ORGANIC VS INORGANIC COMPOUNDS Another way of classifying












- Slides: 19

ORGANIC VS. INORGANIC COMPOUNDS Another way of classifying chemical compounds…

Every compound in the world is classified as either Organic , or Inorganic. Did you know that well over 50% of all known compounds on earth are Organic compounds? ?

Organic Compounds • Organic compounds contain carbon (C) (and usually hydrogen (H)) • Sometimes other types of atoms near carbon on the periodic table are also attached, especially: nitrogen, oxygen, sulfur, phosphorus and the halogens

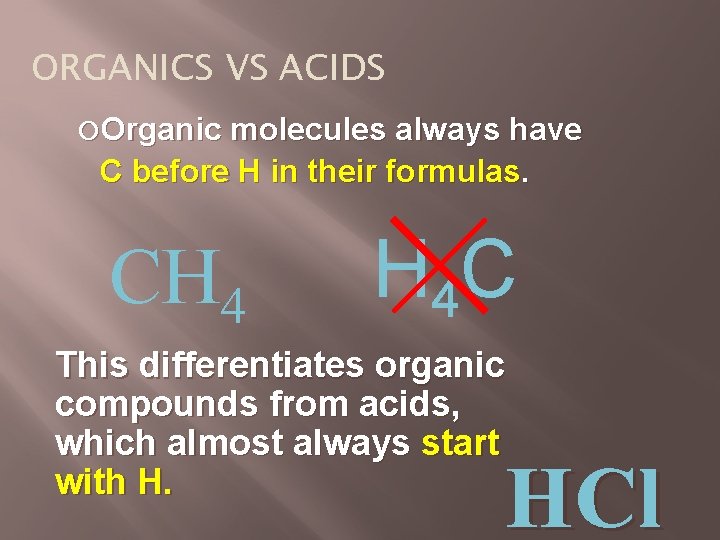
ORGANICS VS ACIDS Organic molecules always have C before H in their formulas. CH 4 H 4 C This differentiates organic compounds from acids, which almost always start with H. HCl

Is that why foods are called “organic”? Organic actually derives from “coming from life”, because the compounds that plants and animals make are all C-compounds This is NOT the meaning of organic foods though! In this case organic foods refer to being produced without chemicals (ironically many of these chemicals are actually ORGANIC compounds!)

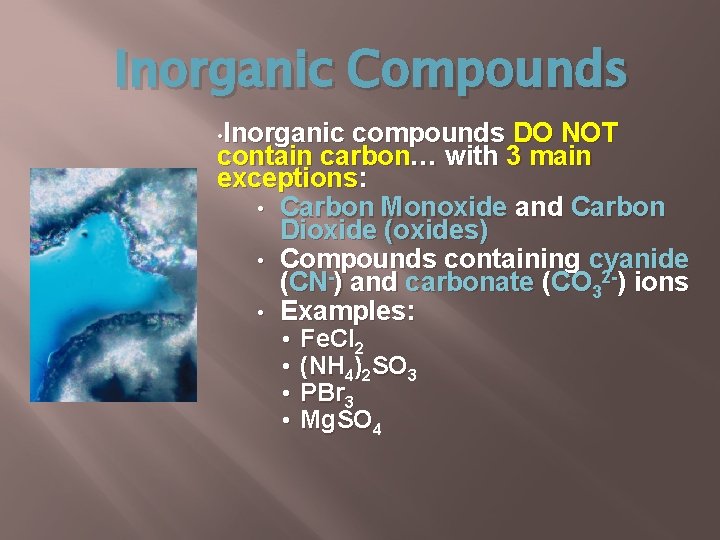
Inorganic Compounds • Inorganic compounds DO NOT contain carbon… with 3 main exceptions: • Carbon Monoxide and Carbon Dioxide (oxides) • Compounds containing cyanide (CN-) and carbonate (CO 32 -) ions • Examples: • • Fe. Cl 2 (NH 4)2 SO 3 PBr 3 Mg. SO 4

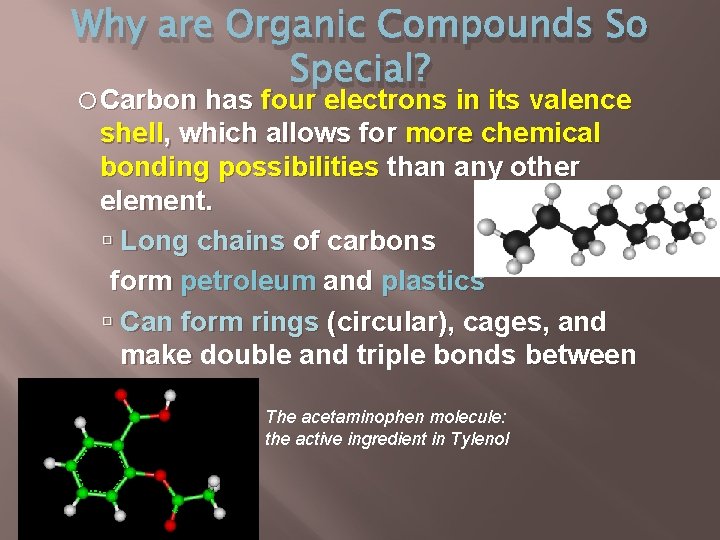
Why are Organic Compounds So Special? Carbon has four electrons in its valence shell, which allows for more chemical bonding possibilities than any other element. Long chains of carbons form petroleum and plastics Can form rings (circular), cages, and make double and triple bonds between carbons The acetaminophen molecule: the active ingredient in Tylenol

There are MANY Types of Organic compounds. We only talk about two. Hydrocarbons Alcohols

Hydrocarbons A hydrocarbon is an organic compound that contains only carbon and hydrogen. Hydrocarbons are based on a carbon “backbone”, or chain, with hydrogen atoms added on the sides

You know about hydrocarbons already! The simplest hydrocarbons are ones that you might recognize… Methane (CH 4)--- main component of natural gas Ethane (C 2 H 6)--- also found in natural gas Propane (C 3 H 8) --- used as a fuel for BBQs Butane (C 4 H 10) --- an extremely flammable fluid used in industrial torches Octane (C 8 H 18)---a combustible liquid in gasoline All hydrocarbons are flammable, and

Alcohols are organic compounds with C, an OH group, and their name ends in ‘ol’. The simplest alcohols are: Methanol (CH 4 O)--- used in labs as a solvent (dissolves other materials) Ethanol (C 2 H 6 O)---is a psychoactive drug (present in alcohol), but is now being considered as a fuel source Isopropyl alcohol (C 3 H 8 O)---rubbing alcohol used to sterilize cuts

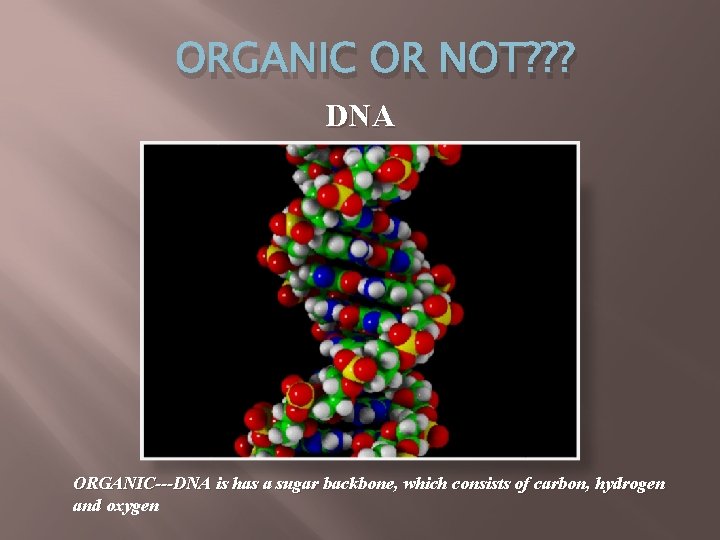
ORGANIC OR NOT? ? ? DNA ORGANIC---DNA is has a sugar backbone, which consists of carbon, hydrogen and oxygen

ORGANIC OR NOT? ? ? Vegetable Oil ORGANIC--- oils and fats are long-chain carbon compounds, which are used by living things as a way of storing energy

ORGANIC OR NOT? ? ? Sulphuric Acid NOT! Sulphuric acid (H 2 SO 4) is the strong acid found in car batteries, but doesn’t contain carbon and isn’t organic

ORGANIC OR NOT? ? ? Corn starch ORGANIC! Starch is produced by plants as a way of storing sugars that they don’t need right away

ORGANIC OR NOT? ? ? Copper (II) Sulphate NOT! Copper (II) sulphate is a beautiful blue crystal, but does not contain carbon and is definitely not organic!

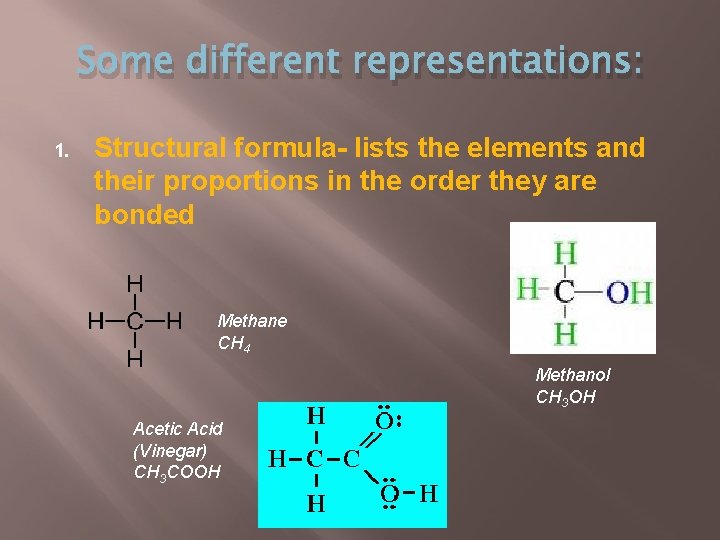
Some different representations: 1. Structural formula- lists the elements and their proportions in the order they are bonded Methane CH 4 Methanol CH 3 OH Acetic Acid (Vinegar) CH 3 COOH

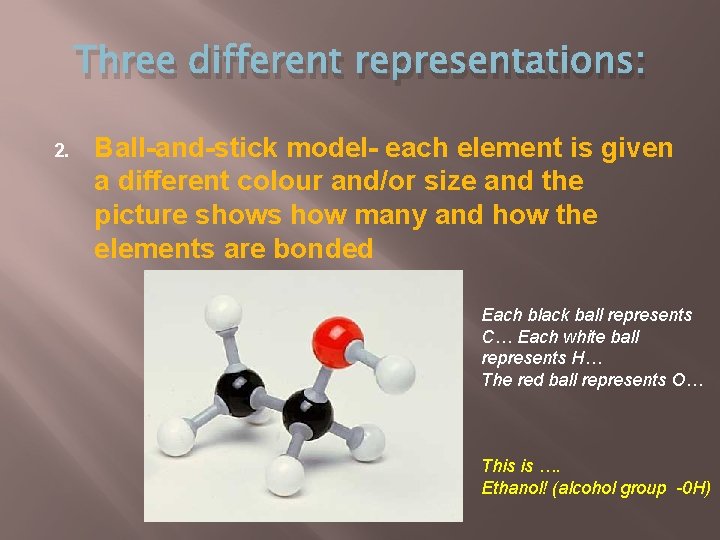
Three different representations: 2. Ball-and-stick model- each element is given a different colour and/or size and the picture shows how many and how the elements are bonded Each black ball represents C… Each white ball represents H… The red ball represents O… This is …. Ethanol! (alcohol group -0 H)

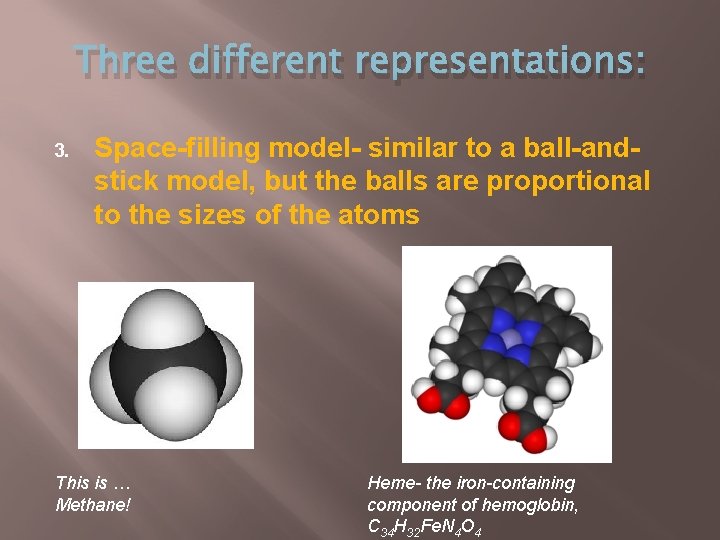
Three different representations: 3. Space-filling model- similar to a ball-andstick model, but the balls are proportional to the sizes of the atoms This is … Methane! Heme- the iron-containing component of hemoglobin, C 34 H 32 Fe. N 4 O 4
 Organic vs inorganic compounds
Organic vs inorganic compounds Organic vs inorganic compounds
Organic vs inorganic compounds Charring test of organic and inorganic compounds
Charring test of organic and inorganic compounds Meaning of the word organic
Meaning of the word organic Organic vs inorganic compounds
Organic vs inorganic compounds Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Organic vs inorganic growth
Organic vs inorganic growth Organic vs inorganic chemistry
Organic vs inorganic chemistry Organic and inorganic cofactors
Organic and inorganic cofactors Asam oksalat rumus kimia
Asam oksalat rumus kimia Organic and inorganic nutrients
Organic and inorganic nutrients Organic vs inorganic chemistry
Organic vs inorganic chemistry Organic and inorganic cofactors
Organic and inorganic cofactors Organic vs inorganic
Organic vs inorganic Which compound is inorganic
Which compound is inorganic Saturated hydrocarbon
Saturated hydrocarbon Smear layer definition
Smear layer definition Organic vs inorganic
Organic vs inorganic Pharmaceutical inorganic chemistry
Pharmaceutical inorganic chemistry Types of matter elements compounds and mixtures
Types of matter elements compounds and mixtures