Nuevos paradigmas en 1 era Lnea del CPNM













- Slides: 41

Nuevos paradigmas en 1 era Línea del CPNM avanzado sin mutaciones drivers. Delvys Rodríguez Abreu, MD Medical Oncology Dept. Hospital Universitario Insular de Gran Canaria. Spain

Conflictos de intereses • He recibido honorarios de Conferencias y Advisory Board de Bristol. Myers-Squibb, Merck Sharp & Dohme, Hoffmann-La Roche, Pierre-Fabre, Novartis, Boehringer, Pfizer, Lilly, Astra-Zeneca.

• Monotherapy

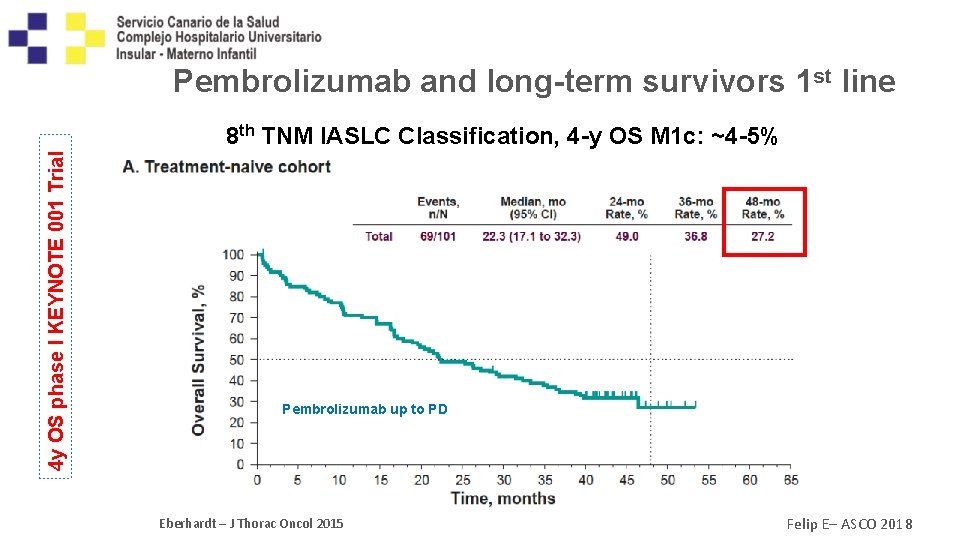
Pembrolizumab and long-term survivors 1 st line 4 y OS phase I KEYNOTE 001 Trial 8 th TNM IASLC Classification, 4 -y OS M 1 c: ~4 -5% PD-L 1 > 50% (N=138) 3 y. OS: 29. 7% Pembrolizumab up to PD Eberhardt – J Thorac Oncol 2015 Felip E– ASCO 2018

• BIRCH: Phase II Trial of Atezolizumab Monotherapy in (TC 2/3 and/or IC 2/3) PD-L 1– Selected Advanced NSCLC 1 c 2 -Year OS Nivolumab or Nivo-IPI From Check. Mate 012 Median duration of survival follow-up = 34. 3 months • Durable survival was observed in all PD-L 1 subgroups 5 Carcereny et al. , BIRCH. WCLC 2017 Goldman JW et al. , CM 012. ASCO 2018

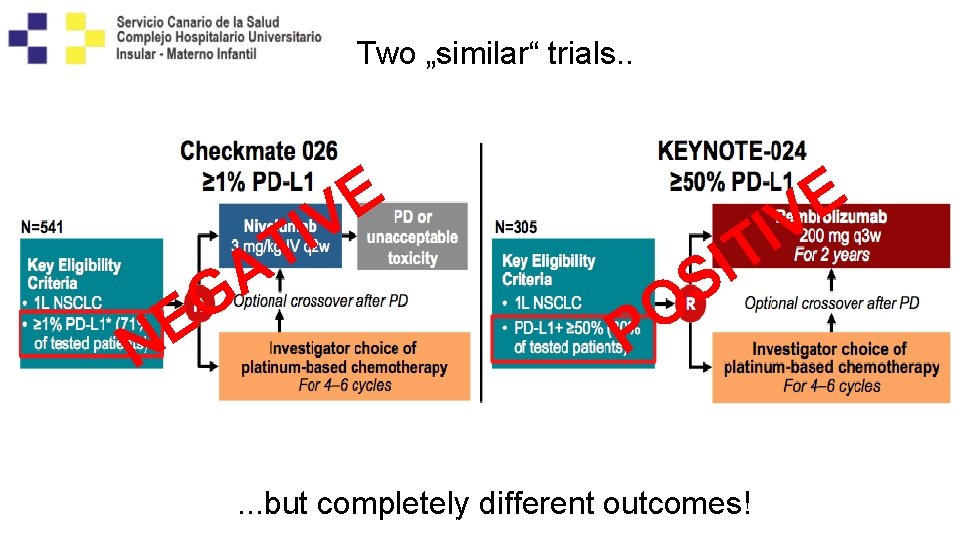
Two „similar“ trials. . E V I T E N A G O P E V I T I S . . . but completely different outcomes! Carbone D et al, NEJM 2017; 376 (25): 2415 -2426; Reck M et al, NEJM 2016; 375 (19):

KEYNOTE-024 Study Confirmed Objective Response Rate Δ 17% P = 0. 0011 60 CR PR 45% 10, 3 vs 6 months ORR, % (95% CI) 50 40 n=6 n = 63 28% 30 n=1 n = 41 20 10 0 Pembrolizumab Chemotherapy Assessed per RECIST v 1. 1 by blinded, independent central review. Data cut-off: May 9, 2016. Reck M, et al. NEJM 2016

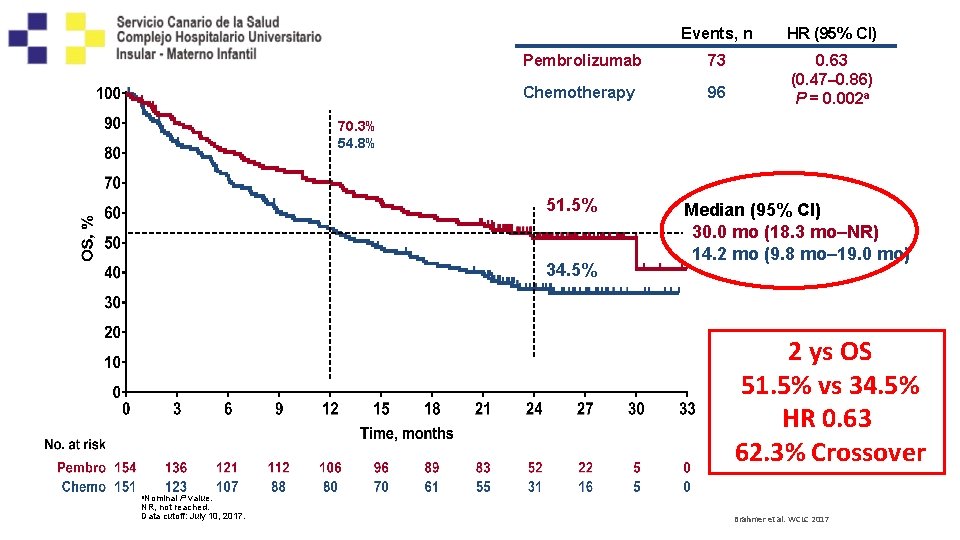
Kaplan-Meier Estimate of OS: Pembrolizumab Updated Analysis Chemotherapy Events, n HR (95% CI) 73 0. 63 (0. 47– 0. 86) P = 0. 002 a 96 70. 3% 54. 8% 51. 5% 34. 5% Median (95% CI) 30. 0 mo (18. 3 mo–NR) 14. 2 mo (9. 8 mo– 19. 0 mo) 2 ys OS 51. 5% vs 34. 5% HR 0. 63 62. 3% Crossover a. Nominal P value. NR, not reached. Data cutoff: July 10, 2017. Brahmer et al. WCLC 2017

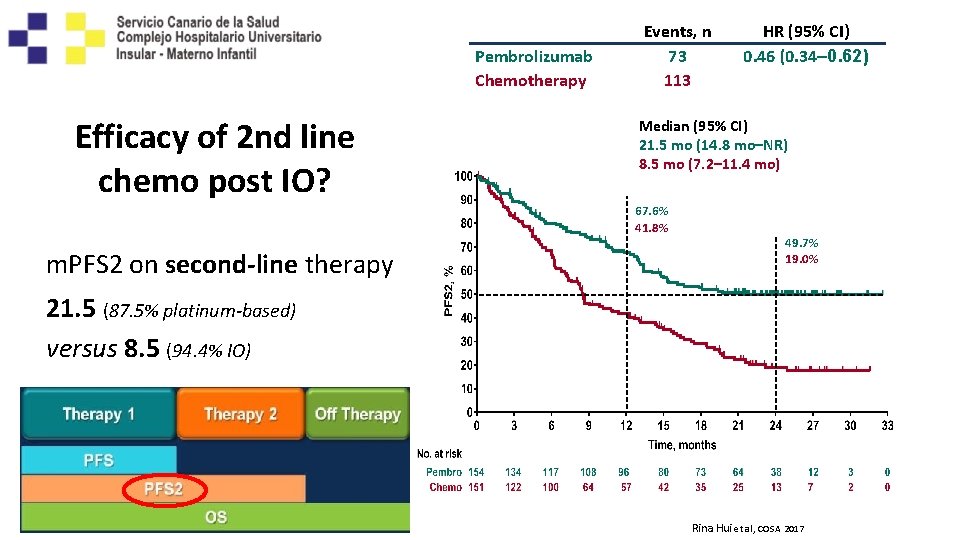
Pembrolizumab Chemotherapy Efficacy of 2 nd line chemo post IO? Events, n 73 113 Median (95% CI) 21. 5 mo (14. 8 mo–NR) 8. 5 mo (7. 2– 11. 4 mo) 67. 6% 41. 8% m. PFS 2 on second-line therapy HR (95% CI) 0. 46 (0. 34– 0. 62) 49. 7% 19. 0% 21. 5 (87. 5% platinum-based) versus 8. 5 (94. 4% IO) Rina Hui et al, COSA 2017

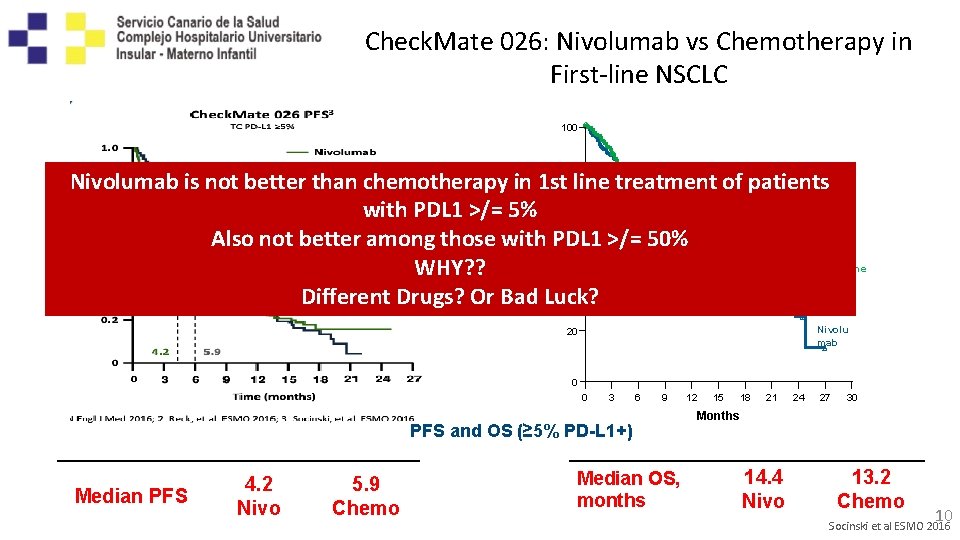
Check. Mate 026: Nivolumab vs Chemotherapy in First-line NSCLC 100 OS (%) Nivolumab is not better than chemotherapy in 1 st 80 line treatment of patients with PDL 1 >/= 5% 60 HR = 1. 02 Also not better among those with PDL 1 >/= 50% Chemothe WHY? ? 40 rapy Different Drugs? Or Bad Luck? Nivolu mab 20 0 0 3 6 9 PFS and OS (≥ 5% PD-L 1+) Median PFS 4. 2 Nivo 5. 9 Chemo Median OS, months 12 15 18 21 24 27 30 Months 14. 4 Nivo 13. 2 Chemo 10 Socinski et al ESMO 2016

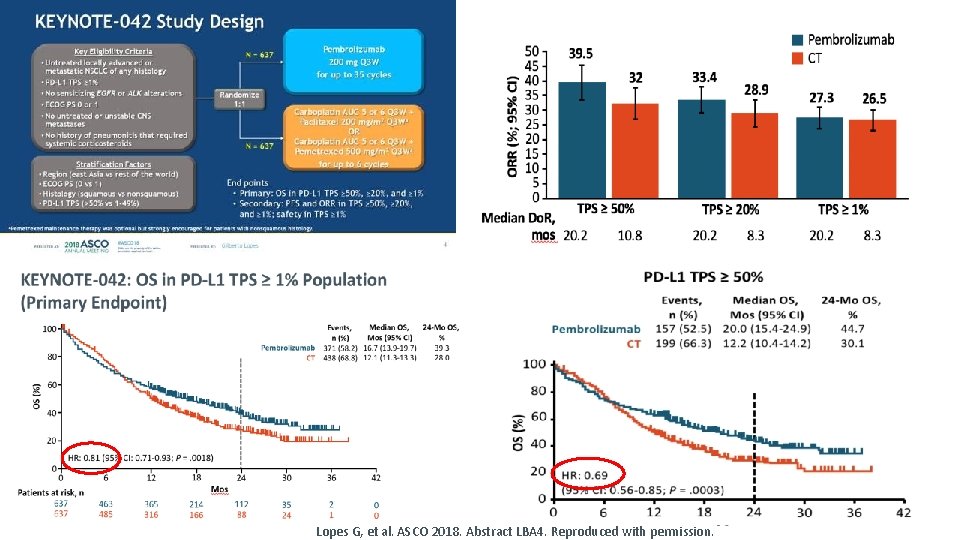
Lopes G, et al. ASCO 2018. Abstract LBA 4. Reproduced with permission.

• Combinations

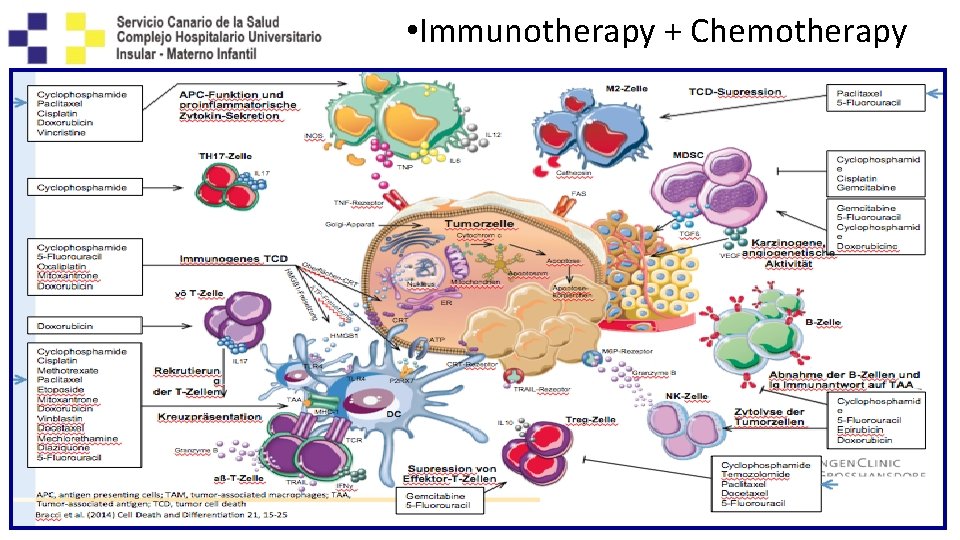
• Immunotherapy + Chemotherapy

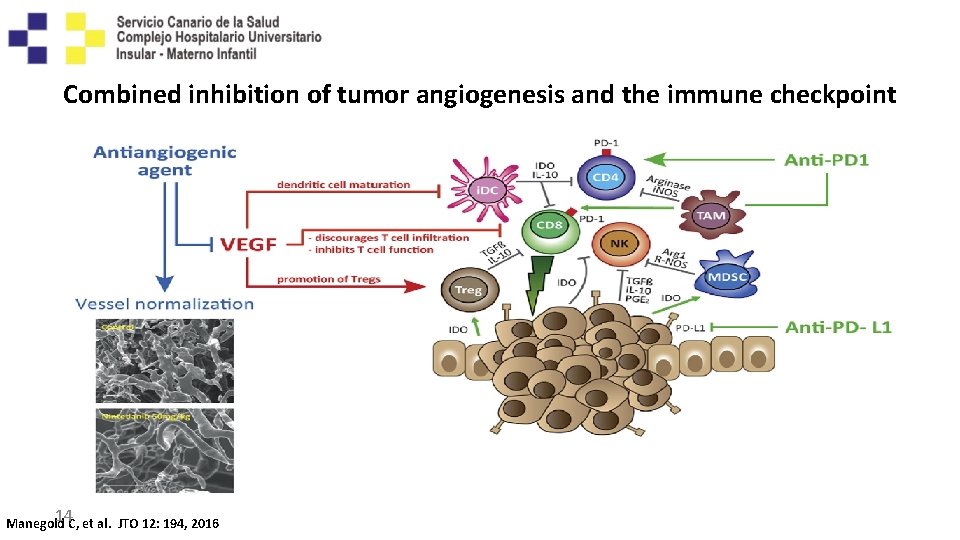
Combined inhibition of tumor angiogenesis and the immune checkpoint 14 Manegold C, et al. JTO 12: 194, 2016

• Non-squamous NSCLC

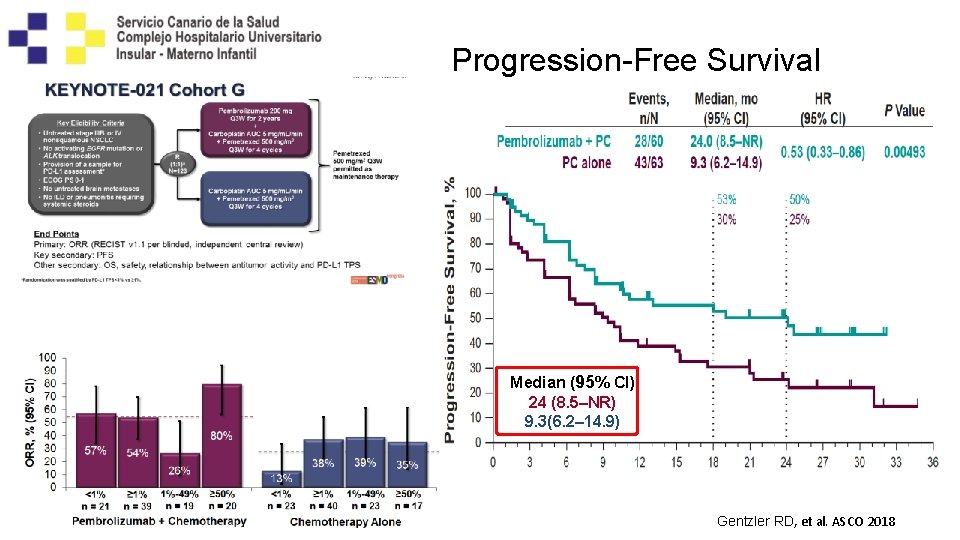
Progression-Free Survival Median ((95% % CI) 24 (8. 5–NR) 24 9. 3(6. 2– 14. 9) Gentzler RD, et al. ASCO 2018

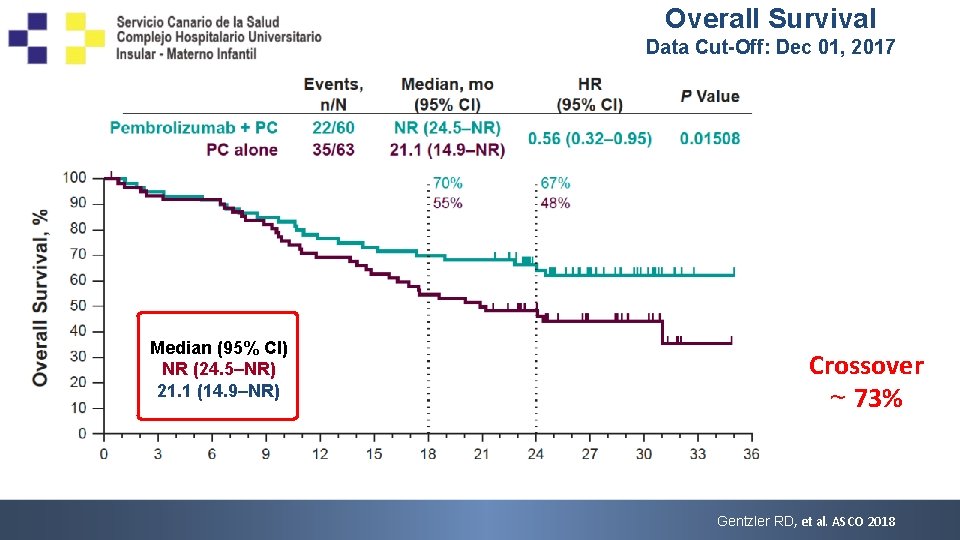
Overall Survival Data Cut-Off: Dec 01, 2017 Median (95% CI) NR (24. 5–NR) 21. 1 (14. 9–NR) • a 24 Crossover ~ 73% additional deaths since primary analysis (pembro + PC, n = 7; PC alone, n = 17). b. P value is descriptive (one-sided P < 0. 025). Gentzler RD, et al. ASCO 2018

May 10, 2017 FDA approved Pembrolizumab in combination with Carbo-Pemetrexed in 1 L NSCLC.

• Phase 3 Trials in Non-squamous NSCLC

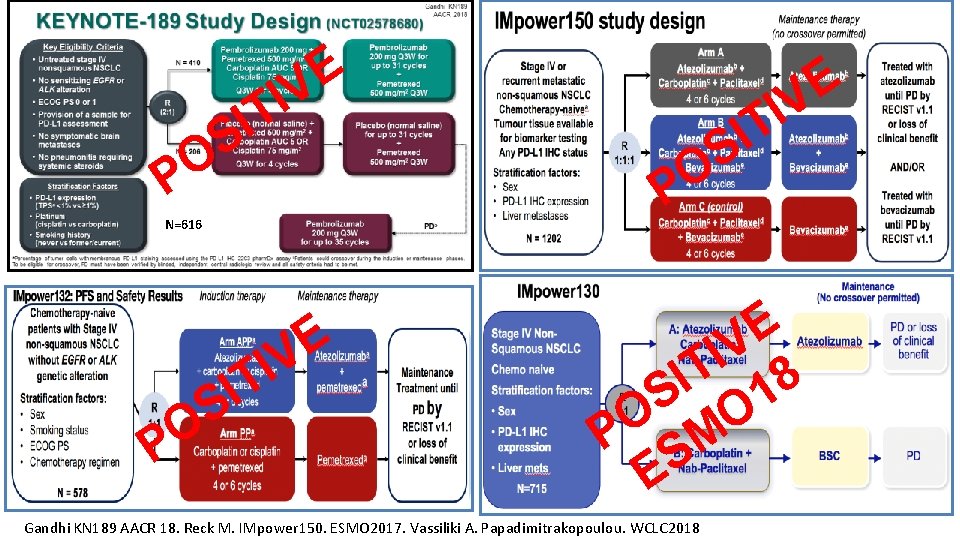
E V I T I S O P N=616 E IV T I S O P E V I T 8 I 1 S O O P SM E Gandhi KN 189 AACR 18. Reck M. IMpower 150. ESMO 2017. Vassiliki A. Papadimitrakopoulou. WCLC 2018

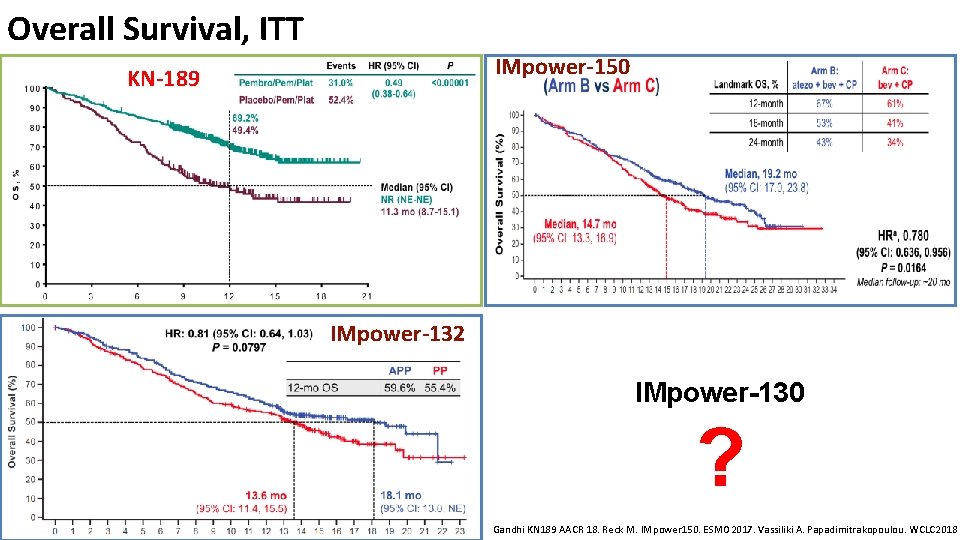
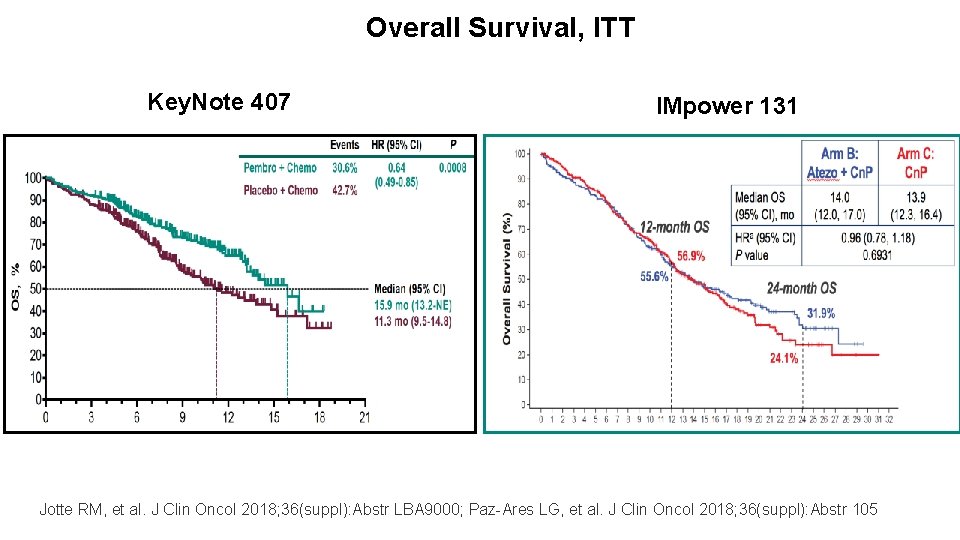
Overall Survival, ITT IMpower-150 KN-189 IMpower-132 IMpower-130 ? Gandhi KN 189 AACR 18. Reck M. IMpower 150. ESMO 2017. Vassiliki A. Papadimitrakopoulou. WCLC 2018

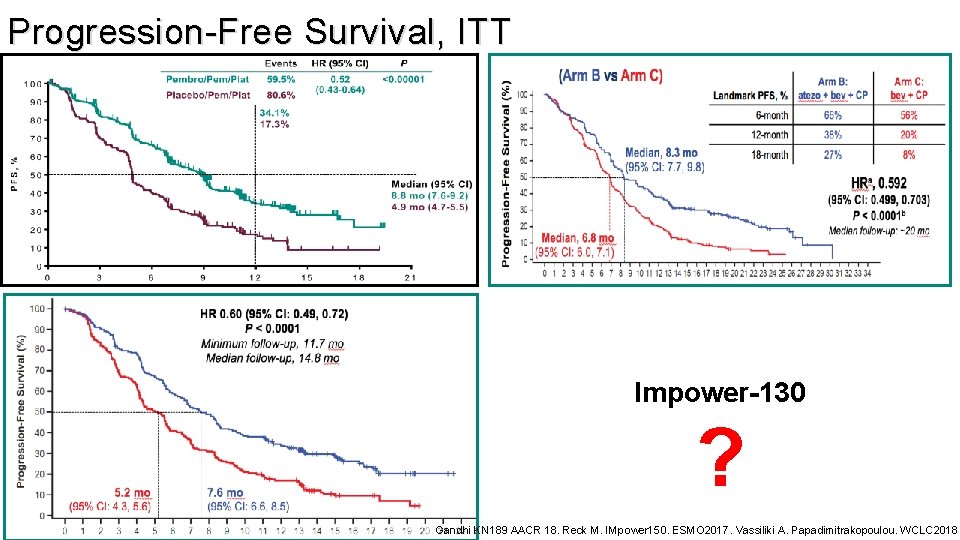
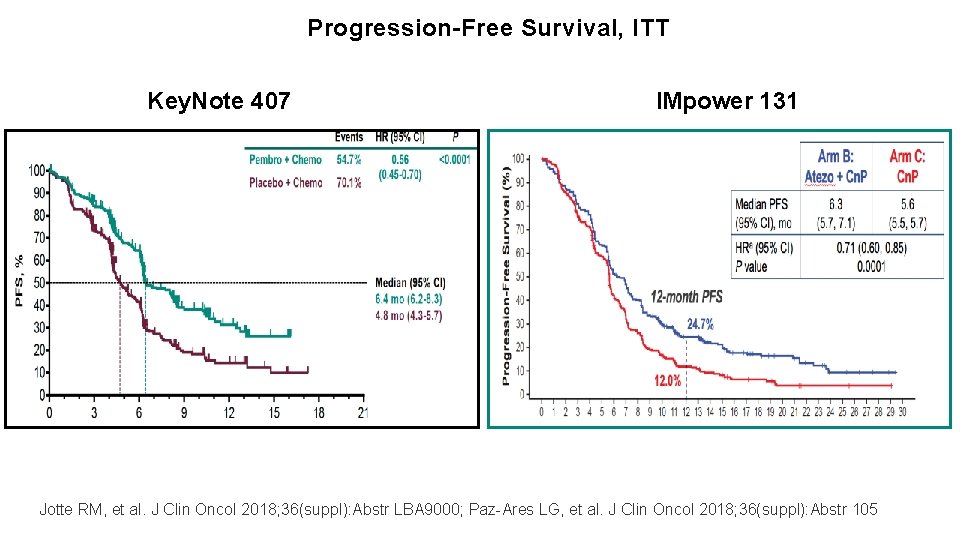
Progression-Free Survival, ITT Impower-130 ? Gandhi KN 189 AACR 18. Reck M. IMpower 150. ESMO 2017. Vassiliki A. Papadimitrakopoulou. WCLC 2018

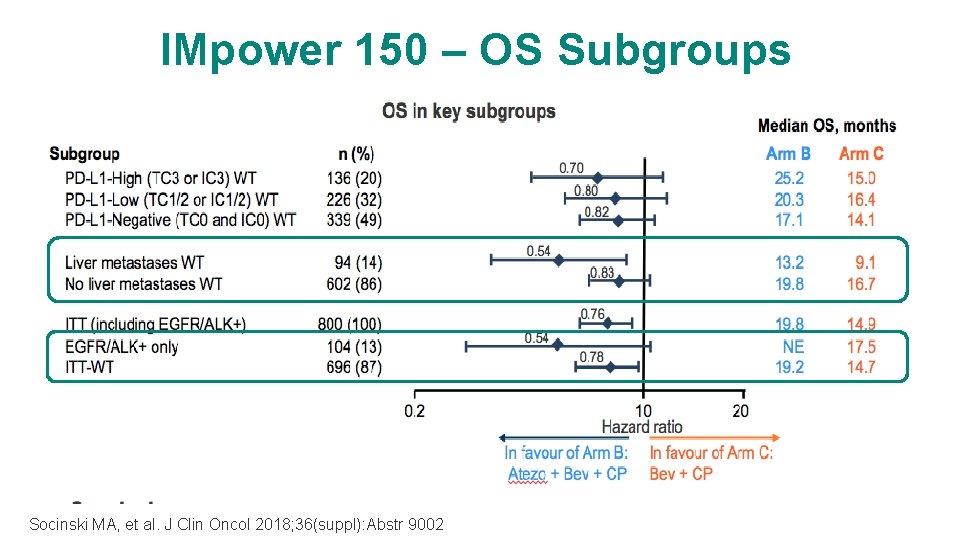
IMpower 150 – OS Subgroups Socinski MA, et al. J Clin Oncol 2018; 36(suppl): Abstr 9002

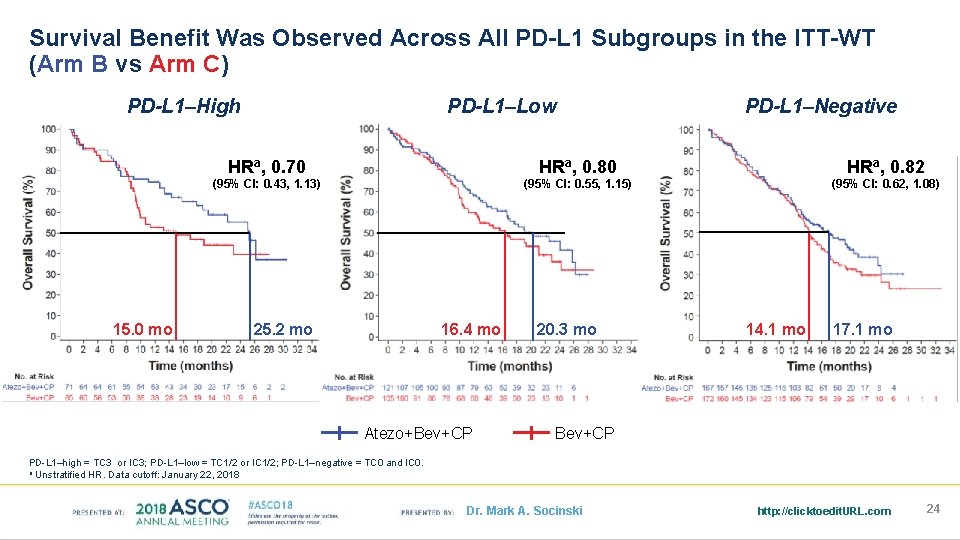
Survival Benefit Was Observed Across All PD-L 1 Subgroups in the ITT-WT (Arm B vs Arm C) PD-L 1–High 15. 0 mo PD-L 1–Low PD-L 1–Negative HRa, 0. 70 HRa, 0. 82 (95% CI: 0. 43, 1. 13) (95% CI: 0. 55, 1. 15) (95% CI: 0. 62, 1. 08) 25. 2 mo 16. 4 mo Atezo+Bev+CP 20. 3 mo 14. 1 mo 17. 1 mo Bev+CP PD-L 1–high = TC 3 or IC 3; PD-L 1–low = TC 1/2 or IC 1/2; PD-L 1–negative = TC 0 and IC 0. a Unstratified HR. Data cutoff: January 22, 2018 Dr. Mark A. Socinski http: //clicktoedit. URL. com 24

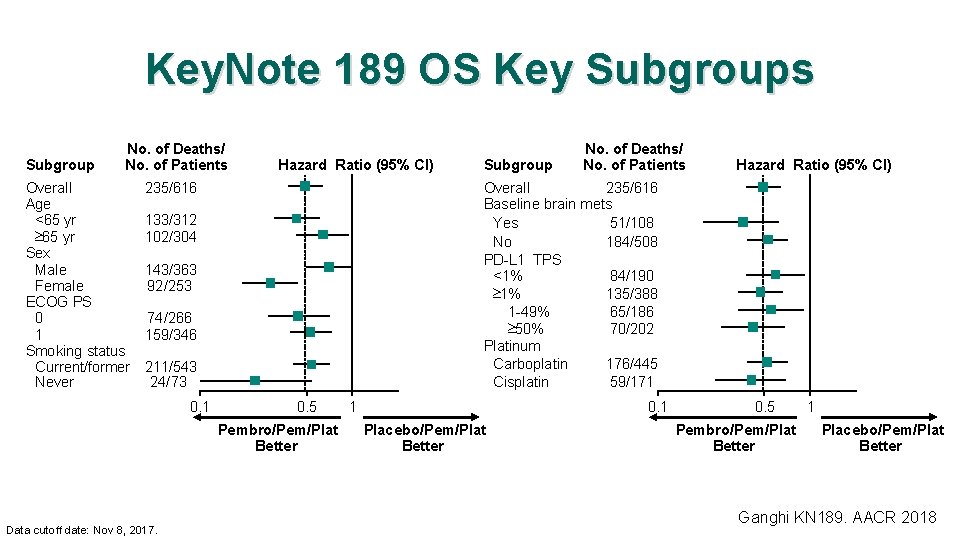
Key. Note 189 OS Key Subgroups Subgroup No. of Deaths/ No. of Patients Overall Age <65 yr ³ 65 yr Sex Male Female ECOG PS 0 1 Smoking status Current/former Never Hazard Ratio (95% CI) 235/616 143/363 92/253 74/266 159/346 211/543 24/73 0. 5 Pembro/Pem/Plat Better Data cutoff date: Nov 8, 2017. Hazard Ratio (95% CI) Overall 235/616 Baseline brain mets Yes 51/108 No 184/508 PD-L 1 TPS <1% 84/190 ³ 1% 135/388 1 -49% 65/186 ³ 50% 70/202 Platinum Carboplatin 176/445 Cisplatin 59/171 133/312 102/304 0. 1 Subgroup No. of Deaths/ No. of Patients 1 0. 1 Placebo/Pem/Plat Better 0. 5 Pembro/Pem/Plat Better 1 Placebo/Pem/Plat Better Ganghi KN 189. AACR 2018

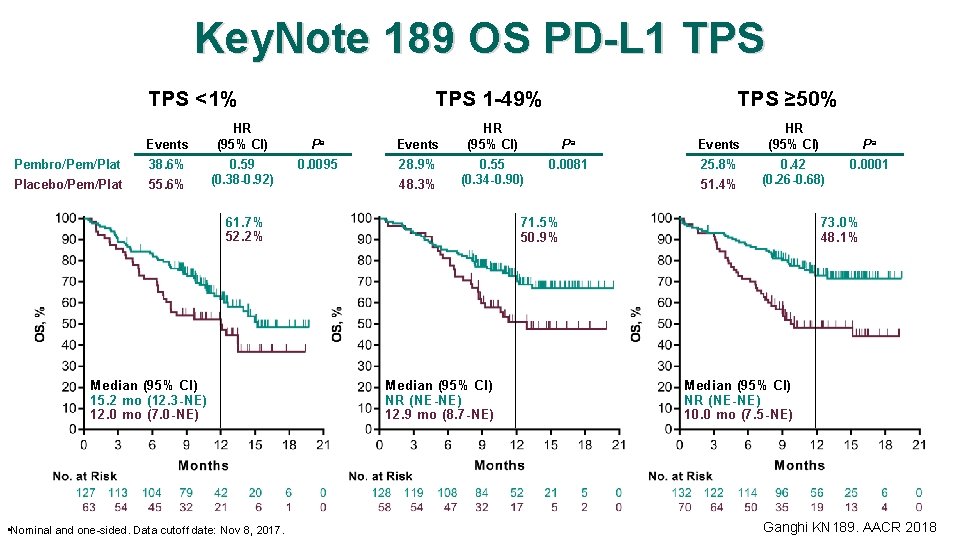
Key. Note 189 OS PD-L 1 TPS <1% Events Pembro/Pem/Plat 38. 6% Placebo/Pem/Plat 55. 6% HR (95% CI) 0. 59 (0. 38 -0. 92) TPS 1 -49% Pa Events 0. 0095 28. 9% 48. 3% HR (95% CI) a Nominal and one-sided. Data cutoff date: Nov 8, 2017. Pa 0. 55 (0. 34 -0. 90) 61. 7% 52. 2% Median (95% CI) 15. 2 mo (12. 3 -NE) 12. 0 mo (7. 0 -NE) TPS ≥ 50% 0. 0081 Events 25. 8% 51. 4% HR (95% CI) 0. 42 (0. 26 -0. 68) 71. 5% 50. 9% Median (95% CI) NR (NE-NE) 12. 9 mo (8. 7 -NE) Pa 0. 0001 73. 0% 48. 1% Median (95% CI) NR (NE-NE) 10. 0 mo (7. 5 -NE) Ganghi KN 189. AACR 2018

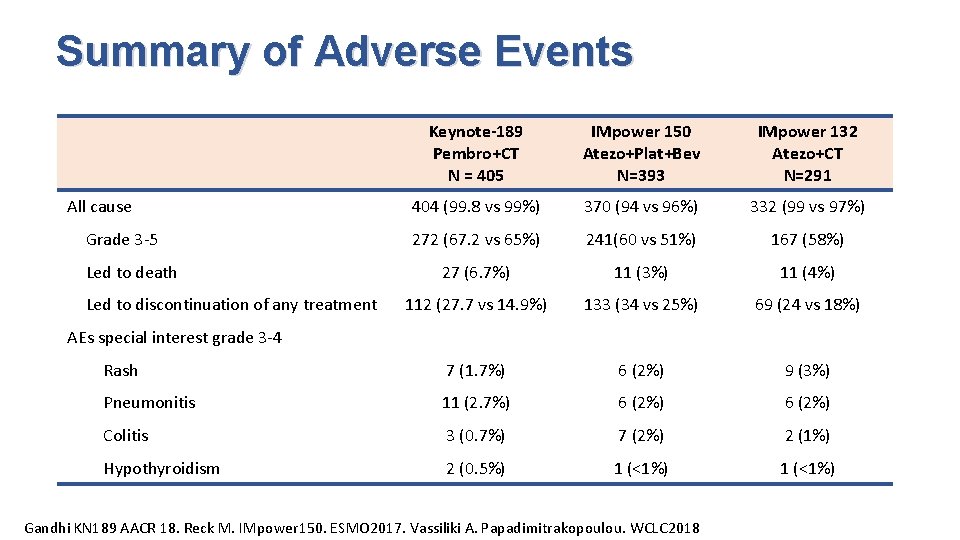
Summary of Adverse Events Keynote-189 Pembro+CT N = 405 IMpower 150 Atezo+Plat+Bev N=393 IMpower 132 Atezo+CT N=291 404 (99. 8 vs 99%) 370 (94 vs 96%) 332 (99 vs 97%) 272 (67. 2 vs 65%) 241(60 vs 51%) 167 (58%) 27 (6. 7%) 11 (3%) 11 (4%) 112 (27. 7 vs 14. 9%) 133 (34 vs 25%) 69 (24 vs 18%) Rash 7 (1. 7%) 6 (2%) 9 (3%) Pneumonitis 11 (2. 7%) 6 (2%) Colitis 3 (0. 7%) 7 (2%) 2 (1%) Hypothyroidism 2 (0. 5%) 1 (<1%) All cause Grade 3 -5 Led to death Led to discontinuation of any treatment AEs special interest grade 3 -4 Gandhi KN 189 AACR 18. Reck M. IMpower 150. ESMO 2017. Vassiliki A. Papadimitrakopoulou. WCLC 2018

THESE ARE PRACTICE CHANGING DATA!!!!

• Squamous NSCLC

Key. Note 407 IMpower 131 E IV T I S O P E V I T I S O P N=559 Jotte RM, et al. J Clin Oncol 2018; 36(suppl): Abstr LBA 9000; Paz-Ares LG, et al. J Clin Oncol 2018; 36(suppl): Abstr 105

Overall Survival, ITT Key. Note 407 IMpower 131 Jotte RM, et al. J Clin Oncol 2018; 36(suppl): Abstr LBA 9000; Paz-Ares LG, et al. J Clin Oncol 2018; 36(suppl): Abstr 105

Progression-Free Survival, ITT Key. Note 407 IMpower 131 Jotte RM, et al. J Clin Oncol 2018; 36(suppl): Abstr LBA 9000; Paz-Ares LG, et al. J Clin Oncol 2018; 36(suppl): Abstr 105

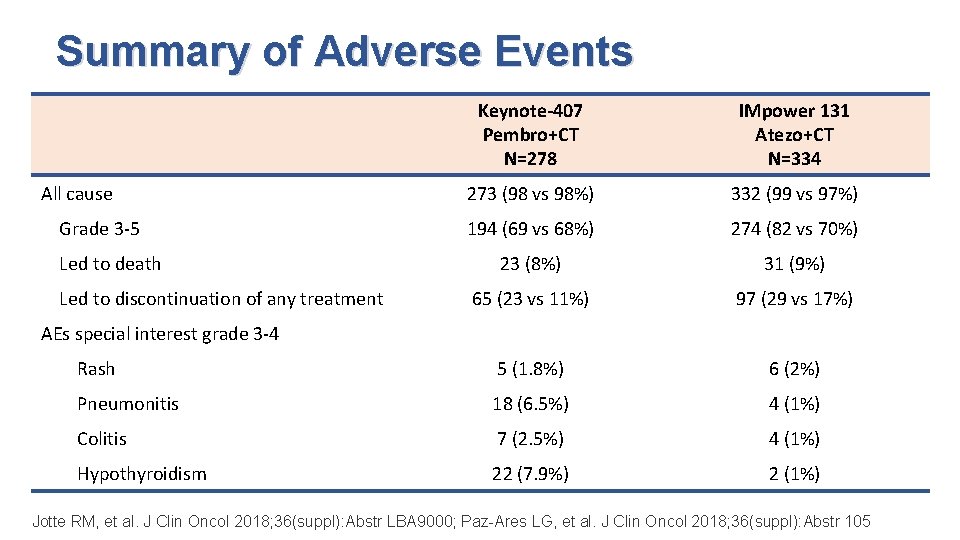
Summary of Adverse Events Keynote-407 Pembro+CT N=278 IMpower 131 Atezo+CT N=334 273 (98 vs 98%) 332 (99 vs 97%) 194 (69 vs 68%) 274 (82 vs 70%) 23 (8%) 31 (9%) 65 (23 vs 11%) 97 (29 vs 17%) Rash 5 (1. 8%) 6 (2%) Pneumonitis 18 (6. 5%) 4 (1%) Colitis 7 (2. 5%) 4 (1%) Hypothyroidism 22 (7. 9%) 2 (1%) All cause Grade 3 -5 Led to death Led to discontinuation of any treatment AEs special interest grade 3 -4 Jotte RM, et al. J Clin Oncol 2018; 36(suppl): Abstr LBA 9000; Paz-Ares LG, et al. J Clin Oncol 2018; 36(suppl): Abstr 105

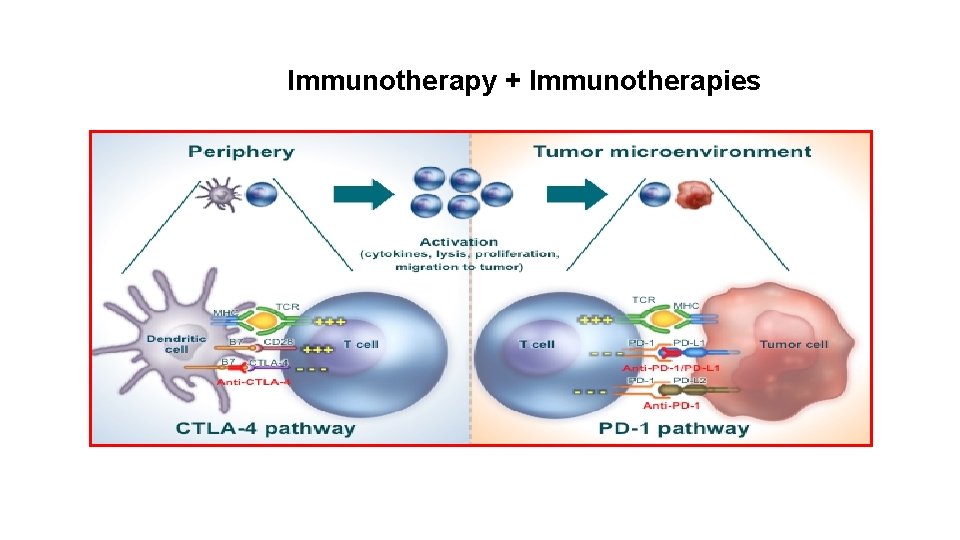
Immunotherapy + Immunotherapies

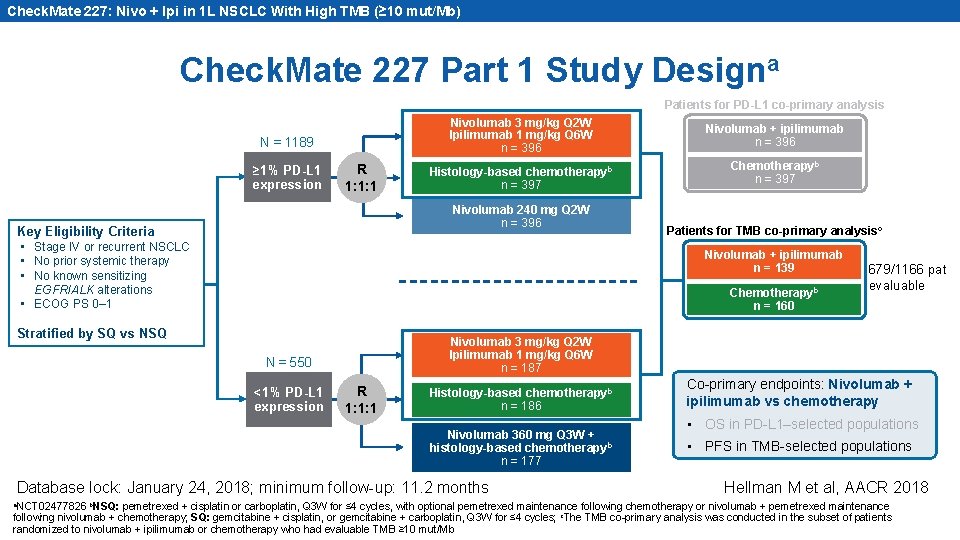
Check. Mate 227: Nivo + Ipi in 1 L NSCLC With High TMB (≥ 10 mut/Mb) Check. Mate 227 Part 1 Study Designa Patients for PD-L 1 co-primary analysis N = 1189 ≥ 1% PD-L 1 expression R 1: 1: 1 Nivolumab 3 mg/kg Q 2 W Ipilimumab 1 mg/kg Q 6 W n = 396 Nivolumab + ipilimumab n = 396 Histology-based chemotherapyb n = 397 Chemotherapyb n = 397 Nivolumab 240 mg Q 2 W n = 396 Key Eligibility Criteria • Stage IV or recurrent NSCLC • No prior systemic therapy • No known sensitizing EGFR/ALK alterations • ECOG PS 0– 1 Nivolumab + ipilimumab n = 139 Chemotherapyb n = 160 Stratified by SQ vs NSQ <1% PD-L 1 expression 679/1166 pat evaluable Nivolumab 3 mg/kg Q 2 W Ipilimumab 1 mg/kg Q 6 W n = 187 N = 550 R 1: 1: 1 Histology-based chemotherapyb n = 186 Nivolumab 360 mg Q 3 W + histology-based chemotherapyb n = 177 Database lock: January 24, 2018; minimum follow-up: 11. 2 months a. NCT 02477826 b. NSQ: Patients for TMB co-primary analysisc Co-primary endpoints: Nivolumab + ipilimumab vs chemotherapy • OS in PD-L 1–selected populations • PFS in TMB-selected populations Hellman M et al, AACR 2018 pemetrexed + cisplatin or carboplatin, Q 3 W for ≤ 4 cycles, with optional pemetrexed maintenance following chemotherapy or nivolumab + pemetrexed maintenance following nivolumab + chemotherapy; SQ: gemcitabine + cisplatin, or gemcitabine + carboplatin, Q 3 W for ≤ 4 cycles; c. The TMB co-primary analysis was conducted in the subset of patients randomized to nivolumab + ipilimumab or chemotherapy who had evaluable TMB ≥ 10 mut/Mb

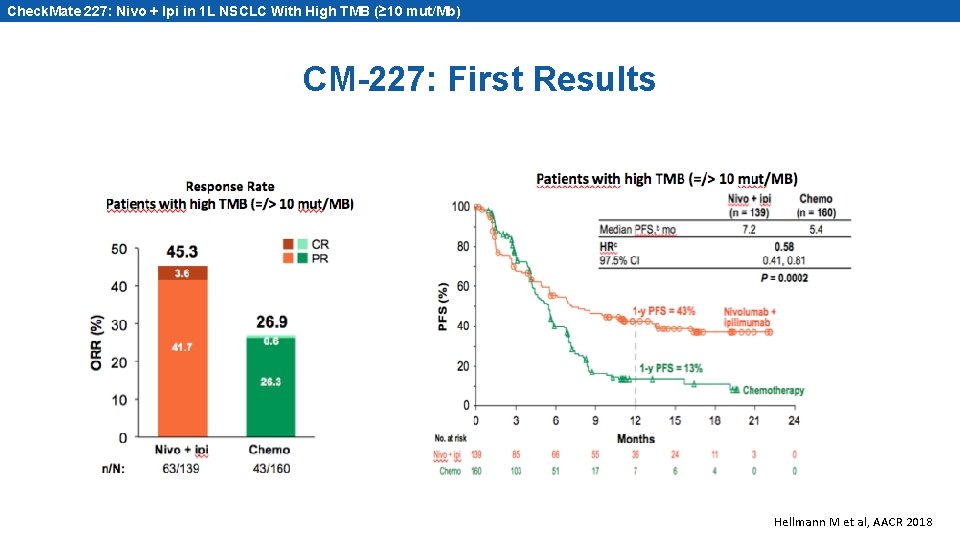
Check. Mate 227: Nivo + Ipi in 1 L NSCLC With High TMB (≥ 10 mut/Mb) CM-227: First Results Hellmann M et al, AACR 2018

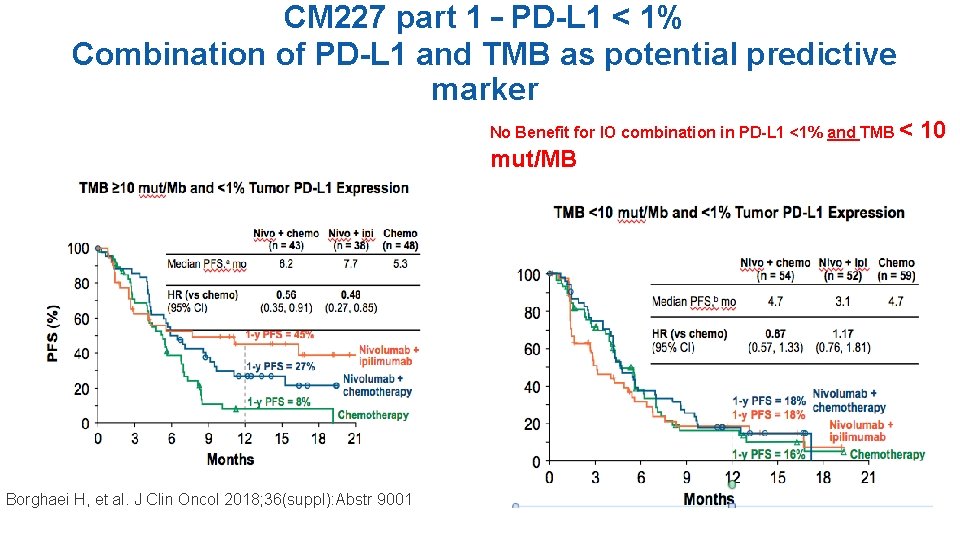
CM 227 part 1 – PD-L 1 < 1% Combination of PD-L 1 and TMB as potential predictive marker No Benefit for IO combination in PD-L 1 <1% and TMB < mut/MB Borghaei H, et al. J Clin Oncol 2018; 36(suppl): Abstr 9001 10

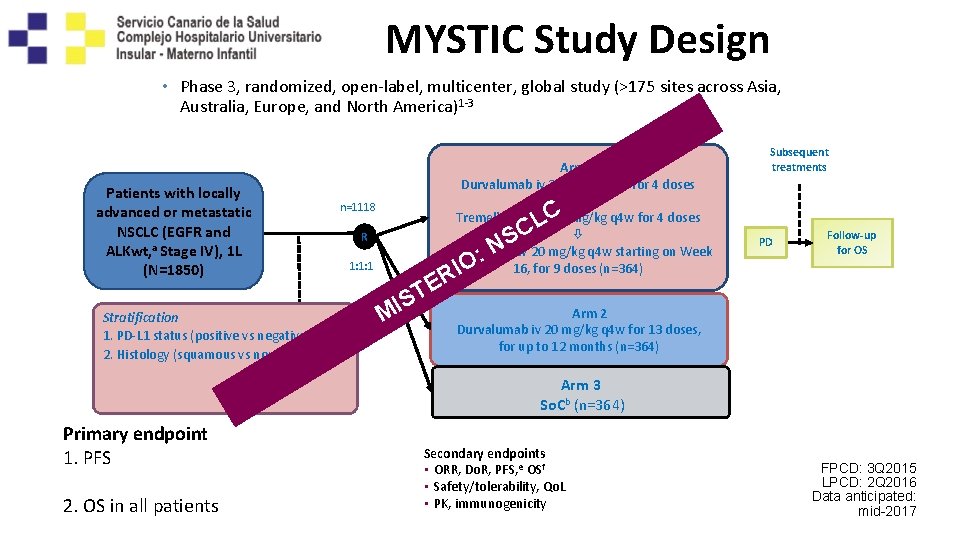
MYSTIC Study Design • Phase 3, randomized, open-label, multicenter, global study (>175 sites across Asia, Australia, Europe, and North America)1 -3 Patients with locally advanced or metastatic NSCLC (EGFR and ALKwt, a Stage IV), 1 L (N=1850) Stratification 1. PD-L 1 status (positive vs negative) 2. Histology (squamous vs nonsquamous) Arm 1 Durvalumab iv 20 mg/kg q 4 w for 4 doses + Tremelimumab iv 1 mg/kg q 4 w for 4 doses Durvalumab iv 20 mg/kg q 4 w starting on Week 16, for 9 doses (n=364) n=1118 R : 1: 1: 1 M E T IS O RI C L C NS Subsequent treatments PD Follow-up for OS Arm 2 Durvalumab iv 20 mg/kg q 4 w for 13 doses, for up to 12 months (n=364) Arm 3 So. Cb (n=364) Primary endpoint 1. PFS in all patients and in PD-L 1(+) patients 2. OS in all patients Secondary endpoints • ORR, Do. R, PFS, e OSf • Safety/tolerability, Qo. L • PK, immunogenicity FPCD: 3 Q 2015 LPCD: 2 Q 2016 Data anticipated: mid-2017

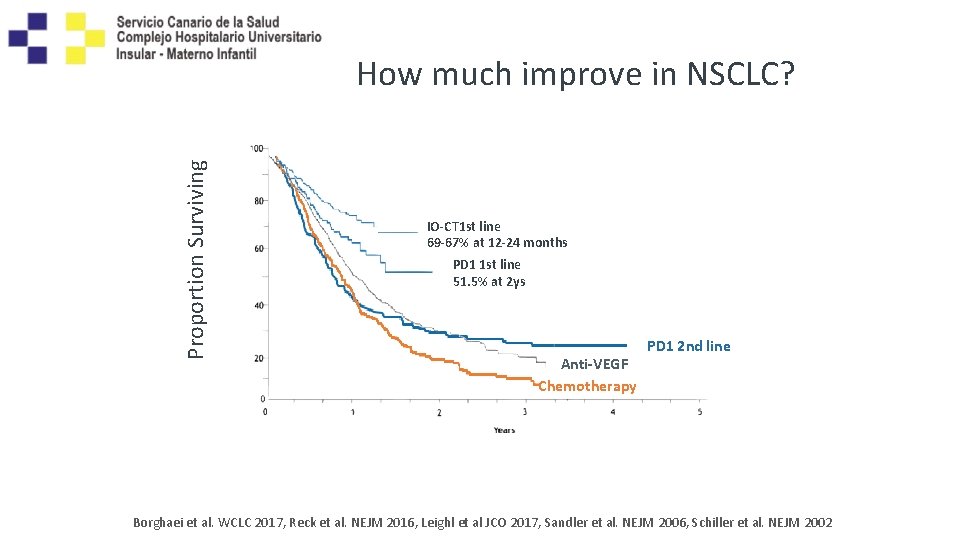
Proportion Surviving How much improve in NSCLC? IO-CT 1 st line 69 -67% at 12 -24 months PD 1 1 st line 51. 5% at 2 ys Anti-VEGF Chemotherapy PD 1 2 nd line Borghaei et al. WCLC 2017, Reck et al. NEJM 2016, Leighl et al JCO 2017, Sandler et al. NEJM 2006, Schiller et al. NEJM 2002

Some take home messages • PEMBROLIZUMAB MONO STILL THE SOC in 1 L NSCLC…PD-L 1>=50% • CT plus IO is a NEW STANDARD OF CARE IN 1 L nonsquamous NSCLC, in all PD-L 1 expression and WILL be a new standard in squamous soon!! • Combos have worse tolerability but no new ir. AEs • We STILL needing betters Biomarkers • Patients access to this NEW OPTIONS of therapy must be our goal!!!

THANK YOU!! Dr. Delvys Rodríguez Abreu Servicio Oncología Médica Hospital Universitario Insular de Gran Canaria drodabr@gobiernodecanarias. org
 Cambios culturales
Cambios culturales Los nuevos paradigmas
Los nuevos paradigmas Nuevos paradigmas educativos
Nuevos paradigmas educativos Nuevos paradigmas educativos
Nuevos paradigmas educativos Nuevos paradigmas educativos
Nuevos paradigmas educativos Banca en lnea
Banca en lnea Lnea
Lnea Lnea
Lnea Quiz 2 the baroque era
Quiz 2 the baroque era Victorian and elizabethan era
Victorian and elizabethan era Creí que era una aventura y en realidad era la vida
Creí que era una aventura y en realidad era la vida Vi uma estrela tão alta
Vi uma estrela tão alta Verbos nuevos
Verbos nuevos Odres nuevos vino nuevo
Odres nuevos vino nuevo Etapas de desarrollo de nuevos productos
Etapas de desarrollo de nuevos productos Nuevos materiales de la edad antigua
Nuevos materiales de la edad antigua Cuentos nuevos
Cuentos nuevos Texto descriptivo
Texto descriptivo Nuevos retos nuevas oportunidades
Nuevos retos nuevas oportunidades Nuevos comienzos en la biblia
Nuevos comienzos en la biblia Bienes de especialidad.
Bienes de especialidad. Ella lleva una sudadera cuando hace frío.
Ella lleva una sudadera cuando hace frío. Nuevos inhibidores de betalactamasas
Nuevos inhibidores de betalactamasas Inhibidores de betalactamasas
Inhibidores de betalactamasas Nuevos grados
Nuevos grados Nuevos anticonvulsivantes
Nuevos anticonvulsivantes Nuevos ambientes de aprendizaje
Nuevos ambientes de aprendizaje Ejemplos de paradigmas gerenciales
Ejemplos de paradigmas gerenciales Paradigma normativo instrumentalista
Paradigma normativo instrumentalista Pionero de paradigmas
Pionero de paradigmas Paradigma conductista
Paradigma conductista Paradigmas de investigacion social
Paradigmas de investigacion social Que es paradigma y sintagma
Que es paradigma y sintagma Paradigmas funcional
Paradigmas funcional Como hacer un paradigma
Como hacer un paradigma Evaluacin
Evaluacin Rationele actor paradigma
Rationele actor paradigma Paradigma curricular
Paradigma curricular L
L Paradigmas
Paradigmas Paradigmas
Paradigmas Paradigmas
Paradigmas