Other Gas Laws Aim What other laws help













- Slides: 16

Other Gas Laws Aim: What other laws help us explain gas behavior? Do Now: Solve the Combined Gas Law Problem on the White Board Homework: Complete worksheet. Show all work. Box in final answers with appropriate units QUIZ – WED CASTLE LEARNING / EXAM - FRIDAY

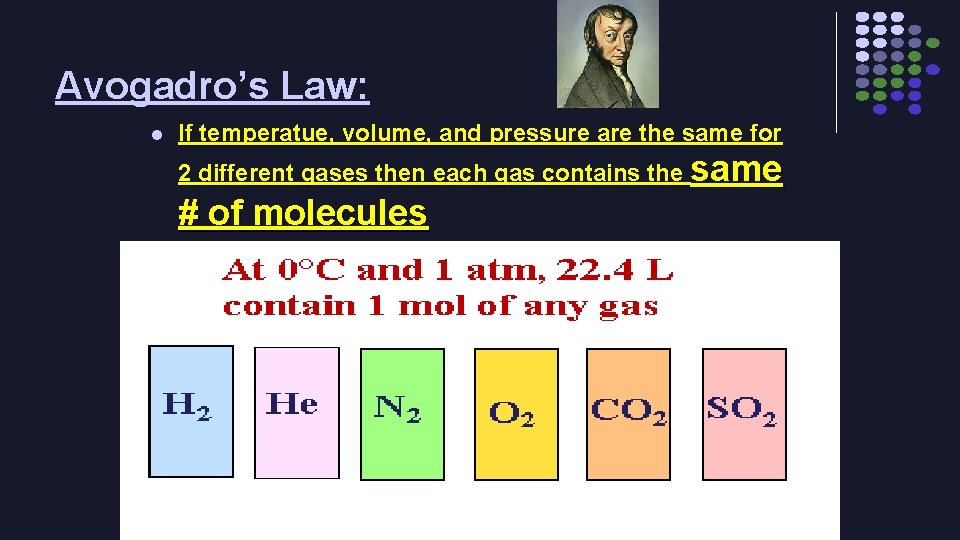
Avogadro’s Hypothesis… *Gases at the same volume, pressure, & temperature have the same number of molecules

Avogadro’s Law: l If temperatue, volume, and pressure are the same for 2 different gases then each gas contains the same # of molecules

Example 1 A 10. 0 liter flask at a given temperature and pressure contains 6. 0 x 1023 molecules of hydrogen gas. Under the same conditions of temperature and pressure, how many molecules would a 10. 0 liter flask of nitrogen gas contain? Example 2 At STP, 1 liter of H 2(g) and 1 liter of He(g) have the same l (a) mass (c) # of atoms l (b) density (d) # of molecules

Example 3 At Standard temperature and pressure, 32. 0 liters of O 2 contain the same number of molecules as: A) 44. 8 L of He B) 32. 0 L of H 2 C) 28. 0 L of N 2 D) 22. 4 L Ar l l Correct Answer: Option B 32. 0 L of H 2

Explain Avogadro’s Law in your own words

Dalton’s Law Partial Pressure (Easy) Ptotal = P 1 + P 2 + P 3 • A container holds three gases: oxygen, carbon dioxide, and helium. The partial pressure of the three gases are 2. 00 atm, 3. 00 atm, and 4. 00 atm, respectively. What is the total pressure inside the container? • 9. 00 atm • Hydrogen gas at a pressure of 1. 5 atm is added to Oxygen gas at 2. 0 atm. What is the total pressure? • 3. 5 atm

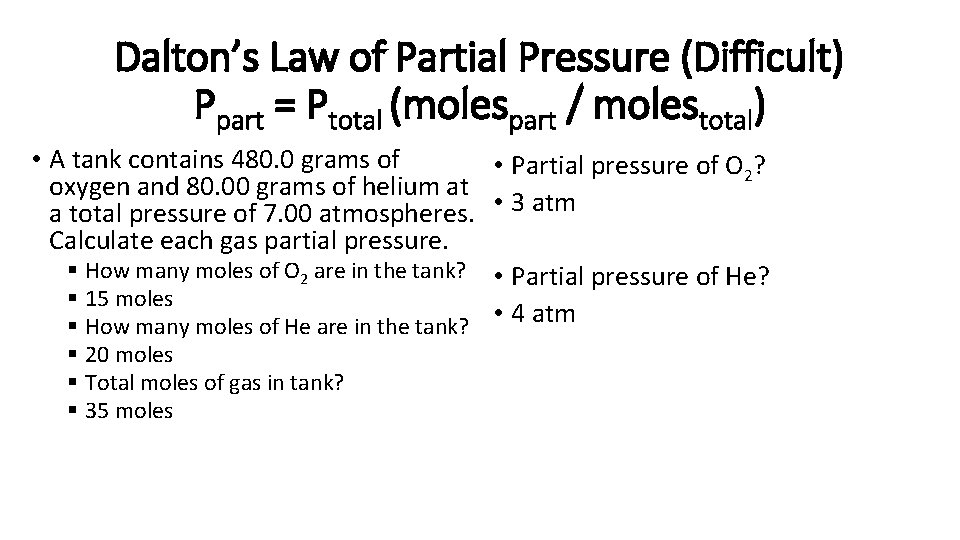
Dalton’s Law of Partial Pressure (Difficult) Ppart = Ptotal (molespart / molestotal) • A tank contains 480. 0 grams of • Partial pressure of O 2? oxygen and 80. 00 grams of helium at a total pressure of 7. 00 atmospheres. • 3 atm Calculate each gas partial pressure. § How many moles of O 2 are in the tank? • Partial pressure of He? § 15 moles § How many moles of He are in the tank? • 4 atm § 20 moles § Total moles of gas in tank? § 35 moles

Dalton’s Law of Partial Pressure (Difficult) Ppart = Ptotal (molespart / molestotal) • A tank contains 5. 00 moles of O 2, 3 moles of neon, 6. 00 moles of H 2 S, and 4. 00 moles of argon at a total pressure of 1620. 0 mm Hg. Calculate the partial pressure of each gas. • • O 2 = 450 Ne = 270 H 2 S = 540 Ar = 360 • A mixture of 14. 0 g of hydrogen, 84 g of nitrogen, and 2. 0 mole of oxygen are placed in a flask. When the partial pressure of the oxygen is 78. 00 mm Hg, what is the total pressure in the flask? • 585 mm Hg

Graham’s Law l Relates the rate of diffusion of gases to their molar masses (GFM) l Diffusion is movement of gas particles from an area of high concentration to an area of low concentration l If someone in a room uses a deodorant spray, it doesn't take long for everyone else in the room to smell it. This is because of diffusion.



Graham’s Law… l The GREATER the molar mass (GFM) of a gas the SLOWER it diffuses l Gases with small GFM diffuse very rapidly Gases with large GFM diffuse slowly l l l Example: At STP which of the following gases will diffuse most rapidly? (1) Cl 2 (2) NH 3 (3) CO 2 (4) N 2

Graham’s Law • At constant temperature, SO 2 molecules have a velocity of 12. 50 m/s. What is the velocity of Hydrogen molecules under the same conditions? • 70. 36 m/s • At constant temperature, sulfur dioxide molecules, SO 2 will diffuse in 34 minutes. How long will it take hydrogen molecules (H 2) under the same conditions? • 6. 04

Grahams Law • A sample of Xenon effuses through a porous container 9. 50 times faster than an unknown gas. What is the molecular mass of this unknown gas? • 11849. 8 g • A sample of Neon effuses through a container 6. 50 times faster than an unknown gas. What is the molecular mass of this unknown gas? • 853 g

Summary • Avogadro's Hypothesis • Daltons Law of Partial Pressure • Graham’s Law of Effusion
 Rules for mad gab
Rules for mad gab My mother makes me chicken poem
My mother makes me chicken poem River
River Master asl unit 2 pdf
Master asl unit 2 pdf Helper
Helper Education through self help is our motto
Education through self help is our motto Freesurfer troubleshooting
Freesurfer troubleshooting Facts about montesquieu
Facts about montesquieu All the gas laws
All the gas laws Ap chemistry gas laws
Ap chemistry gas laws Lussac's law worksheet answer key
Lussac's law worksheet answer key Kmt gas laws
Kmt gas laws Direct or indirect relationship
Direct or indirect relationship N in ideal gas law
N in ideal gas law Gas laws
Gas laws Different gas laws
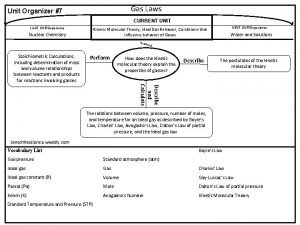
Different gas laws Gas laws graphic organizer
Gas laws graphic organizer