Nuclear Chemistry The study of nuclear reactions and











- Slides: 19

Nuclear Chemistry The study of nuclear reactions and their uses By: Robyn Louis & Alyssa Williams

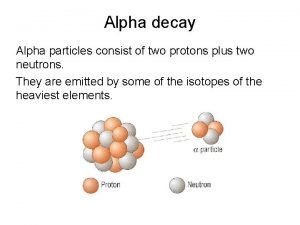
Discovery of Radioactivity ✘Henri Becquerel discovered radioactivity ✘In the late 19 th century Ernest Rutherford discovered that there were two types of radiation; alpha(��) and beta(��). Later on, Paul Villard discovered the gamma ray (��).

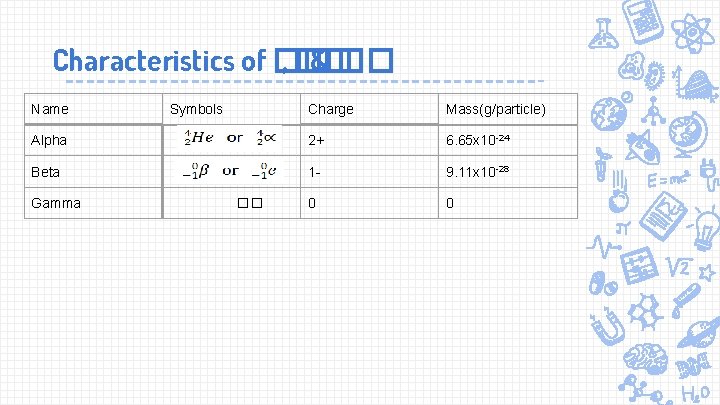
Characteristics of �� , �� & �� Name Charge Mass(g/particle) Alpha 2+ 6. 65 x 10 -24 Beta 1 - 9. 11 x 10 -28 0 0 Gamma Symbols ��

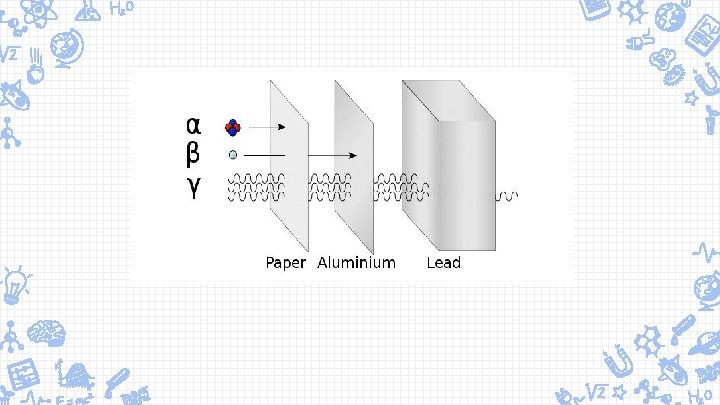
Radioactivity ✘gamma rays are a form of electromagnetic radiation and is the most penetrating allowing it to absorb the most energy , then beta and lastly alpha ✘radiation energy transferred to any material to be absorbed meaning that energy is being absorbed


Nuclear Reactions and Radioactive Decay ✘Radioactivity is the result of a natural change of an isotope from one element to another ✘alpha particle emissions causes a decrease of two units in atomic number and four units in mass number ✘beta particle emissions cause no change in atomic number and an increase of one unit in mass number ✘gamma rays cause no change

Radioactive Decay ✘ radioactive isotopes decay in order to form a radioactive product which causes several nuclear reactions which is ended by a nonradioactive isotope ✘ positron emission: a particle with the same mass as an electron but with the opposite sign. ✘ electron capture : nucleus captures an electron from the electron cloud surrounding the nucleus(same mass, atomic number reduced by 1)

Continued. . . ✘ Too many neutrons = spontaneous �� production ✘ Too many protons= spontaneous positron production ✘ When a nuclide has ≥ 84 protons it tends to undergo radioactive decay ✘ Nuclides with odd protons or neutrons are more likely to undergo radioactive decay ✘ Proton/neutron numbers 2, 8, 20, 28, 50, 82

Nuclear Binding Energy (Eb) ✘Einstein determined that matter is energy and that rest energy is the amount of energy with in a piece of matter; mass is a form of energy ✘the measure of energy to seperate the nucleus of an atom into protons and neutrons ✘E=mc 2 or Δc 2 ; c=speed of light=3 x 108 m/s △E=-4. 54 x 10 -12

Continued. . . ✘The larger the bind, the more stable the nuclei is ✘Higher the bind the more mass is turned into energy to bind the nucleons together

Fission ✘fission: splitting a heavy nuclei into 2 smaler nuclei with smaller mass numbers →mass of product < mass of reactants (missing mass = energy) ✘Material that starts reaction is the product and can start another reaction ✘Minimum amount of nuclide that provides the number of neutrons to

Fusion ✘Combining two light nuclei to form a heavier , more stable nucleus ✘energy from H bomb

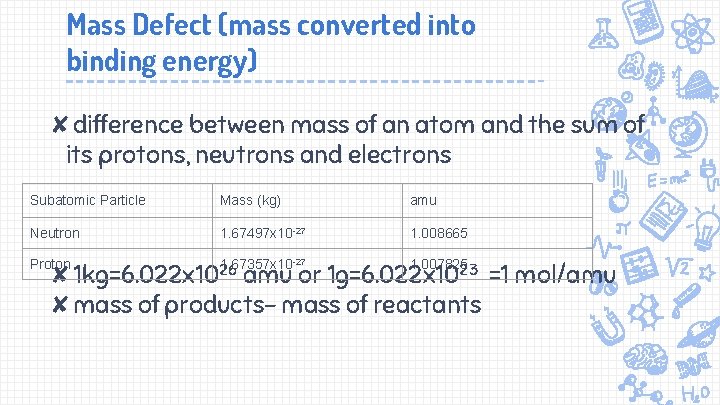
Mass Defect (mass converted into binding energy) ✘difference between mass of an atom and the sum of its protons, neutrons and electrons Subatomic Particle Mass (kg) amu Neutron 1. 67497 x 10 -27 1. 008665 Proton 1. 67357 x 10 ✘ 1 kg=6. 022 x 1026 amu -27 1. 007825 or 1 g=6. 022 x 1023 =1 mol/amu ✘mass of products- mass of reactants

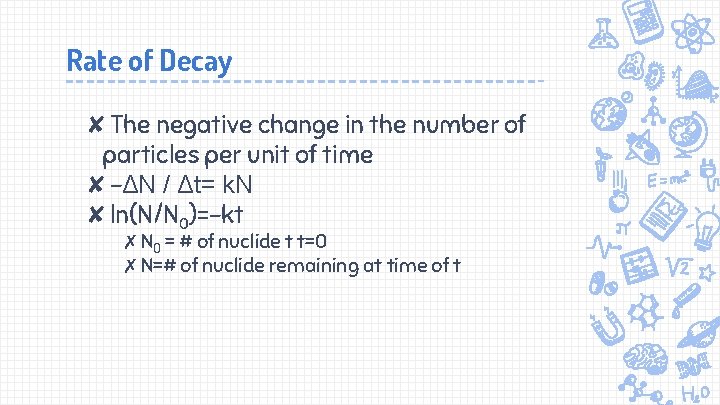
Rate of Decay ✘The negative change in the number of particles per unit of time ✘-ΔN / Δt= k. N ✘ln(N/N 0)=-kt ✗N 0 = # of nuclide t t=0 ✗N=# of nuclide remaining at time of t

Half Life Time required for the # of nuclides to reach half the original value ✘t 1/2=ln(2)/k=. 693/k

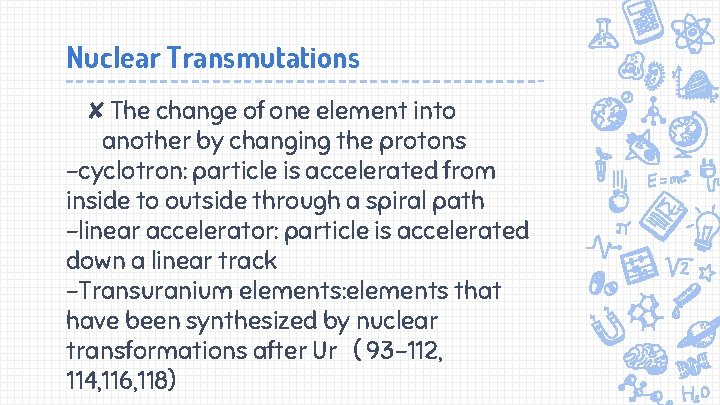
Nuclear Transmutations ✘The change of one element into another by changing the protons -cyclotron: particle is accelerated from inside to outside through a spiral path -linear accelerator: particle is accelerated down a linear track -Transuranium elements: elements that have been synthesized by nuclear transformations after Ur ( 93 -112, 114, 116, 118)

Detection/ Use of Radioactivity ✘Detection →geiger counter: Argon becomes ionized and when struck by a high energy particle electrical energy is amplified and radioactivity intensity is shown →scintillation counter: zinc sulfide give off light when struck by high energy particle from radioactive decay

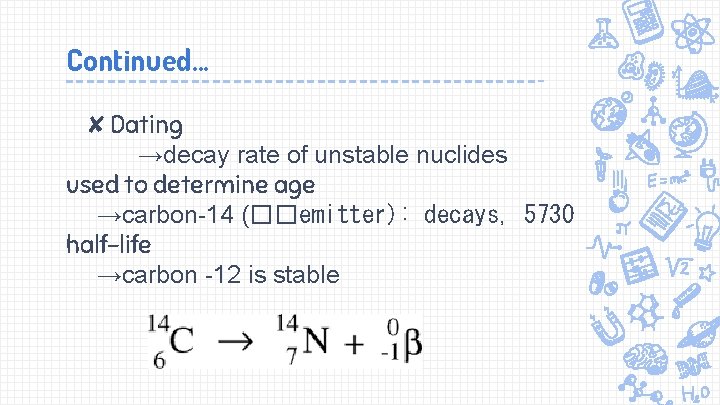
Continued. . . ✘Dating →decay rate of unstable nuclides used to determine age →carbon-14 (��emitter): decays, 5730 half-life →carbon -12 is stable

ASSIGNMENT Pg. 1091 -12 Pg. 1092 -18, 22, 30 Pg. 1093 -46 Pg. 1094 -52
 Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Nuclear decays and reactions section 2
Nuclear decays and reactions section 2 Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Redox reaction
Redox reaction Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions U 235 fission products
U 235 fission products Nuclear transmutation equation
Nuclear transmutation equation Balancing nuclear reactions
Balancing nuclear reactions Balance nuclear equations
Balance nuclear equations Flerov laboratory of nuclear reactions
Flerov laboratory of nuclear reactions Decay equation of uranium 238
Decay equation of uranium 238 Two types of nuclear reactions
Two types of nuclear reactions Bombardment reactions
Bombardment reactions Nuclear reactions are at
Nuclear reactions are at Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear 4 types of chemical reactions
4 types of chemical reactions