NUEVAS PERSPECTIVAS EN TERAPIA PRECISIN EN CPNM AVANZADO






- Slides: 49

NUEVAS PERSPECTIVAS EN TERAPIA PRECISIÓN EN CPNM AVANZADO: EGFR, ALK, … Enriqueta Felip Vall d’Hebron University Hospital, Barcelona, Spain #SEOM 2018

Disclosure information Dr Felip has previously acted in an advisory role or speaker’s bureau for Abb. Vie, Astra. Zeneca, Blueprint Medicines, Boehringer-Ingelheim, Bristol. Myers Squibb, Eli Lilly, Guardanthealth, Merck Sharp & Dohme, Novartis, Pfizer, Roche and Takeda #SEOM 2018

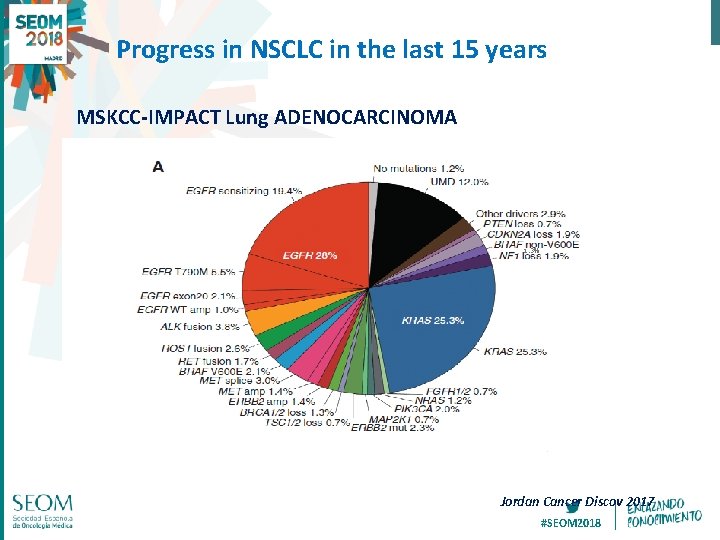
Progress in NSCLC in the last 15 years MSKCC-IMPACT Lung ADENOCARCINOMA 1. 3% Jordan Cancer Discov 2017 #SEOM 2018

EGFR overview #SEOM 2018

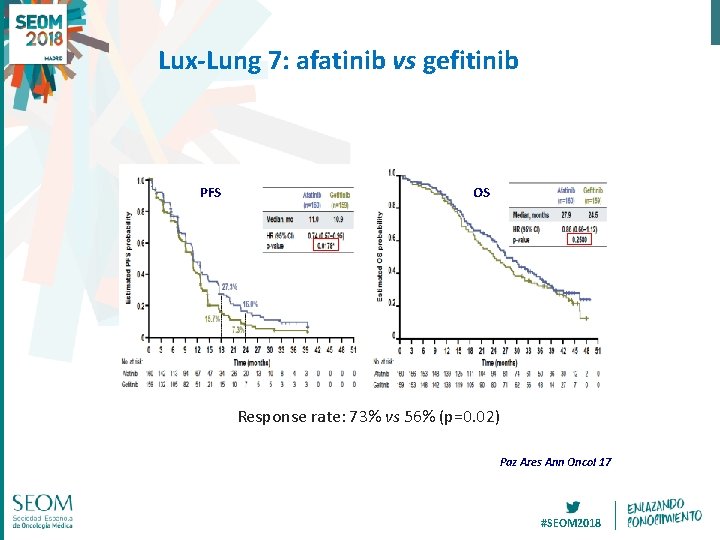
Lux-Lung 7: afatinib vs gefitinib PFS OS Response rate: 73% vs 56% (p=0. 02) Paz Ares Ann Oncol 17 #SEOM 2018

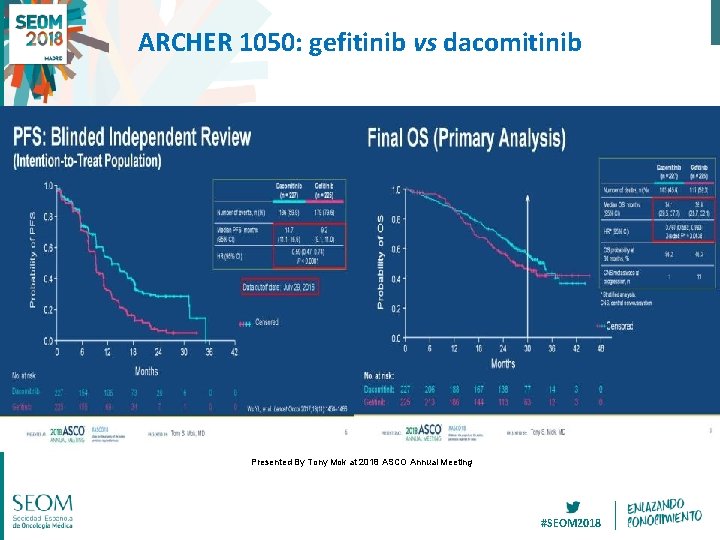
ARCHER 1050: gefitinib vs dacomitinib Presented By Tony Mok at 2018 ASCO Annual Meeting #SEOM 2018

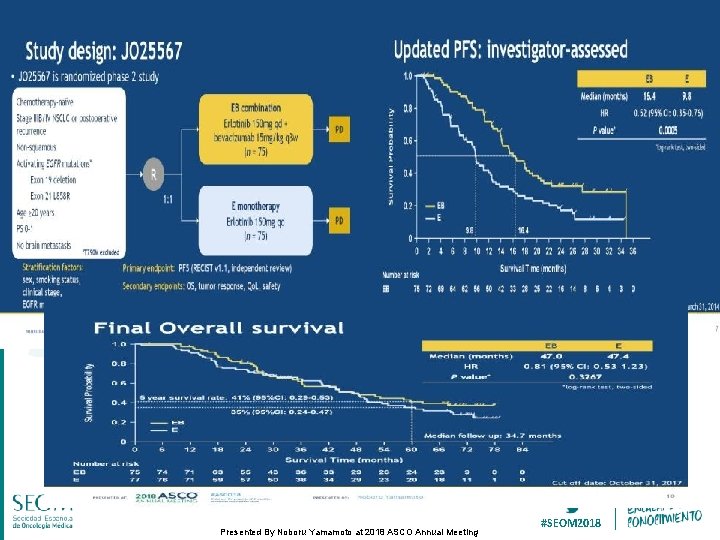
Study design: JO 25567 Presented By Noboru Yamamoto at 2018 ASCO Annual Meeting #SEOM 2018

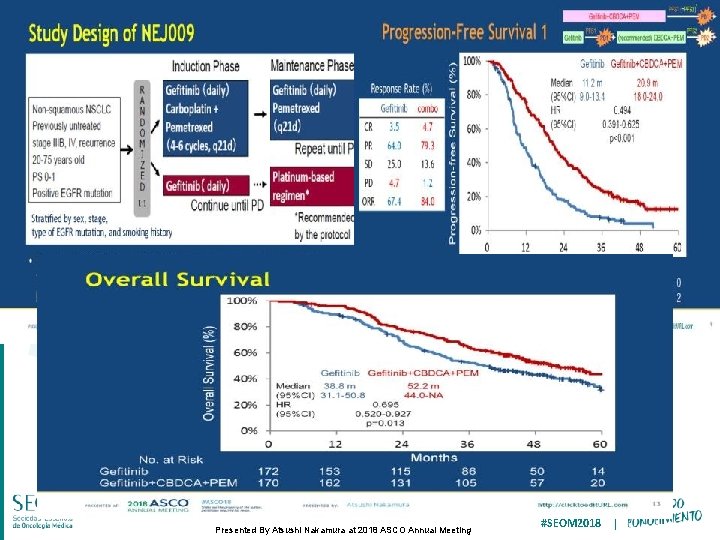
Study Design of NEJ 009 Presented By Atsushi Nakamura at 2018 ASCO Annual Meeting #SEOM 2018

#SEOM 2018

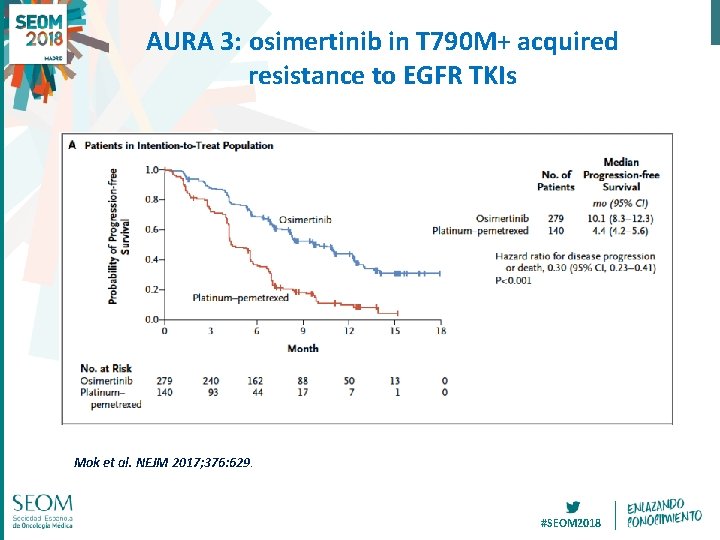
AURA 3: osimertinib in T 790 M+ acquired resistance to EGFR TKIs Mok et al. NEJM 2017; 376: 629. #SEOM 2018

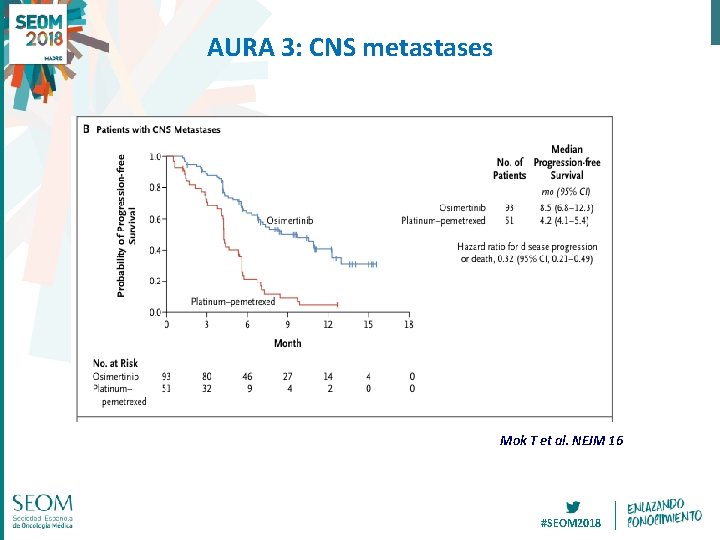
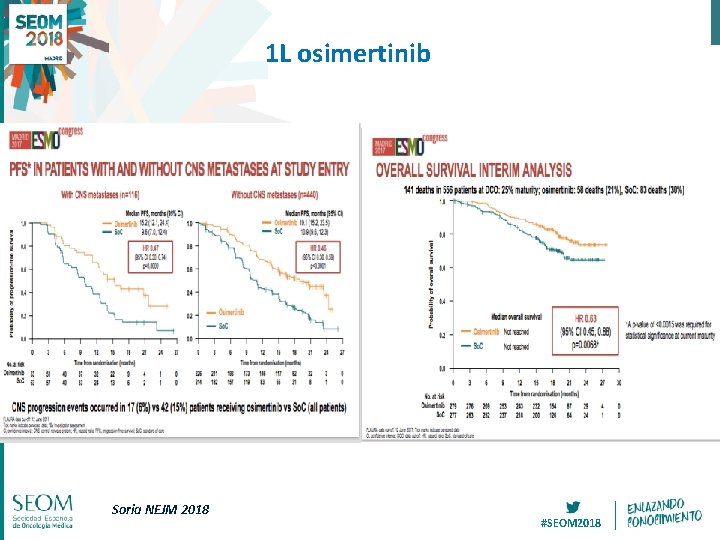
AURA 3: CNS metastases Mok T et al. NEJM 16 #SEOM 2018

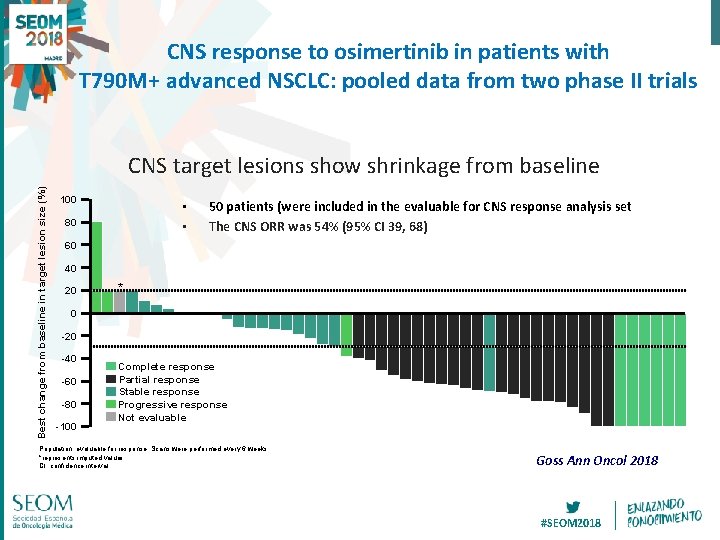
CNS response to osimertinib in patients with T 790 M+ advanced NSCLC: pooled data from two phase II trials Best change from baseline in target lesion size (%) CNS target lesions show shrinkage from baseline 100 • • 80 50 patients (were included in the evaluable for CNS response analysis set The CNS ORR was 54% (95% CI 39, 68) 60 40 20 * 0 -20 -40 -60 -80 -100 Complete response Partial response Stable response Progressive response Not evaluable Population: evaluable for response. Scans were performed every 6 weeks *represents imputed values CI, confidence interval Goss Ann Oncol 2018 #SEOM 2018

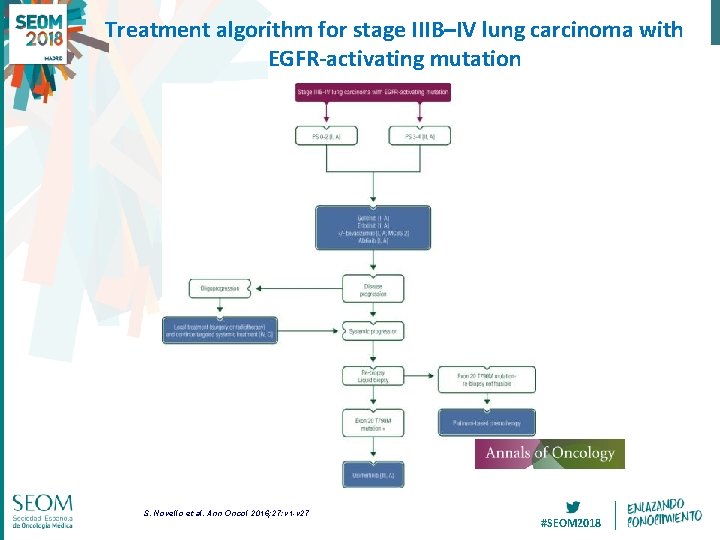
Treatment algorithm for stage IIIB–IV lung carcinoma with EGFR-activating mutation S. Novello et al. Ann Oncol 2016; 27: v 1 -v 27 #SEOM 2018

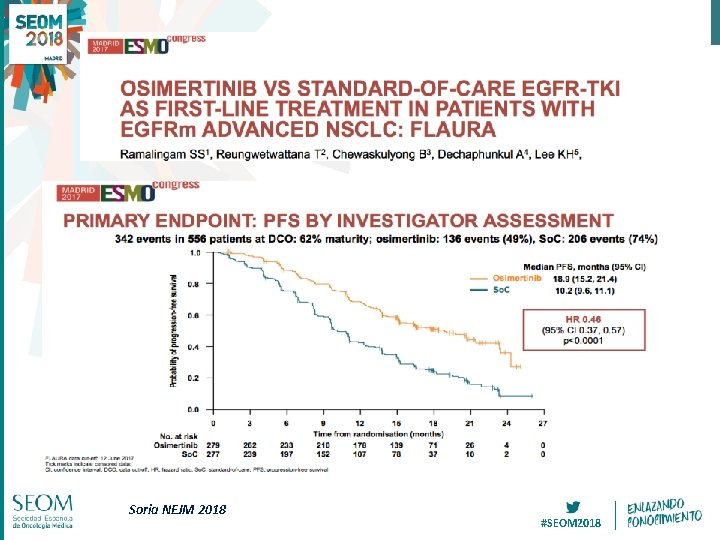
Soria NEJM 2018 #SEOM 2018

1 L osimertinib Soria NEJM 2018 #SEOM 2018

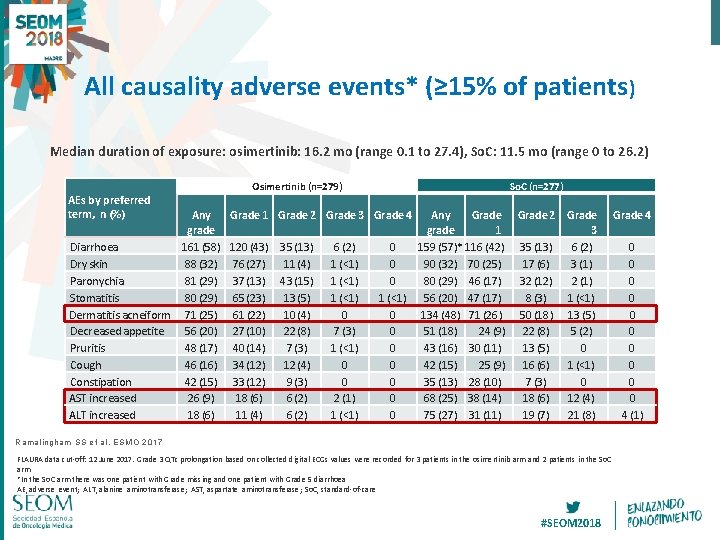
All causality adverse events* (≥ 15% of patients) Median duration of exposure: osimertinib: 16. 2 mo (range 0. 1 to 27. 4), So. C: 11. 5 mo (range 0 to 26. 2) AEs by preferred term, n (%) Any grade Diarrhoea 161 (58) Dry skin 88 (32) Paronychia 81 (29) Stomatitis 80 (29) Dermatitis acneiform 71 (25) Decreased appetite 56 (20) Pruritis 48 (17) Cough 46 (16) Constipation 42 (15) AST increased 26 (9) ALT increased 18 (6) Osimertinib (n=279) So. C (n=277) Grade 1 Grade 2 Grade 3 Grade 4 120 (43) 35 (13) 76 (27) 11 (4) 37 (13) 43 (15) 65 (23) 13 (5) 61 (22) 10 (4) 27 (10) 22 (8) 40 (14) 7 (3) 34 (12) 12 (4) 33 (12) 9 (3) 18 (6) 6 (2) 11 (4) 6 (2) 1 (<1) 0 7 (3) 1 (<1) 0 0 2 (1) 1 (<1) Any Grade 2 grade 1 0 159 (57)* 116 (42) 35 (13) 0 90 (32) 70 (25) 17 (6) 0 80 (29) 46 (17) 32 (12) 1 (<1) 56 (20) 47 (17) 8 (3) 0 134 (48) 71 (26) 50 (18) 0 51 (18) 24 (9) 22 (8) 0 43 (16) 30 (11) 13 (5) 0 42 (15) 25 (9) 16 (6) 0 35 (13) 28 (10) 7 (3) 0 68 (25) 38 (14) 18 (6) 0 75 (27) 31 (11) 19 (7) Grade 3 6 (2) 3 (1) 2 (1) 1 (<1) 13 (5) 5 (2) 0 1 (<1) 0 12 (4) 21 (8) Grade 4 Ramalingham SS et al, ESMO 2017 FLAURA data cut-off: 12 June 2017. Grade 3 QTc prolongation based on collected digital ECGs values were recorded for 3 patients in the osimertinib arm and 2 patients in the So. C arm *In the So. C arm there was one patient with Grade missing and one patient with Grade 5 diarrhoea AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; So. C, standard-of-care 16#SEOM 2018 0 0 0 0 0 4 (1)

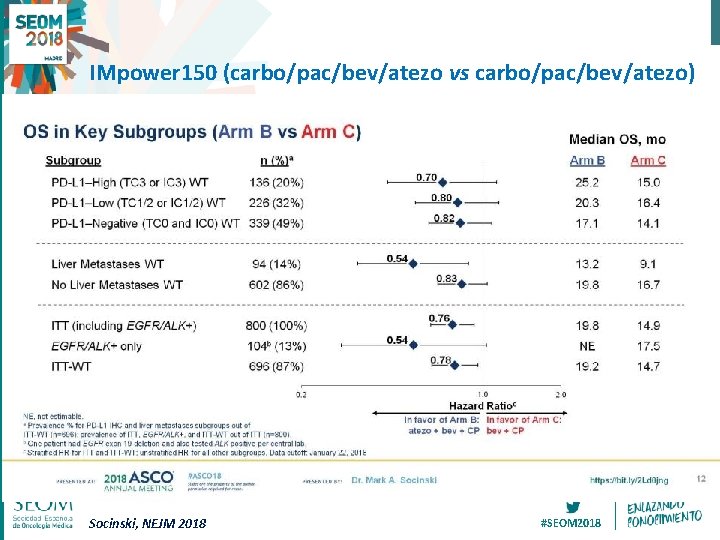
IMpower 150 (carbo/pac/bev/atezo vs carbo/pac/bev/atezo) OS in Key Subgroups (Arm B vs Arm C) Socinski, NEJM 2018 #SEOM 2018

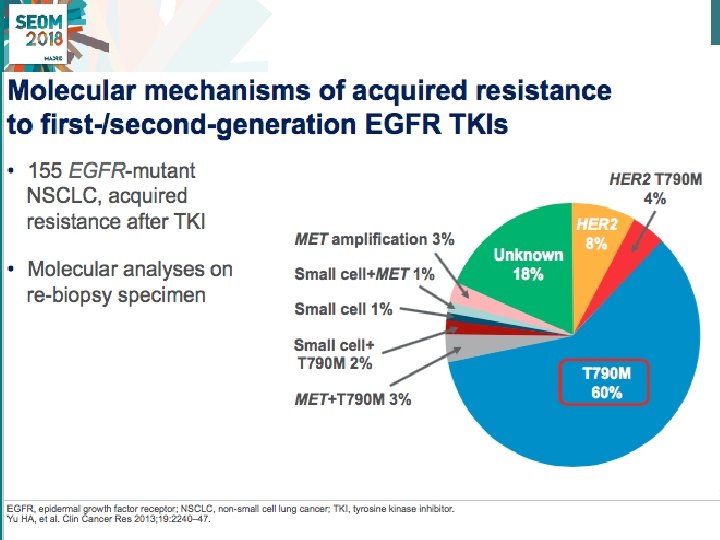
New paradigm on the treatment of patients with EGFRm+ NSCLC • EGFRm, a disease with a number of available options • EGFRTKIs, RR > 70%, PFS >10 mo, in 1 st line and 2 nd line (T 790 M+ pts) • Should 1 -2 nd generation EGFR TKI remain the 1 L treatment or should we use 1 L osimertinib? • Clear need for EGFR determinations in diagnostic and re-biopsy tumor tissue / liquid biopsues #SEOM 2018

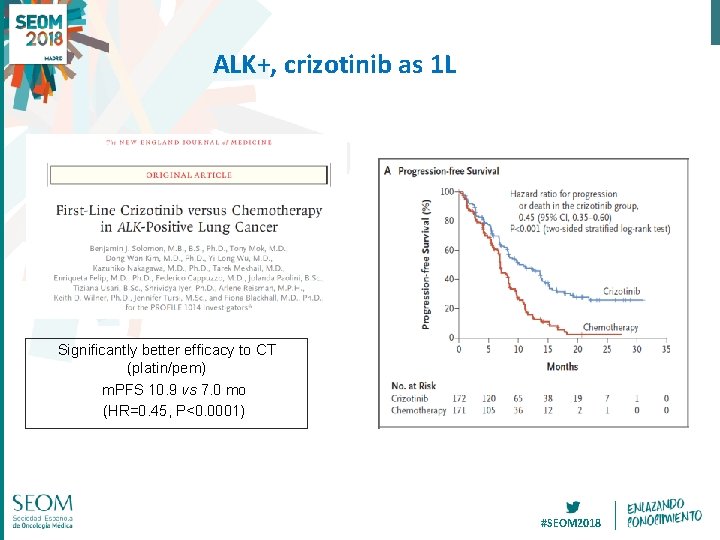
ALK+, crizotinib as 1 L Significantly better efficacy to CT (platin/pem) m. PFS 10. 9 vs 7. 0 mo (HR=0. 45, P<0. 0001) #SEOM 2018

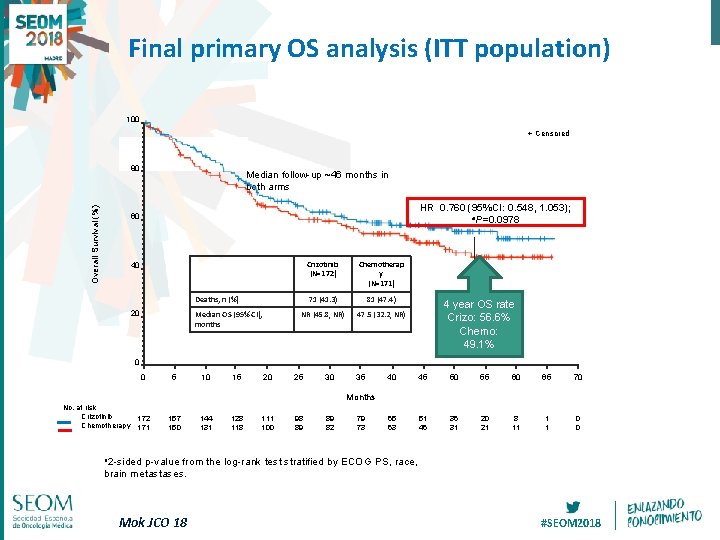
Final primary OS analysis (ITT population) 100 + Censored Overall Survival (%) 80 Median follow-up ~46 months in both arms HR 0. 760 (95%CI: 0. 548, 1. 053); a. P=0. 0978 60 Crizotinib (N=172) Chemotherap y (N=171) 71 (41. 3) 81 (47. 4) NR (45. 8, NR) 47. 5 (32. 2, NR) 40 Deaths, n (%) 20 Median OS (95% CI), months 4 year OS rate Crizo: 56. 6% Chemo: 49. 1% 0 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 65 63 51 46 36 31 20 21 8 11 1 1 0 0 Months No. at risk Crizotinib 172 Chemotherapy 171 157 150 144 131 128 111 100 98 89 89 82 79 73 a 2 -sided p-value from the log-rank test stratified by ECOG PS, race, brain metastases. Mok JCO 18 #SEOM 2018

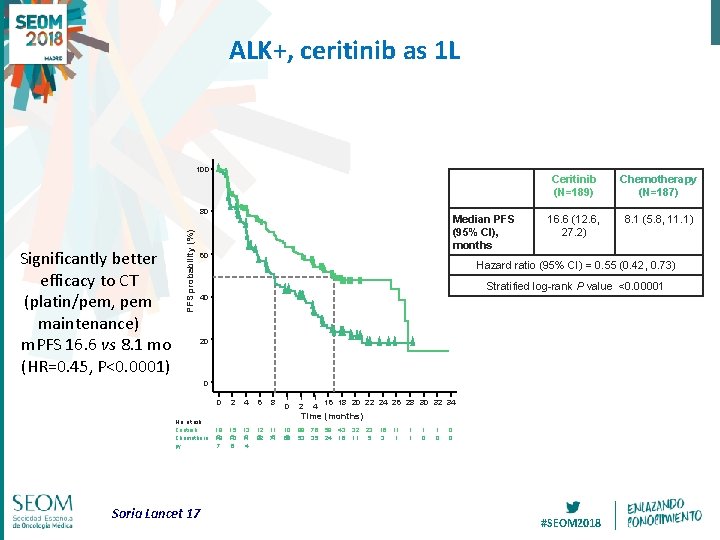
ALK+, ceritinib as 1 L 100 Significantly better efficacy to CT (platin/pem, pem maintenance) m. PFS 16. 6 vs 8. 1 mo (HR=0. 45, P<0. 0001) PFS probability (%) 80 Median PFS (95% CI), months 60 Ceritinib (N=189) Chemotherapy (N=187) 16. 6 (12. 6, 27. 2) 8. 1 (5. 8, 11. 1) Hazard ratio (95% CI) = 0. 55 (0. 42, 0. 73) Stratified log-rank P value <0. 00001 40 20 0 0 No. at risk Ceritinib Chemothera py Soria Lancet 17 18 9 18 7 2 15 5 13 6 4 13 9 11 4 6 12 5 82 8 11 6 71 1 0 1 2 1 4 16 18 20 22 24 26 28 30 32 34 10 5 60 98 53 76 35 59 24 Time (months) 43 16 32 11 23 5 16 3 11 1 1 0 0 0 #SEOM 2018

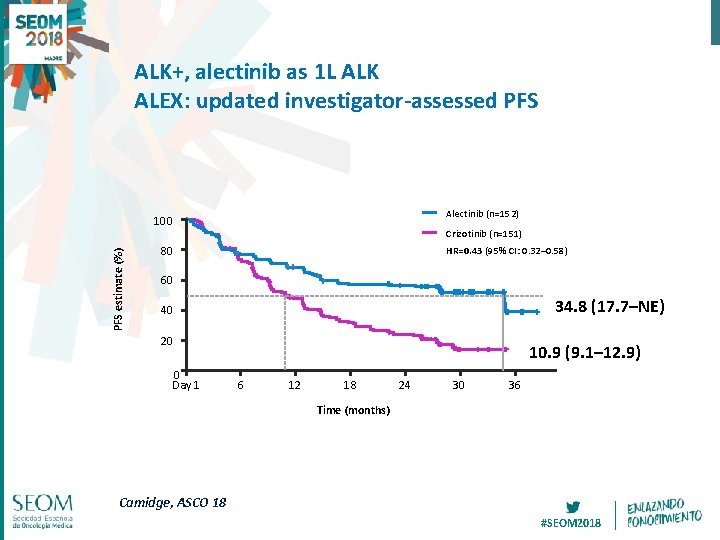
ALK+, alectinib as 1 L ALK ALEX: updated investigator-assessed PFS Alectinib (n=152) PFS estimate (%) 100 Crizotinib (n=151) HR=0. 43 (95% CI: 0. 32– 0. 58) 80 60 34. 8 (17. 7–NE) 40 20 0 Day 1 10. 9 (9. 1– 12. 9) 6 12 18 24 30 36 Time (months) Camidge, ASCO 18 #SEOM 2018

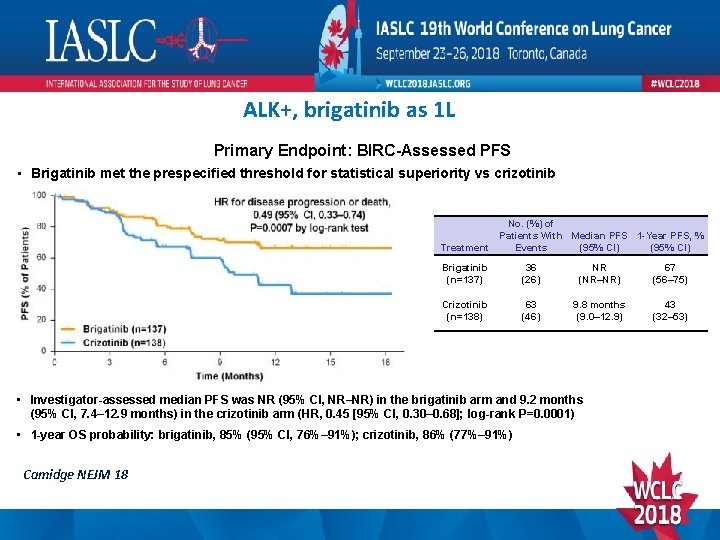
ALK+, brigatinib as 1 L Primary Endpoint: BIRC-Assessed PFS • Brigatinib met the prespecified threshold for statistical superiority vs crizotinib Treatment No. (%) of Patients With Median PFS 1 -Year PFS, % Events (95% CI) Brigatinib (n=137) 36 (26) NR (NR–NR) 67 (56– 75) Crizotinib (n=138) 63 (46) 9. 8 months (9. 0– 12. 9) 43 (32– 53) • Investigator-assessed median PFS was NR (95% CI, NR–NR) in the brigatinib arm and 9. 2 months (95% CI, 7. 4– 12. 9 months) in the crizotinib arm (HR, 0. 45 [95% CI, 0. 30– 0. 68]; log-rank P=0. 0001) • 1 -year OS probability: brigatinib, 85% (95% CI, 76%– 91%); crizotinib, 86% (77%– 91%) Camidge NEJM 18

1 L ALK+ NSCLC –After IASLC 18: + Brigatinib 1 L #SEOM 2018

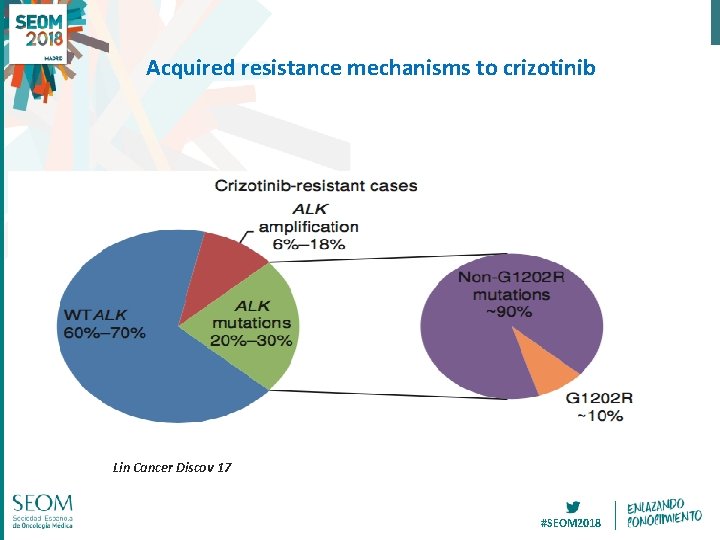
Acquired resistance mechanisms to crizotinib Lin Cancer Discov 17 #SEOM 2018

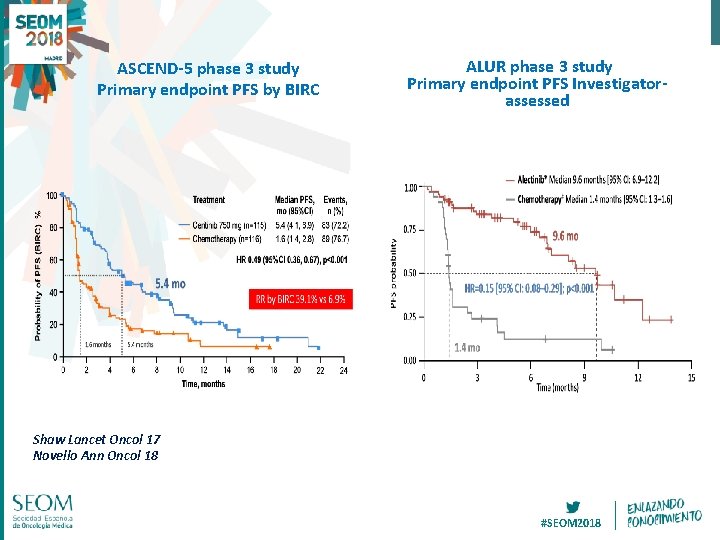
ASCEND-5 phase 3 study Primary endpoint PFS by BIRC ALUR phase 3 study Primary endpoint PFS Investigatorassessed Shaw Lancet Oncol 17 Novello Ann Oncol 18 #SEOM 2018

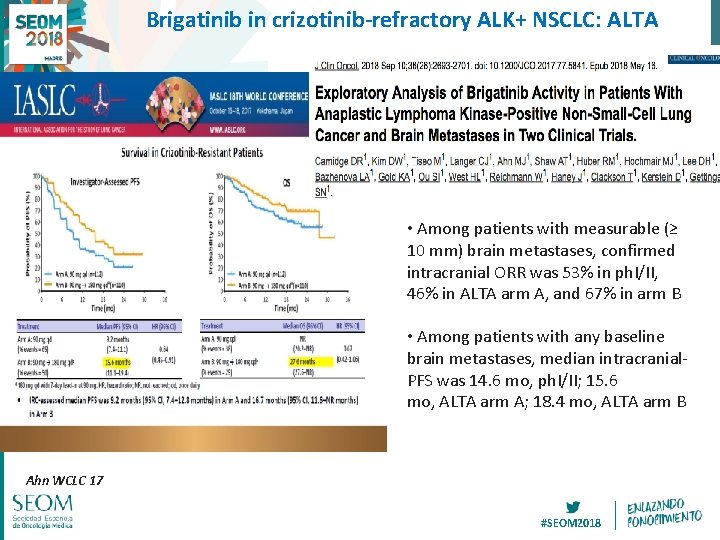
Brigatinib in crizotinib-refractory ALK+ NSCLC: ALTA • Among patients with measurable (≥ 10 mm) brain metastases, confirmed intracranial ORR was 53% in ph. I/II, 46% in ALTA arm A, and 67% in arm B • Among patients with any baseline brain metastases, median intracranial. PFS was 14. 6 mo, ph. I/II; 15. 6 mo, ALTA arm A; 18. 4 mo, ALTA arm B Ahn WCLC 17 #SEOM 2018

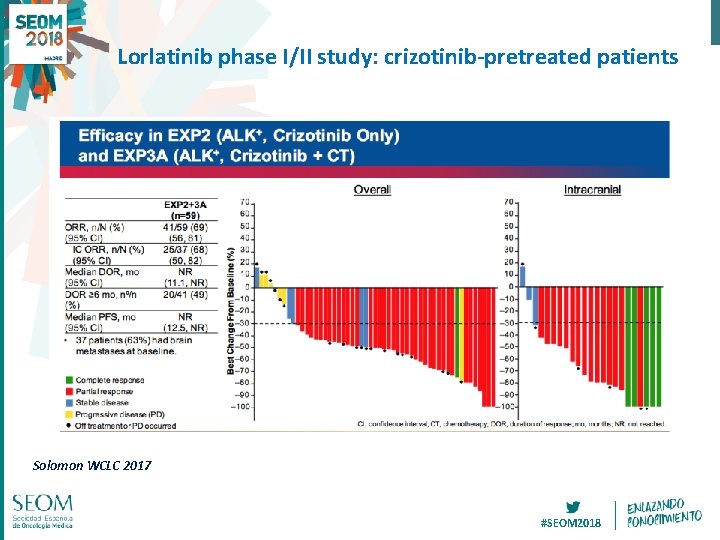
Lorlatinib phase I/II study: crizotinib-pretreated patients Solomon WCLC 2017 #SEOM 2018

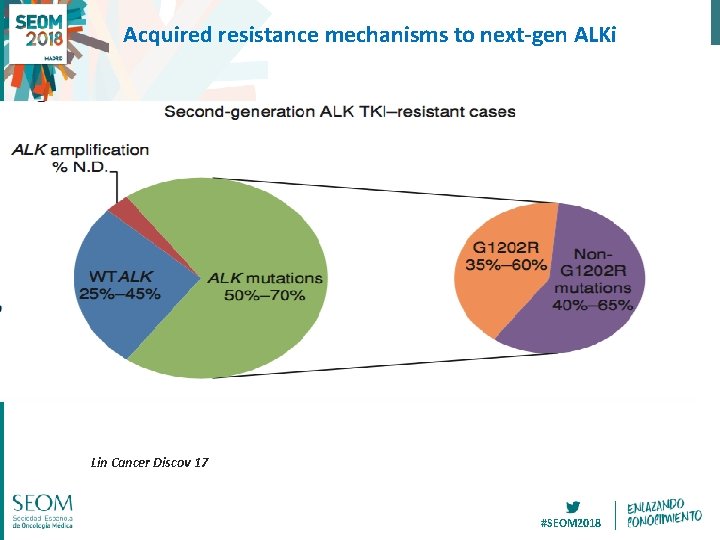
Acquired resistance mechanisms to next-gen ALKi Lin Cancer Discov 17 #SEOM 2018

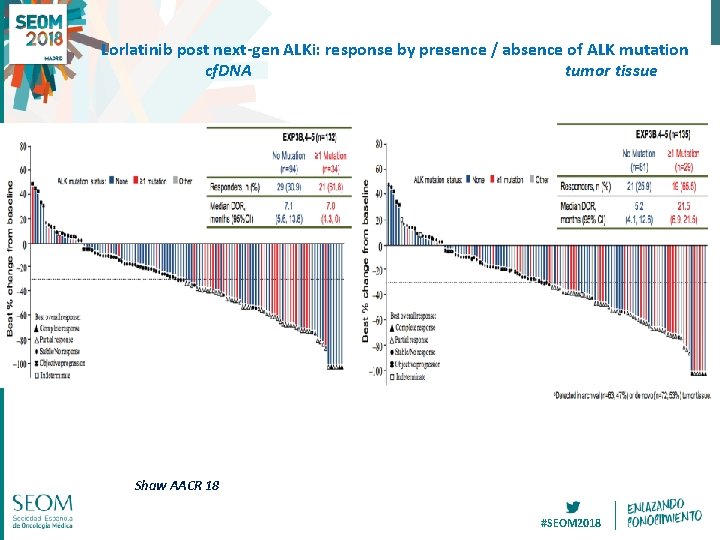
Lorlatinib post next-gen ALKi: response by presence / absence of ALK mutation cf. DNA tumor tissue Shaw AACR 18 #SEOM 2018

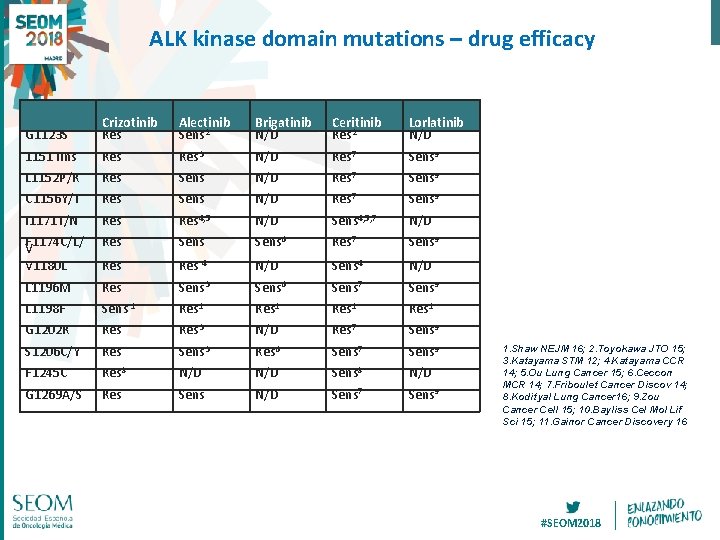
ALK kinase domain mutations – drug efficacy G 1123 S Crizotinib Res Alectinib Sens 2 Brigatinib N/D Ceritinib Res 2 Lorlatinib N/D 1151 Tins Res 3 N/D Res 7 Sens 9 L 1152 P/R Res Sens N/D Res 7 Sens 9 C 1156 Y/T Res Sens N/D Res 7 Sens 9 I 1171 T/N Res 4, 5 N/D Sens 4, 5, 7 N/D F 1174 C/L/ V V 1180 L Res Sens 6 Res 7 Sens 9 Res 4 N/D Sens 4 N/D L 1196 M Res Sens 3 Sens 6 Sens 7 Sens 9 L 1198 F Sens 1 Res 1 G 1202 R Res 3 N/D Res 7 Sens 9 S 1206 C/Y Res Sens 3 Res 6 Sens 7 Sens 9 F 1245 C Res 8 N/D Sens 8 N/D G 1269 A/S Res Sens N/D Sens 7 Sens 9 1. Shaw NEJM 16; 2. Toyokawa JTO 15; 3. Katayama STM 12; 4. Katayama CCR 14; 5. Ou Lung Cancer 15; 6. Ceccon MCR 14; 7. Friboulet Cancer Discov 14; 8. Kodityal Lung Cancer 16; 9. Zou Cancer Cell 15; 10. Bayliss Cel Mol Lif Sci 15; 11. Gainor Cancer Discovery 16 #SEOM 2018

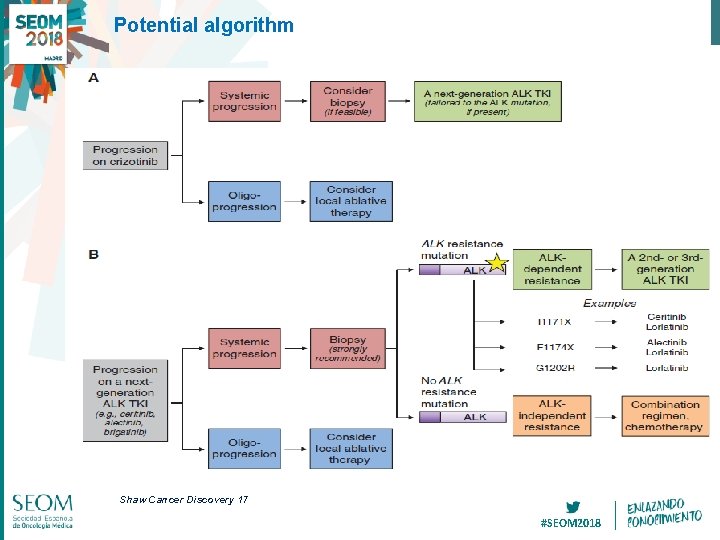
Potential algorithm Shaw Cancer Discovery 17 #SEOM 2018

Stage IV ALK+ NSCLC • ALKi have favourably transformed the course of disease for ALK+ patients o After crizotinib failure: next-gen ALKi, active o Standard therapy at PD after next-gen ALKi is not well defined • Re-biopsies / liquid biopsy encouraged in patients with PD to ALKi to better understand resistances mechanisms and develop future therapeutic approaches #SEOM 2018

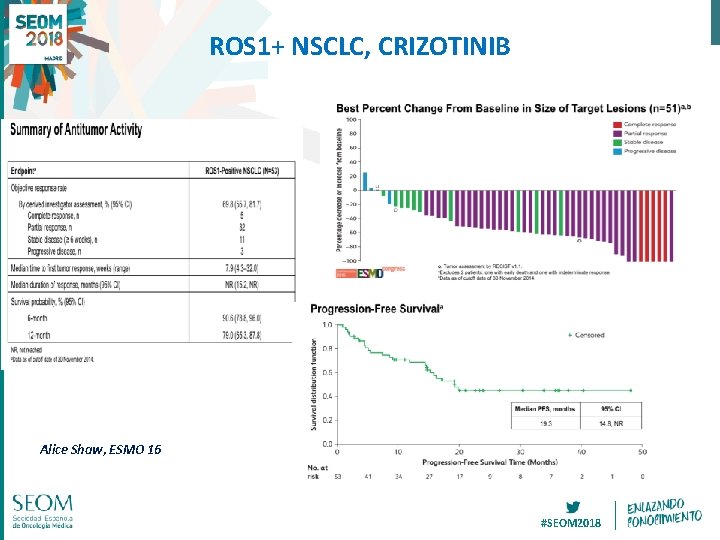
ROS 1+ NSCLC, CRIZOTINIB Alice Shaw, ESMO 16 #SEOM 2018

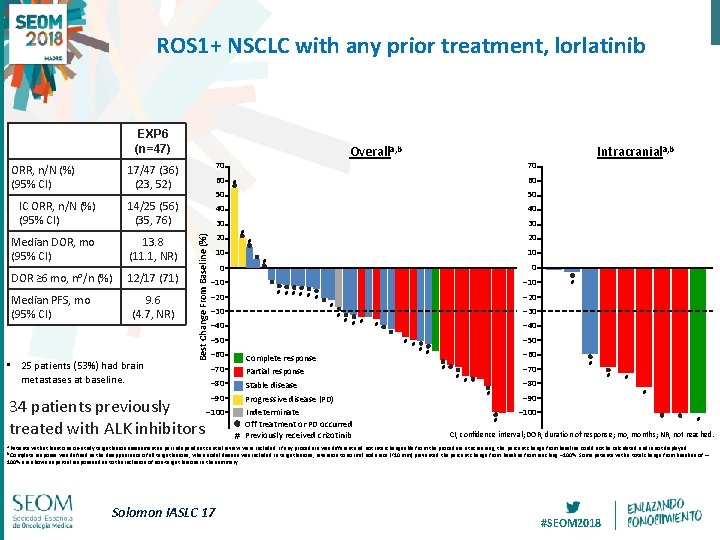
ROS 1+ NSCLC with any prior treatment, lorlatinib EXP 6 (n=47) Overalla, b ORR, n/N (%) (95% CI) 17/47 (36) (23, 52) 70 IC ORR, n/N (%) (95% CI) 14/25 (56) (35, 76) 50 50 40 40 13. 8 (11. 1, NR) DOR ≥ 6 mo, n⁰/n (%) 12/17 (71) Median PFS, mo (95% CI) 9. 6 (4. 7, NR) • 25 patients (53%) had brain metastases at baseline. 70 60 # 30 Best Change From Baseline (%) Median DOR, mo (95% CI) 60 30 # 20 20 # 10 10 # 0 0 ‒ 10 # # # ‒ 20 # ‒ 40 ‒ 30 # # # ‒ 50 Complete response ‒ 70 Partial response ‒ 80 Stable disease 34 patients previously ‒ 100 treated with ALK inhibitors ‒ 40 # # # ‒ 60 # ‒ 20 # ‒ 30 ‒ 90 Intracraniala, b Progressive disease (PD) Indeterminate Off treatment or PD occurred # Previously received crizotinib ‒ 50 # # ‒ 60 ‒ 70 # # # ‒ 80 # # ‒ 90 # # ‒ 100 # CI, confidence interval; DOR, duration of response; mo, months; NR, not reached. a Patients with at least one on-study target lesion assessment as per independent central review were included. If any procedure was different and not interchangeable from the procedure at screening, the percent change from baseline could not be calculated and is not displayed. b Complete response was defined as the disappearance of all target lesions; when nodal disease was included in target lesions, reversion to normal node size (<10 mm) prevented the percent change from baseline from reaching – 100%. Some patients with a total change from baseline of – 100% are shown as partial responses due to the inclusion of non-target lesions in the summary. Solomon IASLC 17 #SEOM 2018

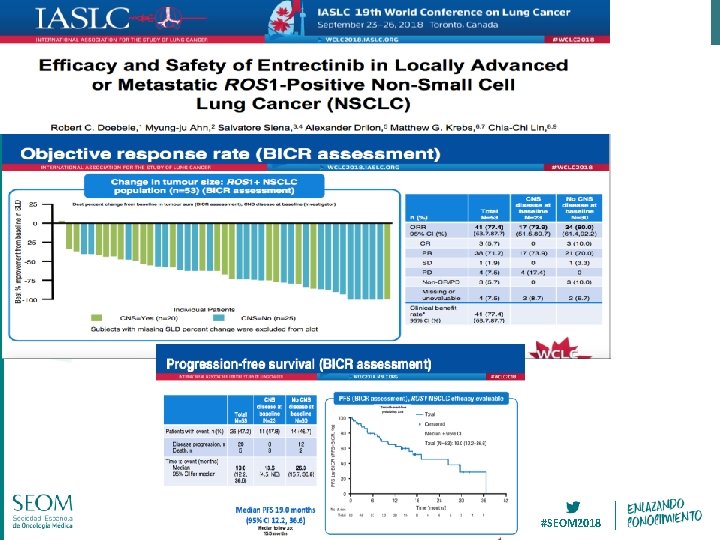
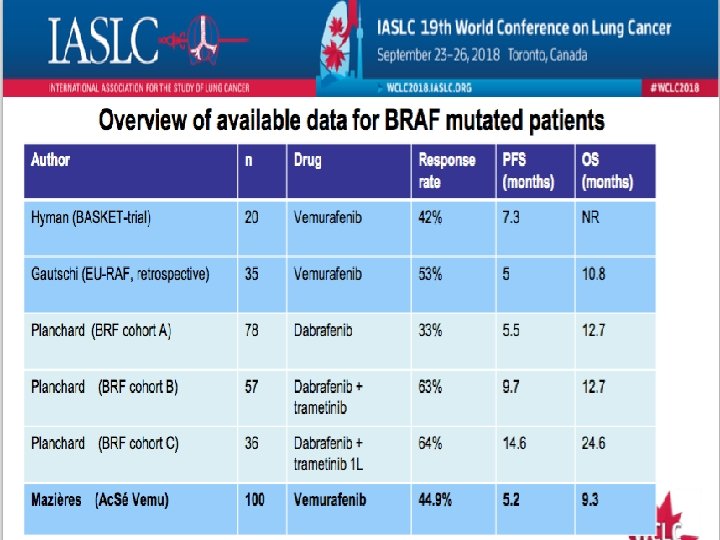
#SEOM 2018

#SEOM 2018

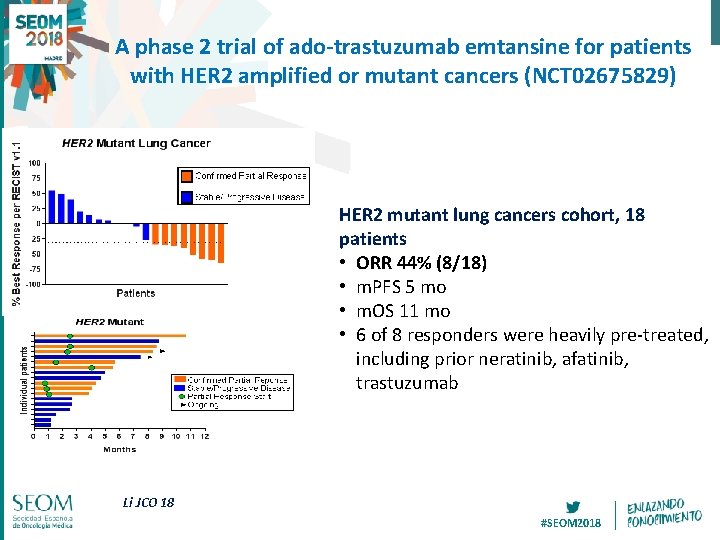
A phase 2 trial of ado-trastuzumab emtansine for patients with HER 2 amplified or mutant cancers (NCT 02675829) HER 2 mutant lung cancers cohort, 18 patients • ORR 44% (8/18) • m. PFS 5 mo • m. OS 11 mo • 6 of 8 responders were heavily pre-treated, including prior neratinib, afatinib, trastuzumab Li JCO 18 #SEOM 2018

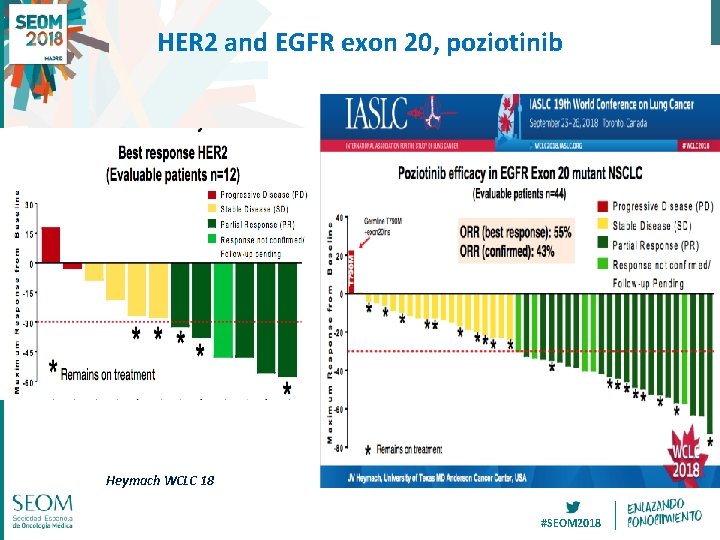
HER 2 and EGFR exon 20, poziotinib Heymach WCLC 18 #SEOM 2018

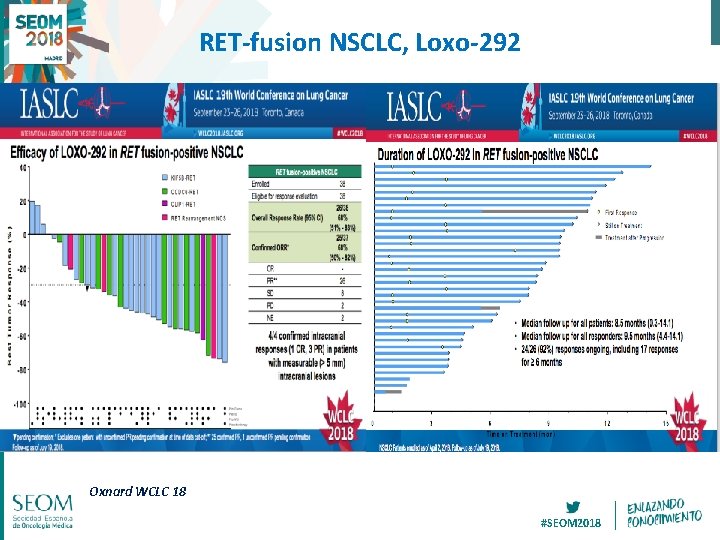
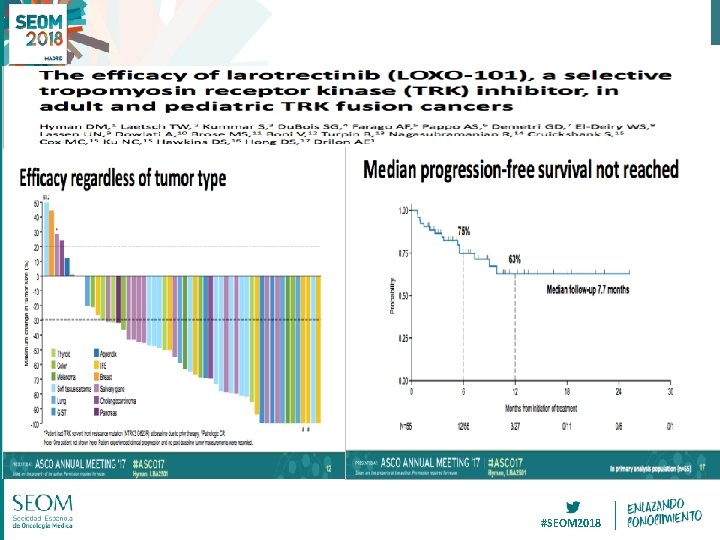
RET-fusion NSCLC, Loxo-292 Oxnard WCLC 18 #SEOM 2018

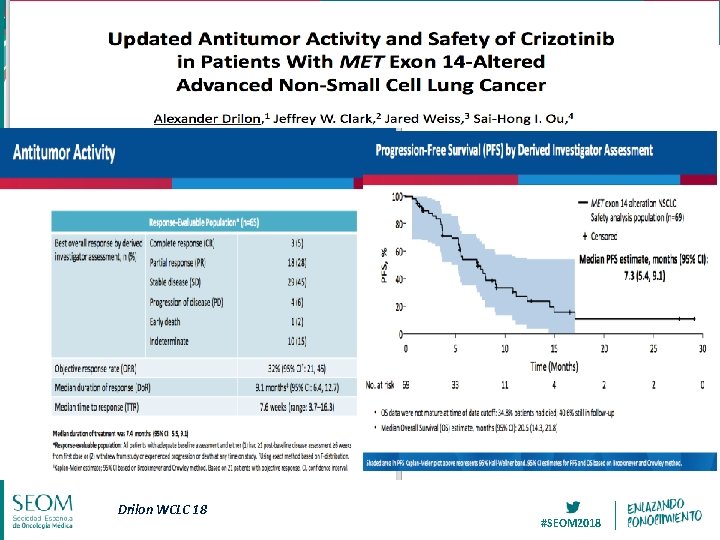
Drilon WCLC 18 #SEOM 2018

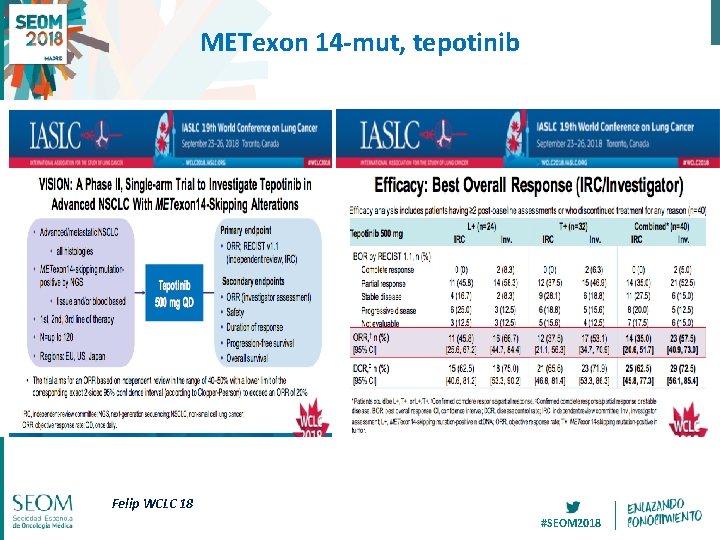
METexon 14 -mut, tepotinib Felip WCLC 18 #SEOM 2018

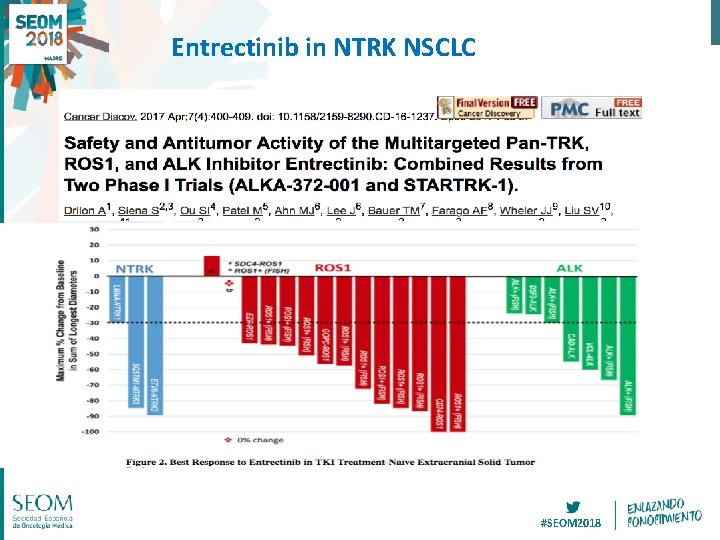
Entrectinib in NTRK NSCLC #SEOM 2018

#SEOM 2018

Biomarkers in lung cancer standard determinations • EGFR mutation status should be systematically analysed in advanced NSCC [I, A] • Molecular EGFR testing is not recommended in pts with a confident diagnosis of SCC, except in never/former light smokers (<15 pack years) [IV, A] • Testing for ALK rearrangement should be systematically carried out in advanced NSCC [II, A] • If possible, multiplex platforms for molecular testing are preferable [III, A] Novello et al. ESMO guidelines. Annals Oncol. 2016 #SEOM 2018

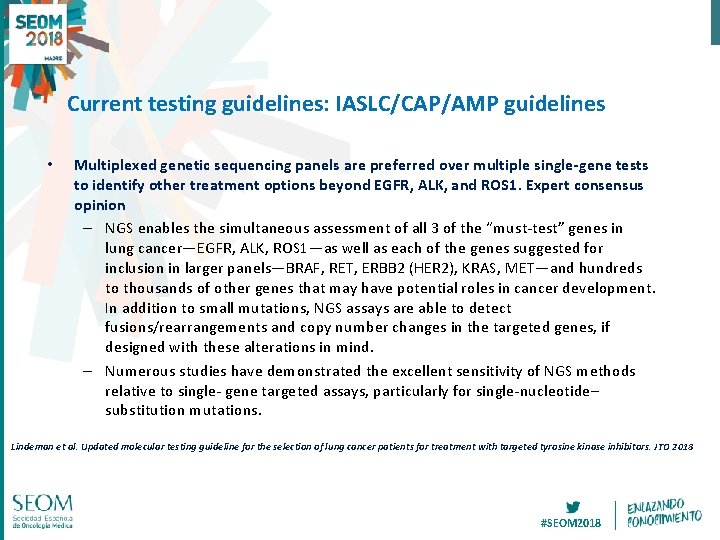
Current testing guidelines: IASLC/CAP/AMP guidelines • Multiplexed genetic sequencing panels are preferred over multiple single-gene tests to identify other treatment options beyond EGFR, ALK, and ROS 1. Expert consensus opinion – NGS enables the simultaneous assessment of all 3 of the “must-test” genes in lung cancer—EGFR, ALK, ROS 1—as well as each of the genes suggested for inclusion in larger panels—BRAF, RET, ERBB 2 (HER 2), KRAS, MET—and hundreds to thousands of other genes that may have potential roles in cancer development. In addition to small mutations, NGS assays are able to detect fusions/rearrangements and copy number changes in the targeted genes, if designed with these alterations in mind. – Numerous studies have demonstrated the excellent sensitivity of NGS methods relative to single- gene targeted assays, particularly for single-nucleotide– substitution mutations. Lindeman et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors. JTO 2018 #SEOM 2018

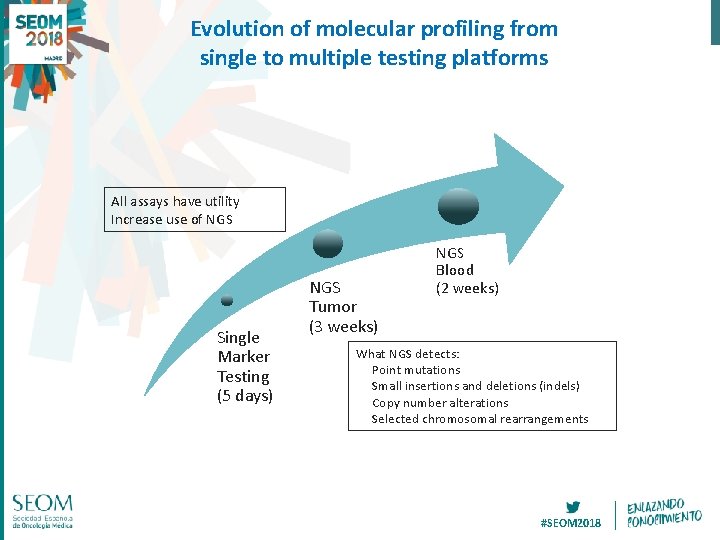
Evolution of molecular profiling from single to multiple testing platforms All assays have utility Increase use of NGS Single Marker Testing (5 days) NGS Tumor (3 weeks) NGS Blood (2 weeks) What NGS detects: Point mutations Small insertions and deletions (indels) Copy number alterations Selected chromosomal rearrangements #SEOM 2018

Precision medicine in NSCLC • NSCLC is divided in subsets by presence of targetable molecular alterations (EGFR, ALK, ROS 1, BRAF, MET, NTRK among others) • Challenges ü Genotyping: NGS ü Effort required to identify patients with uncommon molecular alterations ü Available treatment options for patients with molecular alterations #SEOM 2018

Gracias!!! efelip@vhebron. net #SEOM 2018
 Nuevas perspectivas 1
Nuevas perspectivas 1 Pasantia rae
Pasantia rae Discipulado avanzado para líderes
Discipulado avanzado para líderes Ajax avanzado
Ajax avanzado Las tres perspectivas basicas
Las tres perspectivas basicas Matt hispano
Matt hispano Perspectiva caballera
Perspectiva caballera Dia de los muertos perspectivas
Dia de los muertos perspectivas Daniel kahneman teoria de las perspectivas
Daniel kahneman teoria de las perspectivas Perspectivas disciplinares
Perspectivas disciplinares Perspectiva caballera definicion
Perspectiva caballera definicion Perspectiva intermedia
Perspectiva intermedia Ginástica para todos: perspectivas no contexto do lazer
Ginástica para todos: perspectivas no contexto do lazer Nuevas validaciones sii
Nuevas validaciones sii Contaminar subjunctive
Contaminar subjunctive Inclusion de nuevas tareas
Inclusion de nuevas tareas Dios hara cosas nuevas
Dios hara cosas nuevas Neuropsicosis de defensa
Neuropsicosis de defensa Insulina glargina esquema
Insulina glargina esquema Les gusta probar ideas teorías y técnicas nuevas
Les gusta probar ideas teorías y técnicas nuevas Contigo aprendi que existen nuevas y mejores emociones
Contigo aprendi que existen nuevas y mejores emociones Las 7 nuevas maravillas del mundo
Las 7 nuevas maravillas del mundo Sobrerrecorrido
Sobrerrecorrido Frases de la vida nuevas 2021
Frases de la vida nuevas 2021 Nuevas tecnologias
Nuevas tecnologias Características generales de las preposiciones ejemplos
Características generales de las preposiciones ejemplos Yo hago nuevas todas las cosas letra
Yo hago nuevas todas las cosas letra Las nuevas palabras fontanarrosa
Las nuevas palabras fontanarrosa El amor de dios - reflexión
El amor de dios - reflexión Nuevos retos nuevas oportunidades
Nuevos retos nuevas oportunidades Terapia do perdão divaldo franco
Terapia do perdão divaldo franco Terapia mrt
Terapia mrt Terapia de pareja
Terapia de pareja Carl rogers quien es
Carl rogers quien es Polifagia paradossa
Polifagia paradossa Via intratecale
Via intratecale Centro siciliano di terapia della famiglia
Centro siciliano di terapia della famiglia Terapia ocupacional uoc
Terapia ocupacional uoc Hivamat kezelés
Hivamat kezelés Terapia dla nieśmiałych
Terapia dla nieśmiałych Meteorismo ruminale terapia
Meteorismo ruminale terapia Laso terapia
Laso terapia Terapia triple helicobacter
Terapia triple helicobacter Terapia intensiva cardiochirurgica monaldi
Terapia intensiva cardiochirurgica monaldi Autorregistro de beck
Autorregistro de beck Terapia gestalt ppt
Terapia gestalt ppt Terapia gestalt 9 zaleceń
Terapia gestalt 9 zaleceń Lokoidi
Lokoidi Motivacion aversiva
Motivacion aversiva Ipoema traumatico
Ipoema traumatico