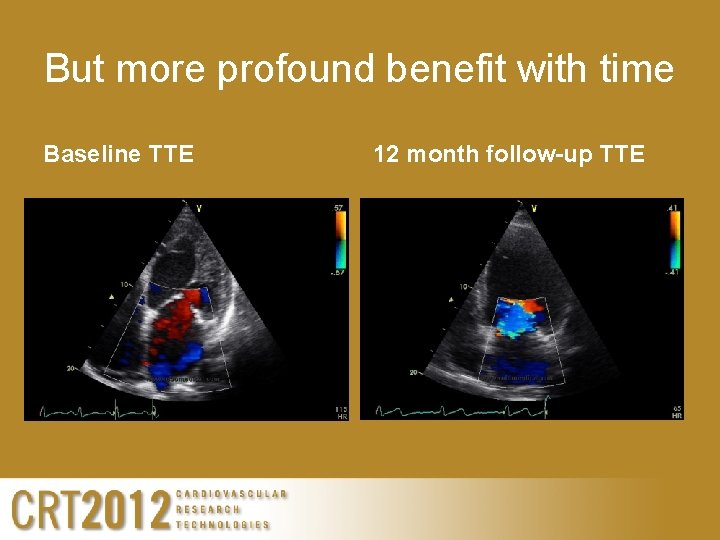
But more profound benefit with time Baseline TTE 12 month follow-up TTE

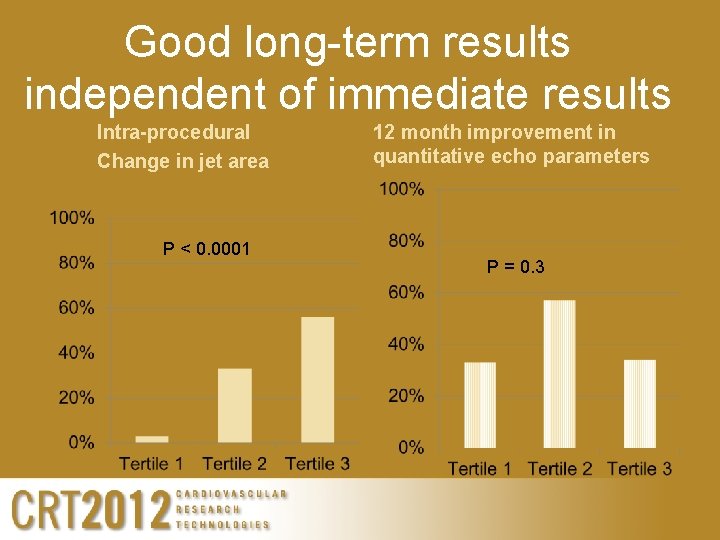
Good long-term results independent of immediate results Intra-procedural Change in jet area P < 0. 0001 12 month improvement in quantitative echo parameters P = 0. 3

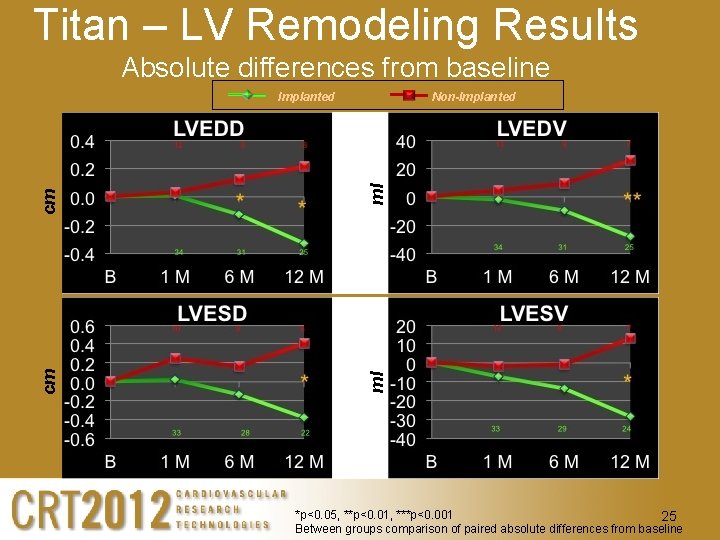
Titan – LV Remodeling Results Absolute differences from baseline ml Non-Implanted ml cm cm Implanted *p<0. 05, **p<0. 01, ***p<0. 001 25 Between groups comparison of paired absolute differences from baseline

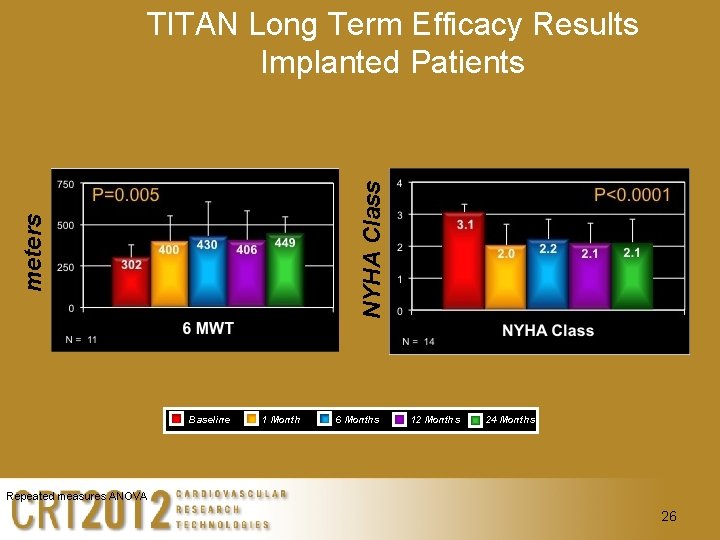
meters NYHA Class TITAN Long Term Efficacy Results Implanted Patients Baseline 1 Month 6 Months 12 Months 24 Months Repeated measures ANOVA 26

Future Directions • Re-initiated OUS studies, with several successful implants • CE Mark renewal – In planning phase for European commercialization • Working with FDA to provide framework for US Pivotal trial

Thank you!

A cosmetic challenge • Occasional loss of anchor wire integrity – Safety maintained: No association with adverse events – Efficacy maintained: Clinical improvement observed through 12 months

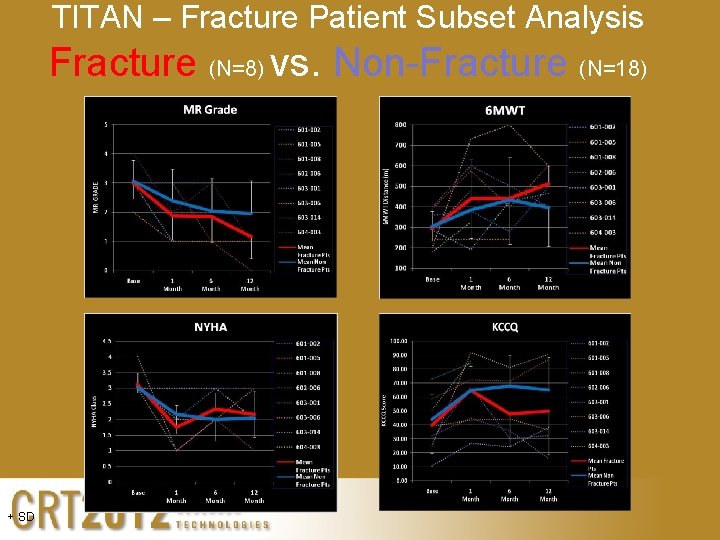
TITAN – Fracture Patient Subset Analysis Fracture (N=8) vs. Non-Fracture (N=18) + SD

Minor device modifications Old XE 2 New XE 2

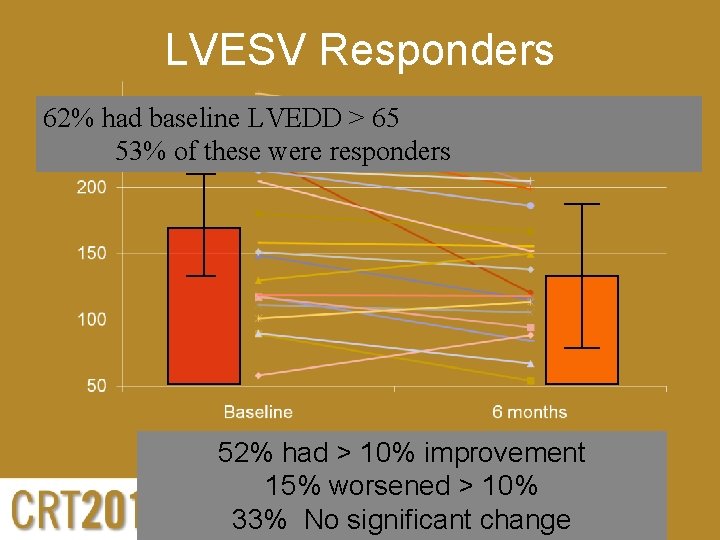
LVESV Responders 62% had baseline LVEDD > 65 53% of these were responders 52% had > 10% improvement 15% worsened > 10% 33% No significant change

“The reports of my death are greatly exaggerated” Mark Twain • While browsing through the popular interventional cardiology website CRTonline. org…. .

Clinical Trial Conclusions • Percutaneous reduction of FMR in the TITAN trial was associated with a 1. 9% 30 day MAE rate • There was a 40% reduction in FMR in the implanted population • Functional improvement was greater in the implanted patients compared to the nonimplanted patients • Reverse remodeling noted in implanted patients 35

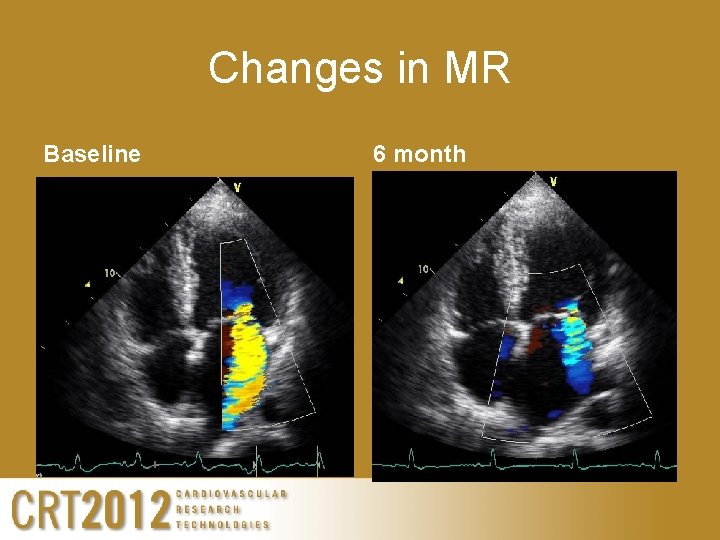
Changes in MR Baseline 6 month

Prevalence of FMR by echo 3 -4+ Patel, Blondheim 2 -4+ Koelling, Cioffi

Worsening clinical parameters with FMR • Mortality Blondheim 1991, Junker 1993, Koelling 2002, Trichon 2003, Cioffi 2005, etc. • Tricuspid regurgitation Blondheim 1991, Koelling 2002 • Hemodynamic parameters Junker 1993 • Increased LV and LA chambers Tada 1987, Strauss 1987 • Exercise performance Tada 1987, Junker 1993 • Symptoms or worse NYHA class Blondheim 1991, Junker 1993, Koelling 2002, Cioffi 2005

Slide Title • Level One bullet – Level Two bullet • Level Three bullet