New Approaches to Cows Milk Allergy during Infancy

















- Slides: 56

New Approaches to Cow’s Milk Allergy during Infancy: Clinical Benefits of an Extensively Hydrolyzed Casein Formula with Lactobacillus rhamnosus GG Craig Jensen, M. D. Section of Gastroenterology, Hepatology and Nutrition Department of Pediatrics Baylor College of Medicine Houston, Texas 1

Disclosures: Consultant/Speaker: Mead Johnson Nutrition 2

Objectives • Distinguish Ig. E and non-Ig. E mediated aspects of cow’s milk allergy (CMA) • Recognize the importance of acquisition of tolerance in the context of CMA • Review the clinical effects of extensively hydrolyzed casein-based formula with Lactobacillus rhamnosus GG (LGG) in infants with CMA 3

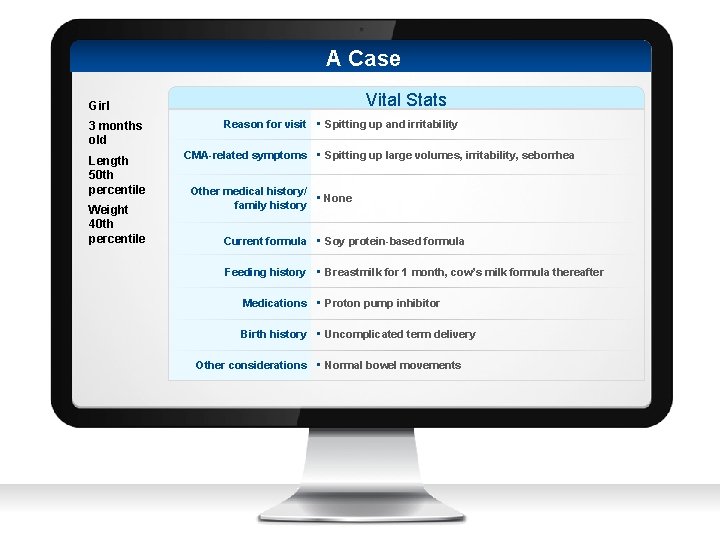
A Case Vital Stats Girl 3 months old Length 50 th percentile Weight 40 th percentile Reason for visit • Spitting up and irritability CMA-related symptoms • Spitting up large volumes, irritability, seborrhea Other medical history/ • None family history Current formula • Soy protein-based formula Feeding history • Breastmilk for 1 month, cow’s milk formula thereafter Medications • Proton pump inhibitor Birth history • Uncomplicated term delivery Other considerations • Normal bowel movements

Another Case Vital Stats Boy Reason for visit • Ongoing blood and mucus in stools 4 months old History of CMA • Diagnosed at 2 months Length 30 th percentile Weight 3 rd percentile CMA-related symptoms • Loose stools, mucus in stools, blood streaks in stools, poor weight gain Other medical history/ • Older sister: allergy to egg family history • Mother: asthma Current formula • Intact cow’s milk–based, lactose-free formula • Began on routine formula, then started on soy formula Feeding history • Then switched to intact cow’s milk–based, lactose-free formula Medications • Simethicone Birth history • Uncomplicated term delivery Other considerations • Gassiness and grunting t.

Cow’s Milk Allergy (CMA): Key Concepts 6

Cow’s Milk Food source for 9000 YEARS Current US consumption: 195 LB/PERSON/YEAR ADVERSE REACTIONS can occur with milk consumption USDA Dairy Products: Per capita consumption, United States. http: //www. ers. usda. gov/data-products/dairy-data. aspx #. U+QWEPt. Rb. To 7

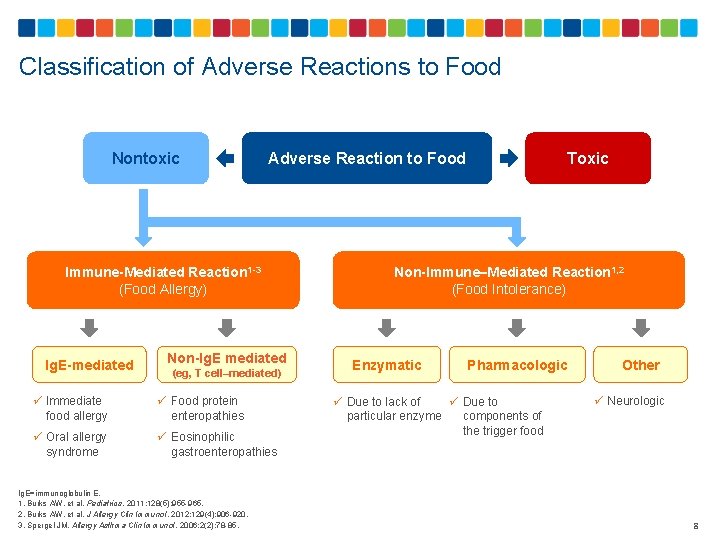
Classification of Adverse Reactions to Food Nontoxic Immune-Mediated Reaction 1 -3 (Food Allergy) Ig. E-mediated Non-Ig. E mediated (eg, T cell–mediated) ü Immediate food allergy ü Food protein enteropathies ü Oral allergy syndrome ü Eosinophilic gastroenteropathies Ig. E=immunoglobulin E. 1. Burks AW, et al. Pediatrics. 2011; 128(5): 955 -965. 2. Burks AW, et al. J Allergy Clin Immunol. 2012; 129(4): 906 -920. 3. Spergel JM. Allergy Asthma Clin Immunol. 2006; 2(2): 78 -85. Toxic Adverse Reaction to Food Non-Immune–Mediated Reaction 1, 2 (Food Intolerance) Enzymatic Pharmacologic ü Due to lack of ü Due to particular enzyme components of the trigger food Other ü Neurologic 8

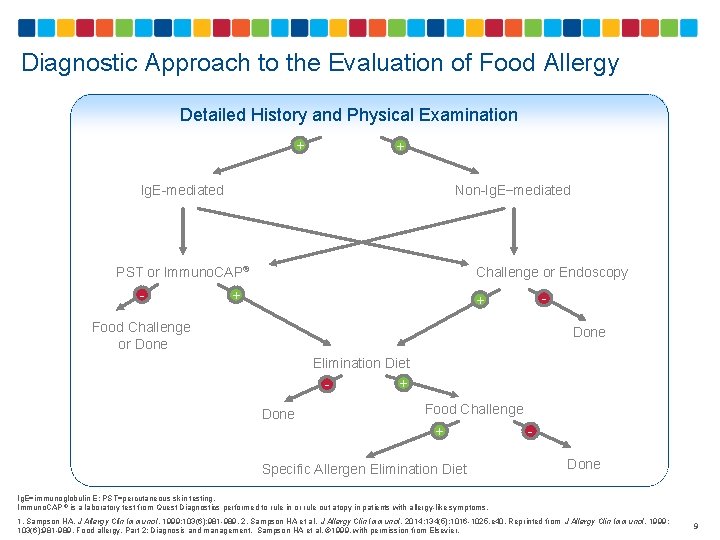
Diagnostic Approach to the Evaluation of Food Allergy Detailed History and Physical Examination + + Ig. E-mediated Non-Ig. E−mediated PST or Immuno. CAP® - Challenge or Endoscopy + - + Food Challenge or Done Elimination Diet + Done Food Challenge + Done Specific Allergen Elimination Diet Ig. E=immunoglobulin E; PST=percutaneous skin testing. Immuno. CAP ® is a laboratory test from Quest Diagnostics performed to rule in or rule out atopy in patients with allergy-like symptoms. 1. Sampson HA. J Allergy Clin Immunol. 1999; 103(6): 981 -989. 2. Sampson HA et al. J Allergy Clin Immunol. 2014; 134(5): 1016 -1025. e 40. Reprinted from J Allergy Clin Immunol. 1999; 103(6): 981 -989. Food allergy. Part 2: Diagnosis and management. Sampson HA et al. © 1999, with permission from Elsevier. 9

Family History and Physical Examination During Early Diagnosis Key observations helpful upfront 1: • Learn about personal and family history of allergic disease • Identify and create a list of suspected foods • Document the precise description of reactions Key symptoms to watch for during a physical examination 1, 2: • Cutaneous: Flushing, hives, angioedema, and eczema • Gastrointestinal: Oropharyngeal pruritus and edema, abdominal cramping, nausea, vomiting, and diarrhea • Pulmonary: Rhinorrhea, laryngeal edema, wheezing, coughing and shortness of breath • Cardiovascular: Hypotension, tachycardia, and arrhythmias • Behavioral: Irritability (preceding or in combination with other symptoms) 1. Sampson HA. J Allergy Clin Immunol. 1999; 103(6): 981 -989. 2. Burks AW, et al. Pediatrics. 2011; 128(5): 955 -965. 10

Important Gastrointestinal Manifestations Associated With Non-Ig. E–mediated Food Allergy • Eosinophilic esophagitis, Gastroenteritis 1, 2: – – Postprandial vomiting, anorexia, abdominal distention, steatorrhea, failure to thrive, weight loss, food impaction, and gastric outlet obstruction A subset with food-induced Ig. E-mediated reactions • Dietary protein enteropathy 2: – – Diarrhea, failure to thrive, abdominal distention, and malabsorption Less frequent anemia, edema, and hypoproteinemia • Dietary protein enterocolitis 2: – Vomiting and diarrhea • Dietary protein proctocolitis 2: – Gross blood in stool + other symptoms • Celiac disease 1: – – Diarrhea, steatorrhea, malabsorption, abdominal distention, flatulence, + nausea and vomiting, failure to thrive, oral ulcers Associated skin disease: dermatitis herpetiformis 1. Spergel JM. Allergy Asthma Clin Immunol. 2006; 2(2): 78 -85. 2. Burks AW, et al. J Allergy Clin Immunol. 2012; 129(4): 906 -920. 11

Description of Allergic Reactions: Timing and Reproducibility Are of the Essence • Key items to note during an early diagnosis 1, 2: – Timing of onset in relation to food ingestion – Symptoms, their severity and duration of reaction – Treatment of reaction – Reproducibility of reaction after ingestion of suspected food – Most recent reaction 1. Sampson HA. J Allergy Clin Immunol. 1999; 103(6): 981 -989. 2. Sampson HA et al. J Allergy Clin Immunol. 2014; 134(5): 1016 -1025. e 40. 12

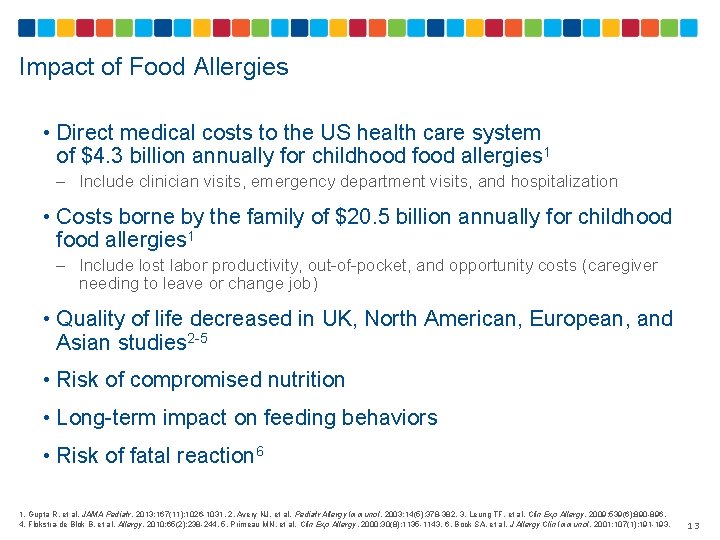
Impact of Food Allergies • Direct medical costs to the US health care system of $4. 3 billion annually for childhood food allergies 1 – Include clinician visits, emergency department visits, and hospitalization • Costs borne by the family of $20. 5 billion annually for childhood food allergies 1 – Include lost labor productivity, out-of-pocket, and opportunity costs (caregiver needing to leave or change job) • Quality of life decreased in UK, North American, European, and Asian studies 2 -5 • Risk of compromised nutrition • Long-term impact on feeding behaviors • Risk of fatal reaction 6 1. Gupta R, et al. JAMA Pediatr. 2013; 167(11): 1026 -1031. 2. Avery NJ, et al. Pediatr Allergy Immunol. 2003; 14(5): 378 -382. 3. Leung TF, et al. Clin Exp Allergy. 2009; 539(6): 890 -896. 4. Flokstra-de Blok B, et al. Allergy. 2010; 65(2): 238 -244. 5. Primeau MN, et al. Clin Exp Allergy. 2000; 30(8): 1135 -1143. 6. Bock SA, et al. J Allergy Clin Immunol. 2001; 107(1): 191 -193. 13

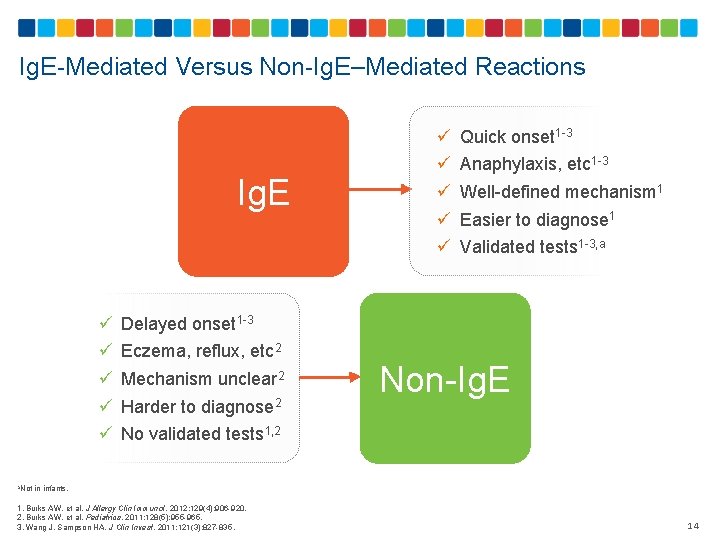
Ig. E-Mediated Versus Non-Ig. E–Mediated Reactions Ig. E ü ü ü ü ü Quick onset 1 -3 Anaphylaxis, etc 1 -3 Well-defined mechanism 1 Easier to diagnose 1 Validated tests 1 -3, a Delayed onset 1 -3 Eczema, reflux, etc 2 Mechanism unclear 2 Harder to diagnose 2 Non-Ig. E No validated tests 1, 2 a. Not in infants. 1. Burks AW, et al. J Allergy Clin Immunol. 2012; 129(4): 906 -920. 2. Burks AW, et al. Pediatrics. 2011; 128(5): 955 -965. 3. Wang J, Sampson HA. J Clin Invest. 2011; 121(3): 827 -835. 14

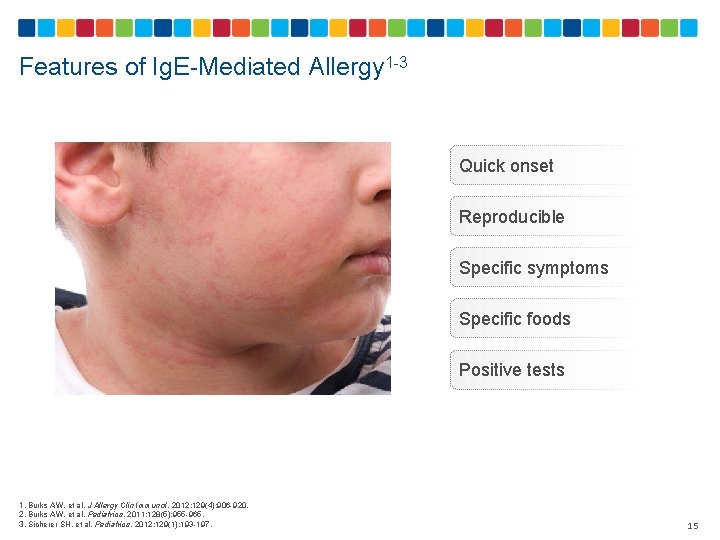
Features of Ig. E-Mediated Allergy 1 -3 Quick onset Reproducible Specific symptoms Specific foods Positive tests 1. Burks AW, et al. J Allergy Clin Immunol. 2012; 129(4): 906 -920. 2. Burks AW, et al. Pediatrics. 2011; 128(5): 955 -965. 3. Sicherer SH, et al. Pediatrics. 2012; 129(1): 193 -197. 15

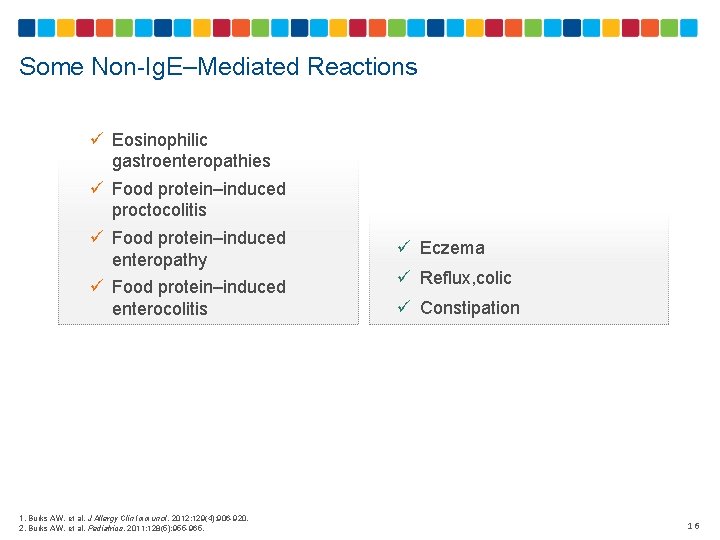
Some Non-Ig. E–Mediated Reactions ü Eosinophilic gastroenteropathies ü Food protein–induced proctocolitis ü Food protein–induced enteropathy ü Food protein–induced enterocolitis 1. Burks AW, et al. J Allergy Clin Immunol. 2012; 129(4): 906 -920. 2. Burks AW, et al. Pediatrics. 2011; 128(5): 955 -965. ü Eczema ü Reflux, colic ü Constipation 16

What Is Primary Prevention and Why Does It Matter? • Primary allergy prevention refers to inhibiting the development of clinical disease before it occurs 1 • Prevalence of food allergy (and other allergic diseases) has increased within the past 2 decades 2, 3 – Peanut allergy: • US: Prevalence of PA in children <18 y. o. increased from 0. 4% in 1997 to 0. 8% in 2002 and 1. 4% in 20082 • UK: Prevalence of PA in children increased from 0. 6% to 1. 8% over the last 2 decades 3 – Tree nut allergy: • US: Prevalence of tree nut allergy in children increased from 0. 2% in 1997 to 1. 1% in 20082 • Food allergy is now considered a public health concern, as the condition is associated with significant morbidity and occasional mortality 4 PA=peanut allergy. 1. Fleischer DM et al. J Allergy Clin Immunol Pract. 2013; 1(1): 29 -36. 2. Sicherer SH et al. J Allergy Clin Immunol 2010; 125(6): 1322 -1326. 3. Hourihane JO et al. J Allergy Clin Immunol 2007; 119: 1197 -1202. 4. Sicherer SH et al. J Allergy Clin Immunol 2014; 133(2): 291 -307. 17

What Factors May Help Explain an Increase in Food Allergy Prevalence? • Changes in Diet 1, 2 – Vitamin D: An association between low Vitamin D levels and increased risk of FA has been suggested 1 – Antioxidants: Some data with asthma but none yet with FA 1 – Obesity: Obesity is associated with an inflammatory state; mostly studied in asthma 1 – Dietary Fat: Despite the earlier results, recent meta-analysis found no clear evidence to support the use of Omega 3 and Omega 6 fatty acids for the primary prevention of atopic allergic disease development or sensitization 2 • Hygiene Hypothesis 3 – Lack of exposure to infectious agents and gut flora increases susceptibility to allergic diseases; limited data for FA, except for mild effect of cesarean delivery FA=food allergy. 1. Sicherer SH et al. J Allergy Clin Immunol. 2014; 133(2): 291 -307. 2. Anandan C et al. Allergy. 2009; 64(6): 840 -848. 3. Kim H et al. Korean J Pediatr. 2013; 56(9): 369 -376. 19

Food Allergy Management Current management of food allergy includes STRICT ALLERGEN AVOIDANCE (exclusion diet) Chapman JA, et al. Ann Allergy Asthma Immunol. 2006; 96(suppl): S 1 -S 68. PHARMACOTHERAPY (in case of accidental exposure to the antigen) 20

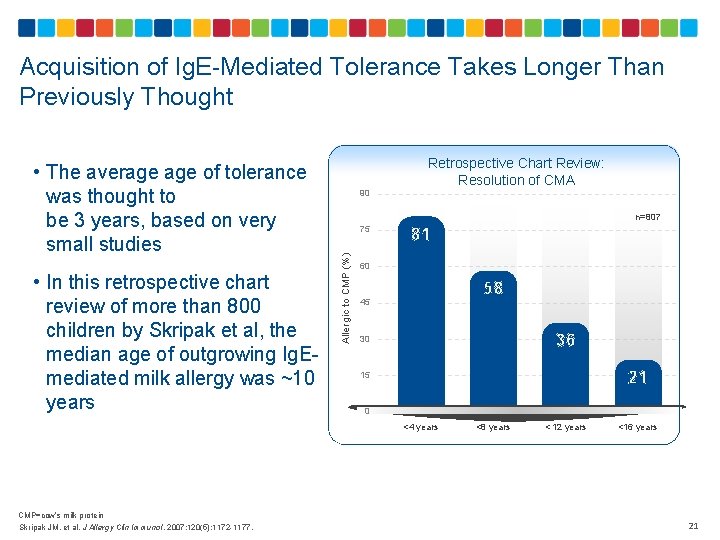
Acquisition of Ig. E-Mediated Tolerance Takes Longer Than Previously Thought • In this retrospective chart review of more than 800 children by Skripak et al, the median age of outgrowing Ig. Emediated milk allergy was ~10 years 90 Retrospective Chart Review: Resolution of CMA n=807 75 Allergic to CMP (%) • The average of tolerance was thought to be 3 years, based on very small studies 81 60 58 45 36 30 21 15 0 <4 years CMP=cow’s milk protein Skripak JM, et al. J Allergy Clin Immunol. 2007; 120(5): 1172 -1177. <8 years < 12 years <16 years 21

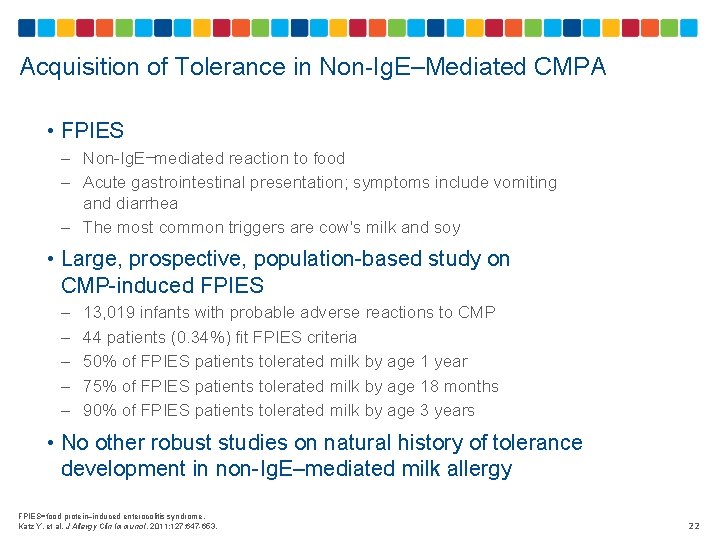
Acquisition of Tolerance in Non-Ig. E–Mediated CMPA • FPIES – Non-Ig. E−mediated reaction to food – Acute gastrointestinal presentation; symptoms include vomiting and diarrhea – The most common triggers are cow's milk and soy • Large, prospective, population-based study on CMP-induced FPIES – – – 13, 019 infants with probable adverse reactions to CMP 44 patients (0. 34%) fit FPIES criteria 50% of FPIES patients tolerated milk by age 1 year 75% of FPIES patients tolerated milk by age 18 months 90% of FPIES patients tolerated milk by age 3 years • No other robust studies on natural history of tolerance development in non-Ig. E–mediated milk allergy FPIES=food protein–induced enterocolitis syndrome. Katz Y, et al. J Allergy Clin Immunol. 2011; 127: 647 -653. 22

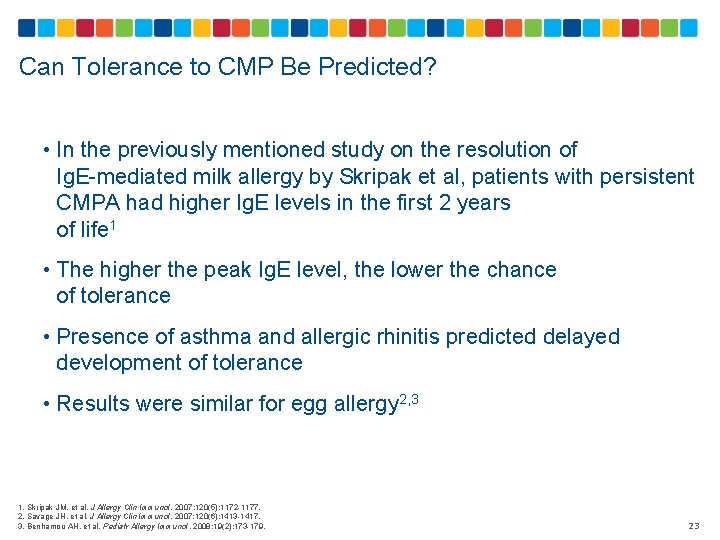
Can Tolerance to CMP Be Predicted? • In the previously mentioned study on the resolution of Ig. E-mediated milk allergy by Skripak et al, patients with persistent CMPA had higher Ig. E levels in the first 2 years of life 1 • The higher the peak Ig. E level, the lower the chance of tolerance • Presence of asthma and allergic rhinitis predicted delayed development of tolerance • Results were similar for egg allergy 2, 3 1. Skripak JM, et al. J Allergy Clin Immunol. 2007; 120(5): 1172 -1177. 2. Savage JH, et al. J Allergy Clin Immunol. 2007; 120(6): 1413 -1417. 3. Benhamou AH, et al. Pediatr Allergy Immunol. 2008; 19(2): 173 -179. 23

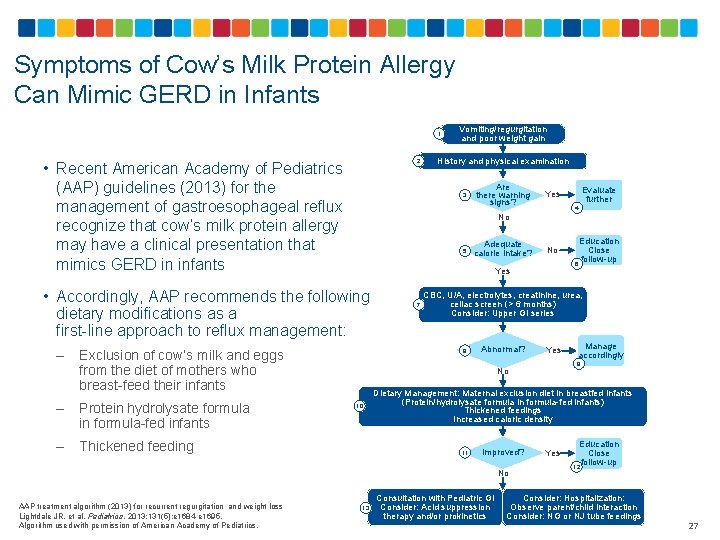
Symptoms of Cow’s Milk Protein Allergy Can Mimic GERD in Infants 1 2 • Recent American Academy of Pediatrics (AAP) guidelines (2013) for the management of gastroesophageal reflux recognize that cow’s milk protein allergy may have a clinical presentation that mimics GERD in infants Protein hydrolysate formula in formula-fed infants – Thickened feeding Are there warning signs? 5 Adequate calorie intake? 4 No Yes 7 8 Abnormal? Yes Dietary Management: Maternal exclusion diet in breastfed infants (Protein/hydrolysate formula in formula-fed infants) Thickened feedings Increased caloric density 11 13 Manage accordingly 9 No 10 Education Close follow-up 6 CBC, U/A, electrolytes, creatinine, urea, celiac screen (> 6 months) Consider: Upper GI series Improved? No AAP treatment algorithm (2013) for recurrent regurgitation and weight loss Lightdale JR, et al. Pediatrics. 2013; 131(5): e 1684 -e 1695. Algorithm used with permission of American Academy of Pediatrics. Evaluate further Yes No Exclusion of cow’s milk and eggs from the diet of mothers who breast-feed their infants – History and physical examination 3 • Accordingly, AAP recommends the following dietary modifications as a first-line approach to reflux management: – Vomiting/regurgitation and poor weight gain Consultation with Pediatric GI Consider: Acid suppression therapy and/or prokinetics Yes Education Close follow-up 12 Consider: Hospitalization: Observe parent/child interaction Consider: NG or NJ tube feedings 27

Key Points 1 Milk allergy can be Ig. E mediated or non-Ig. E mediated 2 Children usually develop tolerance to milk by late childhood 3 The age of tolerance development is hard to predict, although the results of initial allergy tests may have some predictive value for Ig. E-mediated allergy 28

PROBIOTICS … 29

What Are Probiotics? • Lactobacillus sp 1, 2 – L acidophilus, L brevis, L delbrueckii, L fermentum, L gasseri, L johnsonii, L paracasei, L plantarum, L reuteri, L rhamnosus GG (LGG), L salivarius • Bifidobacterium sp 1, 2 – B bifidum, B breve, B infantis, B longum, B adolescentis, B lactis (Bb 12) • Other microbes 2 – Escherichia coli Nissle 1917, Saccharomyces cerevisiae, Enterococcus sp 1. FAO /WHO. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Oct 2001. http: //www. who. int/foodsafety/publications/fs_management/probiotics/en/ 2. Holzapfel WH, et al. Am J Clin Nutr. 2001; 73(suppl): 365 S-373 S. 30

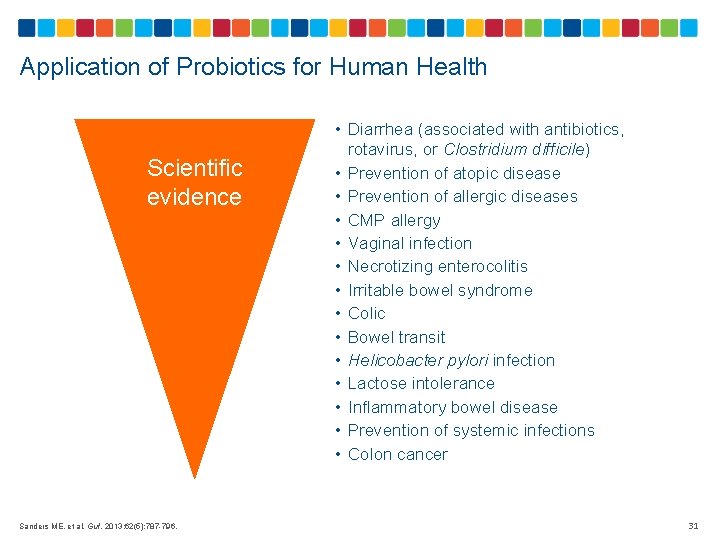
Application of Probiotics for Human Health Scientific evidence Sanders ME, et al. Gut. 2013; 62(5): 787 -796. • Diarrhea (associated with antibiotics, rotavirus, or Clostridium difficile) • Prevention of atopic disease • Prevention of allergic diseases • CMP allergy • Vaginal infection • Necrotizing enterocolitis • Irritable bowel syndrome • Colic • Bowel transit • Helicobacter pylori infection • Lactose intolerance • Inflammatory bowel disease • Prevention of systemic infections • Colon cancer 31

Probiotics Affect Allergies in Multiple Ways • Degradation/structural modification of enteral antigens 1 • Normalization of the properties of aberrant indigenous microbiota and gut barrier functions 2 • Regulation of the secretion of inflammatory mediators and promotion of immune system development 1 • Prevention of food allergy through promotion of endogenous barrier mechanisms and alleviation of intestinal inflammation 2 • Stimulation of immune response and reduction of serum Ig. E levels 3 • Reduction of T helper 2 (Th 2) cytokine response 2 1. Isolauri E, et al. Gastroenterol Clin North Am. 2012; 41(4): 747 -762. 2. Butel MJ. Med Mal Infect. 2014; 44(1): 1 -8. 3. Kim HJ, et al. Korean J Pediatr. 2013; 56(9): 369 -376. 32

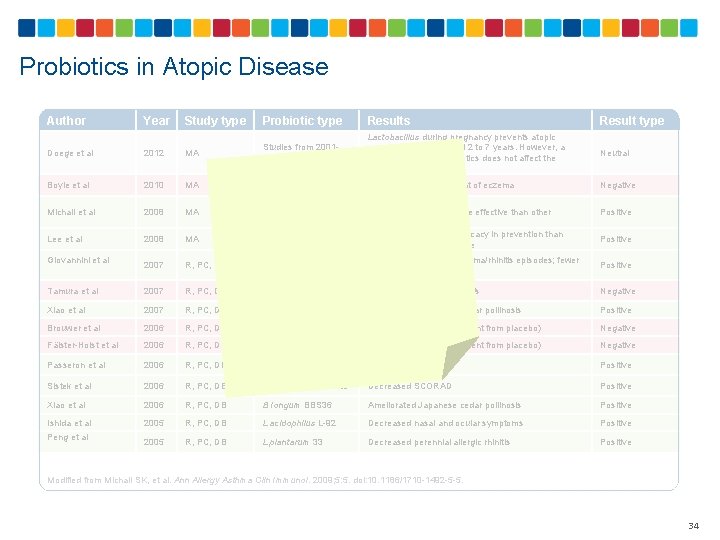
Probiotics in Sensitization and Allergic Diseases Author Year Study type Probiotic type Ou et al 2013 R, PC, DB LGG Ou et al 2011 R, PC, DB LGG Dotterud et al 2010 R, C, DB LGG, L acidophilus La-5, B lactis Bb 12 Administration during pregnancy reduced the severity of maternal AD but not the incidence of childhood allergic sensitization or AD No significant effects on sensitization, development of AD, and maternal Ig. E levels Reduced the cumulative incidence of AD, but had no effect on atopic sensitization Rose et al 2010 R, PC, DB LGG No significant differences in SCORAD indices Negative Wickens et al 2008 R, PC, DB Lower Ig. E-associated eczema Positive Osborn et al 2007 MA No current evidence to support the administration of probiotics to prevent eczema Negative Abrahamsson et al 2007 R, C, DB L reuteri Decreased Ig. E-associated eczema Positive Kalliomäki et al 2007 R, PC, DB LGG Decreased AD Positive Kukkonen et al 2007 R, PC, DB LGG, MIX Lower Ig. E-associated diseases and eczema Positive Taylor et al 2007 R, PC, DB No change in AD rates or SCORAD Negative Taylor et al 2007 R, PC, DB No change in AD Negative Lodinova- Zadnikova 2004 C E coli Decreased long-term incidence of allergy Positive Kalliomäki et al 2003 R, PC, DB LGG Decreased AD Positive Lodinova- Zadnikova 2003 C E coli Decreased allergy development Positive Kalliomäki et al 2001 R, PC, DB LGG Decreased AD Positive Rautava et al 2001 R, PC, DB LGG Decreased AD Positive L rhamnosus 19070 -2 + L reuteri DSM 122460 Studies from 19662001 L acidophilus LAVRI- A 1 Results Result type Neutral R, randomized; PC, placebo controlled; DB, double blind; AD, atopic dermatitis; C, controlled; SCORAD, severity scoring of atopic dermatitis; MA, meta-analysis Modified from Michail SK, et al. Ann Allergy Asthma Clin Immunol. 2008; 5: 5. doi: 10. 1186/1710 -1492 -5 -5. 33

Probiotics in Atopic Disease Author Year Study type Probiotic type Results Result type Neutral Negative Doege et al 2012 MA Studies from 20012009 Lactobacillus during pregnancy prevents atopic eczema in children aged 2 to 7 years. However, a mixture of various probiotics does not affect the development of AD Boyle et al 2010 MA Studies from 20032008 No evidence on treatment of eczema Michail et al 2008 MA Studies from 19972007 Lee et al 2008 MA Studies from 19972007 R, PC, DB L casei DN-114001 Longer time free from asthma/rhinitis episodes; fewer rhinitis episodes Positive Tamura et al 2007 R, PC, DB L casei strain Shirota No change in allergic rhinitis Negative Xiao et al 2007 R, PC, DB B Iongum BBS 36 Ameliorated Japanese cedar pollinosis Positive Brouwer et al 2006 R, PC, DB LGG Lower SCORAD (no different from placebo) Negative Fälster-Holst et al 2006 R, PC, DB LGG Lower SCORAD (no different from placebo) Negative Decreased SCORAD Positive Giovannini et al Lower SCORAD LGG appeared to be more effective than other probiotic preparations Probiotics show better efficacy in prevention than treatment of atopic disease Positive Passeron et al 2006 R, PC, DB L rhamnosus Lcr 35 and prebiotic Sistek et al 2006 R, PC, DB L rhamnosus, B lactis Decreased SCORAD Positive Xiao et al 2006 R, PC, DB B Iongum BBS 36 Ameliorated Japanese cedar pollinosis Positive Ishida et al 2005 R, PC, DB L acidophilus L-92 Decreased nasal and ocular symptoms Positive 2005 R, PC, DB L plantarum 33 Decreased perennial allergic rhinitis Positive Peng et al Modified from Michail SK, et al. Ann Allergy Asthma Clin Immunol. 2009; 5: 5. doi: 10. 1186/1710 -1492 -5 -5. 34

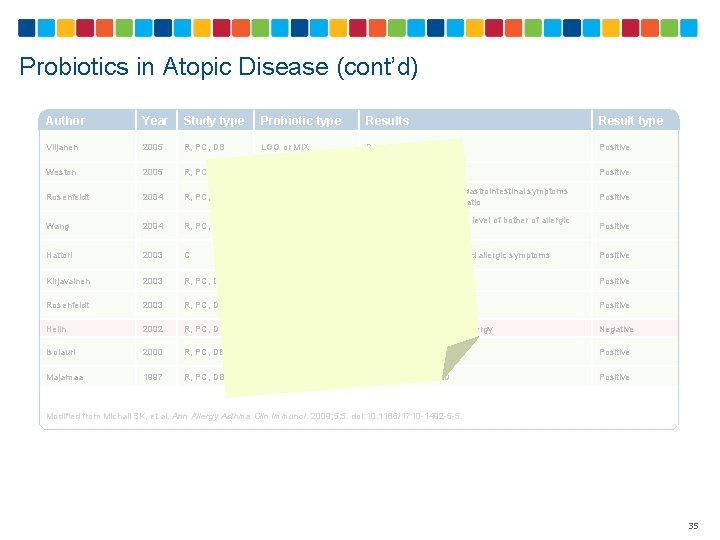
Probiotics in Atopic Disease (cont’d) Author Year Study type Probiotic type Results Result type Viljanen 2005 R, PC, DB LGG or MIX Decreased SCORAD Positive Weston 2005 R, PC, DB L fermentum Decreased SCORAD Positive Rosenfeldt 2004 R, PC, DB L rhamnosus and L reuteri Decreased frequency of gastrointestinal symptoms and lactulose: mannitaol ratio Positive Wang 2004 R, PC, DB L paracasei Decreased frequency and level of bother of allergic rhinitis Positive Hattori 2003 C B breve M-16 V Ameliorated cutaneous and allergic symptoms Positive Kirjavainen 2003 R, PC, DB LGG Decreased SCORAD Positive Rosenfeldt 2003 R, PC, DB L rhamnosus and L reuteri Decreased SCORAD Positive Helin 2002 R, PC, DB LGG No effect on birch pollen allergy Negative Isolauri 2000 R, PC, DB B lactis (Bb-12) or LGG Decreased SCORAD Positive Majamaa 1997 R, PC, DB LGG Decreased SCORAD Positive Modified from Michail SK, et al. Ann Allergy Asthma Clin Immunol. 2009; 5: 5. doi: 10. 1186/1710 -1492 -5 -5. 35

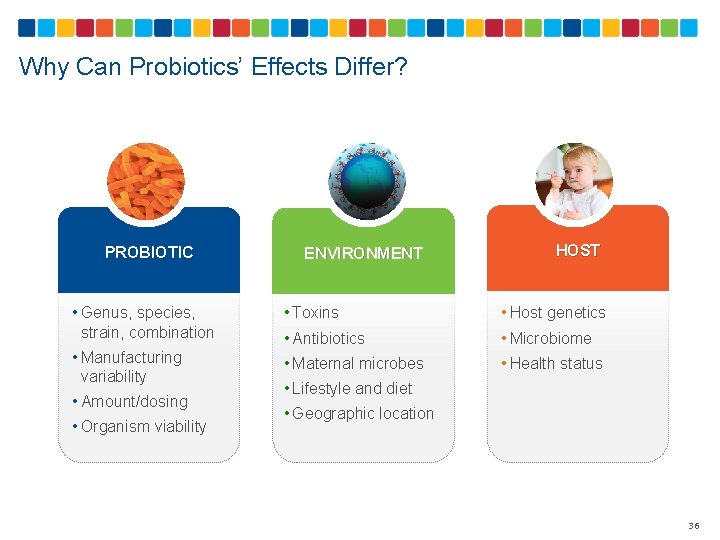
Why Can Probiotics’ Effects Differ? PROBIOTIC ENVIRONMENT HOST • Genus, species, strain, combination • Toxins • Host genetics • Antibiotics • Microbiome • Manufacturing variability • Maternal microbes • Health status • Amount/dosing • Organism viability • Lifestyle and diet • Geographic location 36

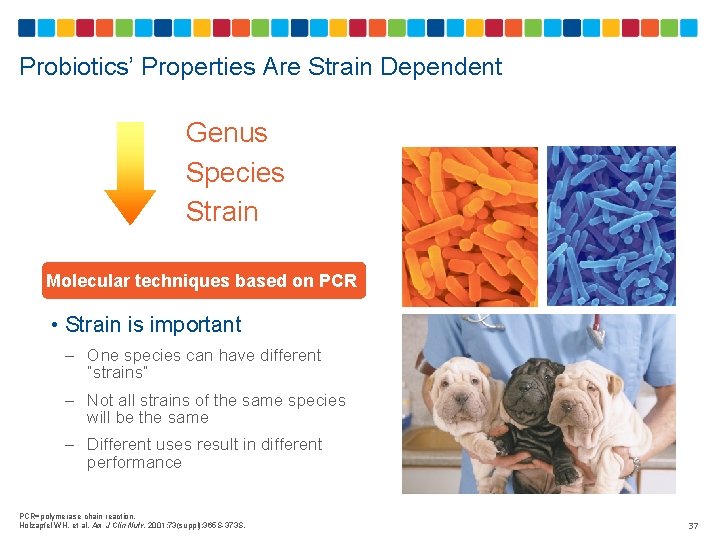
Probiotics’ Properties Are Strain Dependent Genus Species Strain Molecular techniques based on PCR • Strain is important – One species can have different “strains” – Not all strains of the same species will be the same – Different uses result in different performance PCR=polymerase chain reaction. Holzapfel WH, et al. Am J Clin Nutr. 2001; 73(suppl): 365 S-373 S. 37

Probiotic Isolates of Different Origins Can Have Different Effects • Study conducted with LGG from 14 different sources and manufacturing processes • All L rhamnosus isolates showed similar tolerance to acid • All isolates were able to bind to human colonic mucus • However, the ability to inhibit the adhesion of pathogens to colonic mucus differed among the L rhamnosus isolates Grześkowiak Ł, et al. Br J Nutr. 2011; 105(6): 887 -894. 38

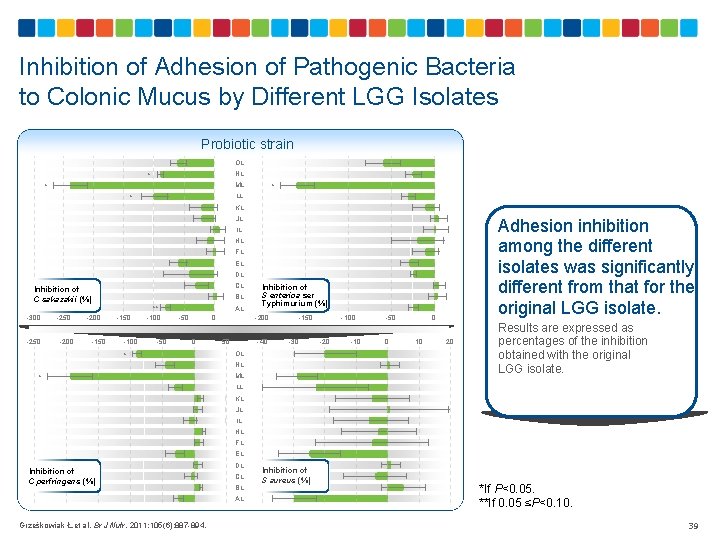
Inhibition of Adhesion of Pathogenic Bacteria to Colonic Mucus by Different LGG Isolates Probiotic strain OL NL * ML * * LL * KL JL Adhesion inhibition among the different isolates was significantly different from that for the original LGG isolate. IL HL FL EL DL CL Inhibition of C sakazakii (%) -300 -250 -200 -150 BL -150 -100 ** -100 -50 AL -50 0 0 * Inhibition of S enterica ser Typhimurium (%) -200 50 -40 -150 -30 OL NL * ML -100 -20 -10 -50 0 0 10 20 Results are expressed as percentages of the inhibition obtained with the original LGG isolate. LL KL JL IL HL FL EL Inhibition of C perfringens (%) DL CL BL AL Grześkowiak Ł, et al. Br J Nutr. 2011; 105(6): 887 -894. Inhibition of S aureus (%) *If P<0. 05. **If 0. 05 ≤P<0. 10. 39

Key Points 1 There is a large amount of data on the preventive and therapeutic effects of probiotics in allergies and atopic diseases, with varying results 2 Results can be affected by several factors, including the type of probiotic, source of probiotic, target group, and probiotic combinations 3 Results from meta-analyses and systematic reviews that combine results of studies with different designs, target groups, and ages should be interpreted with caution 40

Lactobacillus rhamnosus GG (LGG, ATCC 53103) • Isolated from a healthy human source • World's most researched probiotic bacterium • Multiple scientifically demonstrated beneficial effects – Lower incidence and shorter duration of diarrhea 1 – Lower incidence of respiratory infections 2, 3 – Increased Ig. A antibody production after rotavirus infection 4 – Enhanced level of rotavirus vaccine-specific antibodies 5 – Decreased risk of atopic eczema in high-risk children 6 1. Szajewska H, et al. Aliment Pharmacol Ther. 2007; 25: 871 -881. 2. Hojsak I, et al. Clin Nutr. 2010; 29: 312 -316. 3. Luoto R, et al. J Allergy Clin Immunol. 2014; 133(2): 405 -413. 4. Majamaa H, et al. J Pediatr Gastroenterol Nutr. 1995; 20(3): 333 -338. 5. Isolauri E, et al. Vaccine. 1995; 13(3): 310 -312. 6. Kalliomaki M, et al. Lancet. 2001; 357(9262): 1076 -1079. 41

The Role of LGG in Allergy • Reported to reduce severity of atopic dermatitis when administered to breastfeeding mothers of infants with atopic eczema/dermatitis due to cow’s milk allergy 1 • May be effective in preventing atopic eczema in high-risk infants when administered postnatally for 6 months and to pregnant mothers 2 • May balance the generation of cytokines (ie, IL-4, IL-5, IL-10, IFN-γ, TGF-β, and TNF-α), which can contribute to downregulation of inflammatory processes 3 -5 IL=interleukin; IFN-γ=interferon gamma; TGF-β=transforming growth factor beta; TNF- α=tumor necrosis factor alpha. 1. Majamaa H, et al. J Allergy Clin Immunol. 1997; 99(2): 179 -185. 2. Kalliomaki, et al. Lancet. 2001; 357(9262): 1076 -1079. 3. Pohjavuori E, et al. J Allergy Clin Immunol. 2004; 114(1): 131 -136. 4. Ghadimi D, et al. Immunobiology. 2008; 213(8): 677 -692. 5. Donato KA, et al. Microbiology. 2010; 156(pt 11): 3288 -3297. 42

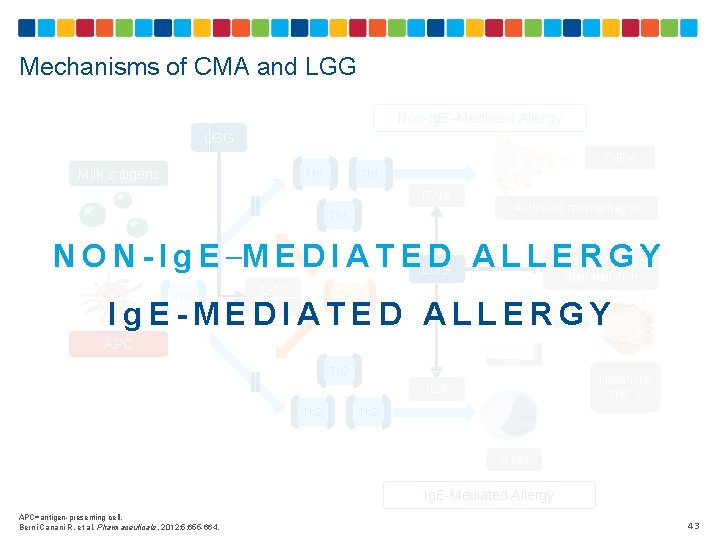
Mechanisms of CMA and LGG Non-Ig. E–Mediated Allergy LGG Milk antigens Th 1 TNF-α IFN-γ Activated macrophages Th 1 N O N - I g E –M E D I A T ELGG D ALLERGY Mast cell/ basophil degranulation IL-10 TGF-ß Th 0 Treg Ig. E APC Ig. E-MEDIATED ALLERGY Th 2 Histamine TNF-α Th 2 IL-4 B cell Ig. E-Mediated Allergy APC=antigen-presenting cell. Berni Canani R, et al. Pharmaceuticals. 2012; 5: 655 -664. 43

Mechanisms of Action for the Protective Effects of LGG • Interaction with other microbes in the gastrointestinal tract – In vitro studies have demonstrated that LGG produces a variety of antimicrobial compounds that inhibit the growth of potential pathogens, including E coli, Salmonella typhi, and Staphylococcus aureus 1, 2 – LGG reduces the adhesion of potentially pathogenic bacteria to the epithelial mucus cell and in vitro 3, 4 • Direct influence on barrier function – LGG supports the integrity of the gut epithelium via several mechanisms in vitro, including modulation of mucus production and strengthening of the epithelial tight junctions 5, 6 – Animal models suggest LGG may help normalize gut permeability 7 1. Carey C, et al. J Microbiol Methods. 2008; 73: 125 -132. 2. Lu R, et al. J Pediatr Gastroenterol Nutr. 2009; 49: 23 -30. 3. Collado MC, et al. Lett Appl Microbiol. 2007; 45(4): 454 -460. 4. Lee YK, et al. Appl Environ Microbiol. 2000; 66(9): 3692 -3697. 5. Donato KA, et al. Microbiology. 2010; 156(pt 11): 3288 -3297. 6. Mattar AF, et al. Pediatr Surg Int. 2002; 18: 586 -590. 7. Isolauri E, et al. Pediatr Res. 1993; 33(6): 548 -553. 44

Mechanisms of Action for the Protective Effects of LGG (cont’d) • Gene expression – Gene expression studies show that probiotic administration of LGG alters the expression of over 400 genes in the duodenal mucosa in patients with esophagitis – Genes affected are those involved in immune response and inflammation, cell growth and differentiation, signaling, and adhesion 1 • Modulation of the immune system – In children with acute diarrhea, administration of LGG enhances the number of antibody-secreting cells 2 – LGG administration is also associated with reduced levels of markers of inflammation in healthy adults 3 1. Di Caro S, et al. Dig Liver Dis. 2005; 37: 320 -329. 2. Kaila M, et al. Pediatr Res. 1992; 32(2): 141 -144. 3. Kekkonen RA, et al. World J Gastroenterol. 2008; 14(13): 2029 -2036. 45

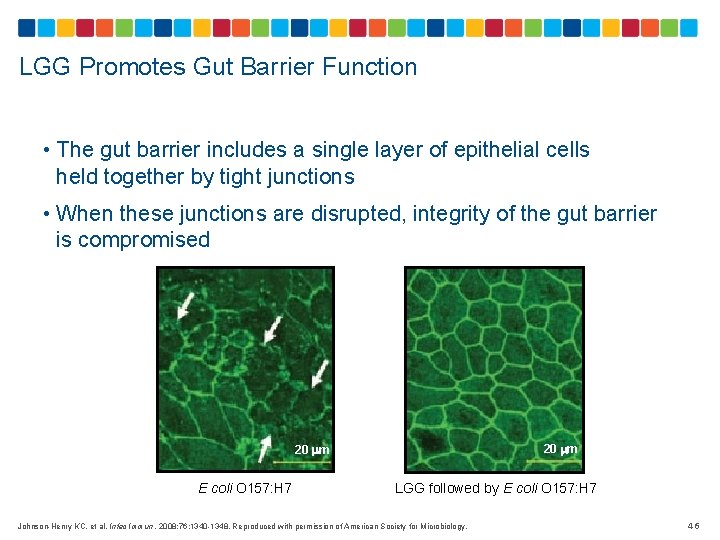
LGG Promotes Gut Barrier Function • The gut barrier includes a single layer of epithelial cells held together by tight junctions • When these junctions are disrupted, integrity of the gut barrier is compromised 20 m E coli O 157: H 7 LGG followed by E coli O 157: H 7 Johnson-Henry KC, et al. Infec Immun. 2008; 76: 1340 -1348. Reproduced with permission of American Society for Microbiology. 46

The Role of LGG in Allergy Management Author Year Probiotic strain Type of allergic disease Result Type Atopic dermatitis (eczema) Kalliomäki et al 2001 LGG Atopic dermatitis Positive Viljanen et al 2005 LGG Atopic eczema/dermatitis syndrome Positive Gruber et al 2007 LGG Atopic dermatitis Negative Kopp et al 2008 LGG Atopic dermatitis Positive Lee et al 2008 Meta-analysis including LGG studies Atopic dermatitis Negative Boyle et al. 2009 Meta-analysis including LGG studies Eczema Negative Kuitunen et al 2009 LGG+ mix Ig. E-associated allergy Positive Rose et al 2010 LGG Atopic dermatitis Positive Food allergy and anaphylaxis Isolauri et al 2002 LGG or B lactis Bb 12 Majamaa et al 1997 LGG Taylor et al 2007 LGG or L acidophilus Food allergy Positive Food-sensitized eczema Positive Cow's milk allergy Positive Allergic rhinitis Kawase et al 2009 LGG + L gasseri Allergic rhinitis Negative Piirainen et al 2008 LGG Allergic rhinitis Negative Asthma Helin et al 2002 LGG Pollen allergy Negative Blümer et al 2007 LGG Allergic asthma Positive Modified from Özdemir. Clin Exp Immunol. 2010; 160(3): 295 -304. 47

Mixed Outcomes of LGG Administration • No effect against mild to moderate atopic dermatitis in infancy when LGG (5 109 CFU twice daily for 12 weeks) was mixed with water and given by spoon 1 • No clinical effect on atopic dermatitis or asthma-related events, and only mild effects on allergic sensitization, when LGG (1010 CFU in capsules, 2 per day for 6 months) was reconstituted with 5 m. L of water and given by spoon 2 • Effective in preventing early atopic disease in children at high risk when LGG (1 1010 CFU for 6 months postnatally) capsules were consumed by mothers and/or mixed with water for infants 3 • Effective in preventing development of eczema during the first 7 years 4 when LGG (1 1010 CFU) capsules were consumed by mothers and/or mixed with water and given by spoon to infants for 6 months postnatally 3 – Follow-up of the cohort showed reduced risk of developing eczema during the first 7 years 4 CFU=colony-forming units. 1. Grüber C, et al. Allergy. 2007; 62(11): 1270 -1276. 2. Rose MA, et al. Clin Exp Allergy. 2010; 40(9): 1398 -1405. 3. Kalliomaki M, et al. Lancet. 2001: 357(9262): 1076 -1079. 4. Kalliomaki M, et al. J Allergy Clin Immunol. 2007; 119(4): 1019 -1021. 48

CMA and LGG: Clinical Study Overview The efficacy of LGG in CMA has been shown in many clinical studies Author Year Key outcomes Feeding groups Effect of LGG vs placebo Majamaa and Isolauri 1997 Skin symptoms Gut barrier function EHCK EHCF + LGG Positive effect Viljanen et al 2005 Skin symptoms EHCF + LGG C+ LGG + mix of 3 probiotic strains LGG may alleviate symptoms of atopic eczema/dermatitis syndrome in Ig. E-sensitized infants but not in non–Ig. E-sensitized infants Brouwer et al 2006 Skin symptoms Nutrilon Pep Nutrilon + LGG Nutrilon + L rhamnosus No effect Baldasarre et al 2010 Gut symptoms Nutramigen® Nutramigen + LGG Improvement of hematochezia and fecal calprotectin Nermes et al 2011 Gut barrier function Nutramigen + LGG Positive effect Lower Ig. A-, Ig. G-, and Ig. M-secreting cells Berni Canani et al 2012 Acquisition of tolerance Nutramigen + LGG Positive effect Accelerates oral tolerance in infants with CMA Acquisition of tolerance Nutramigen + LGG Hydrolyzed rice formula Soy formula Amino acid–based formula Positive effect Accelerates oral tolerance in infants with CMA Berni Canani et al 2013 • LGG provides significant improvements in gut and skin symptoms and gut barrier function, and significantly accelerates acquisition of tolerance to cow’s milk protein 49

Lactobacillus rhamnosus GG (LGG) and Extensively Hydrolyzed Casein Formula 50

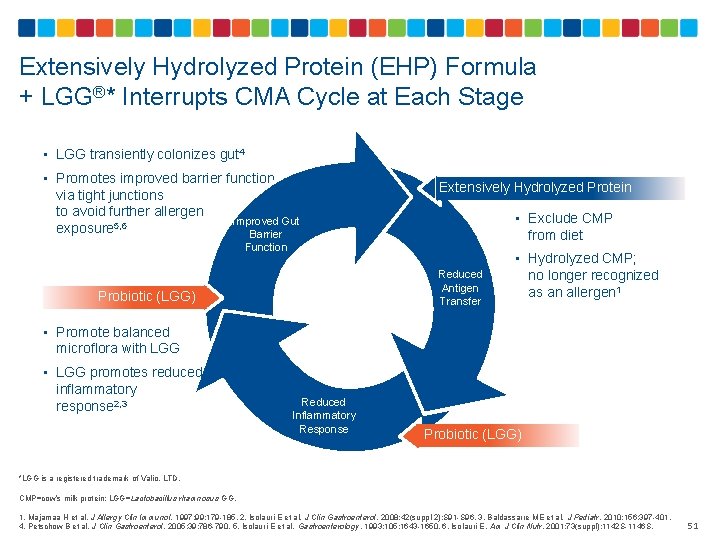
Extensively Hydrolyzed Protein (EHP) Formula + LGG®* Interrupts CMA Cycle at Each Stage • LGG transiently colonizes gut 4 • Promotes improved barrier function via tight junctions to avoid further allergen Improved Gut exposure 5, 6 Barrier Extensively Hydrolyzed Protein • Exclude CMP from diet Function Reduced Antigen Transfer Probiotic (LGG) • Hydrolyzed CMP; no longer recognized as an allergen 1 • Promote balanced microflora with LGG • LGG promotes reduced inflammatory response 2, 3 Reduced Inflammatory Response Probiotic (LGG) *LGG is a registered trademark of Valio, LTD. CMP=cow’s milk protein; LGG=Lactobacillus rhamnosus GG. 1. Majamaa H et al. J Allergy Clin Immunol. 1997; 99: 179 -185. 2. Isolauri E et al. J Clin Gastroenterol. 2008; 42(suppl 2): S 91 -S 96. 3. Baldassarre ME et al. J Pediatr. 2010; 156: 397 -401. 4. Petschow B et al. J Clin Gastroenterol. 2005; 39: 786 -790. 5. Isolauri E et al. Gastroenterology. 1993; 105: 1643 -1650. 6. Isolauri E. Am J Clin Nutr. 2001; 73(suppl): 1142 S-1146 S. 51

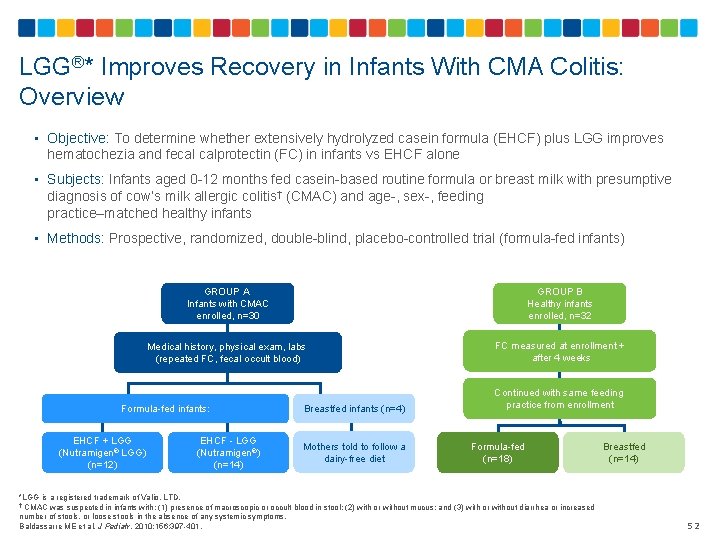
LGG®* Improves Recovery in Infants With CMA Colitis: Overview • Objective: To determine whether extensively hydrolyzed casein formula (EHCF) plus LGG improves hematochezia and fecal calprotectin (FC) in infants vs EHCF alone • Subjects: Infants aged 0 -12 months fed casein-based routine formula or breast milk with presumptive diagnosis of cow’s milk allergic colitis† (CMAC) and age-, sex-, feeding practice–matched healthy infants • Methods: Prospective, randomized, double-blind, placebo-controlled trial (formula-fed infants) GROUP A Infants with CMAC enrolled, n=30 GROUP B Healthy infants enrolled, n=32 Medical history, physical exam, labs (repeated FC, fecal occult blood) FC measured at enrollment + after 4 weeks Formula-fed infants: EHCF + LGG (Nutramigen® LGG) (n=12) EHCF - LGG (Nutramigen®) (n=14) Breastfed infants (n=4) Mothers told to follow a dairy-free diet Continued with same feeding practice from enrollment Formula-fed (n=18) *LGG is a registered trademark of Valio, LTD. † CMAC was suspected in infants with: (1) presence of macroscopic or occult blood in stool; (2) with or without mucus; and (3) with or without diarrhea or increased number of stools, or loose stools in the absence of any systemic symptoms. Baldassarre ME et al. J Pediatr. 2010; 156: 397 -401. Breastfed (n=14) 52

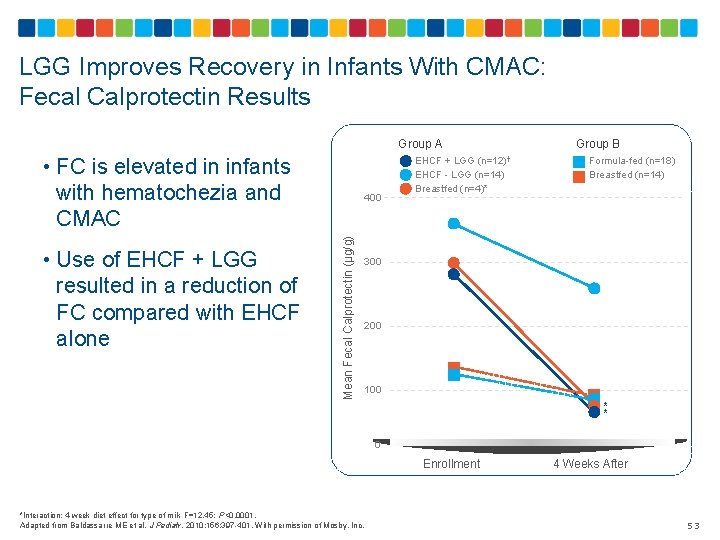
LGG Improves Recovery in Infants With CMAC: Fecal Calprotectin Results • FC is elevated in infants with hematochezia and CMAC Mean Fecal Calprotectin (µg/g) • Use of EHCF + LGG resulted in a reduction of FC compared with EHCF alone 400 Group A Group B EHCF + LGG (n=12)† EHCF - LGG (n=14) Breastfed (n=4)* Formula-fed (n=18) Breastfed (n=14) 300 200 100 * * 0 Enrollment *Interaction: 4 -week diet effect for type of milk F=12. 45; P<0. 0001. Adapted from Baldassarre ME et al. J Pediatr. 2010; 156: 397 -401. With permission of Mosby, Inc. 4 Weeks After 53

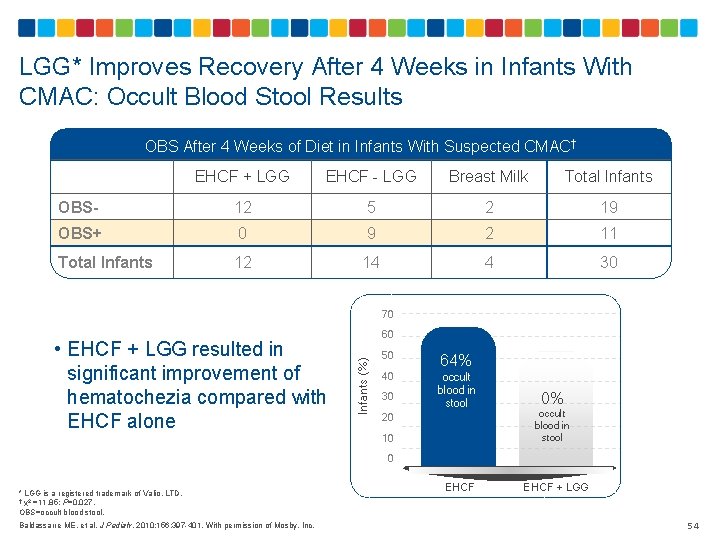
LGG* Improves Recovery After 4 Weeks in Infants With CMAC: Occult Blood Stool Results OBS After 4 Weeks of Diet in Infants With Suspected CMAC† EHCF + LGG EHCF - LGG Breast Milk Total Infants OBS- 12 5 2 19 OBS+ 0 9 2 11 Total Infants 12 14 4 30 70 Infants (%) • EHCF + LGG resulted in significant improvement of hematochezia compared with EHCF alone 60 50 64% 40 occult blood in stool 30 20 10 0% occult blood in stool 0 * LGG is a registered trademark of Valio, LTD. † χ2 =11. 85; P=0. 027. OBS=occult blood stool. Baldassarre ME, et al. J Pediatr. 2010; 156: 397 -401. With permission of Mosby, Inc. EHCF + LGG 54

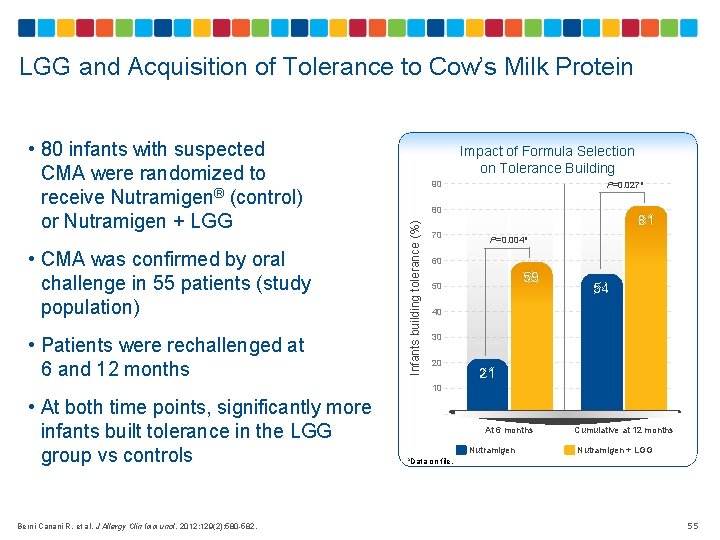
LGG and Acquisition of Tolerance to Cow’s Milk Protein • CMA was confirmed by oral challenge in 55 patients (study population) • Patients were rechallenged at 6 and 12 months Impact of Formula Selection on Tolerance Building 90 P=0. 027 a 80 Infants building tolerance (%) • 80 infants with suspected CMA were randomized to receive Nutramigen® (control) or Nutramigen + LGG 81 70 P=0. 004 a 60 59 50 54 40 30 20 21 10 • At both time points, significantly more infants built tolerance in the LGG group vs controls Berni Canani R, et al. J Allergy Clin Immunol. 2012; 129(2): 580 -582. At 6 months Nutramigen a. Data on file Cumulative at 12 months Nutramigen + LGG . 55

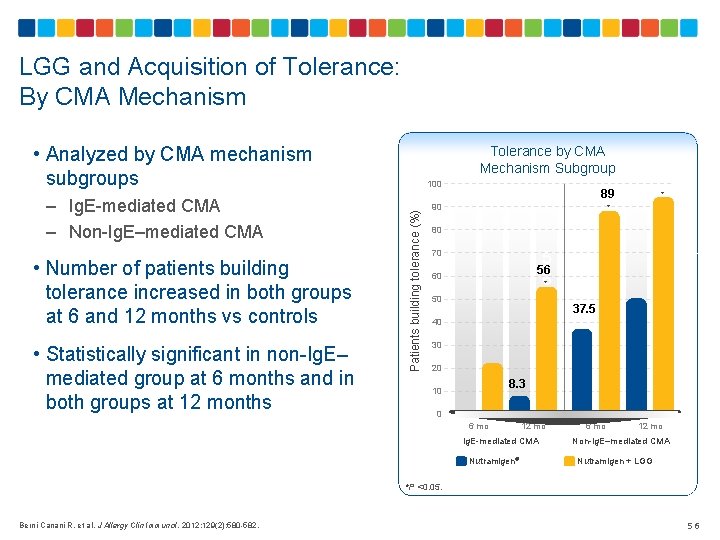
LGG and Acquisition of Tolerance: By CMA Mechanism • Analyzed by CMA mechanism subgroups • Number of patients building tolerance increased in both groups at 6 and 12 months vs controls • Statistically significant in non-Ig. E– mediated group at 6 months and in both groups at 12 months 100 Patients building tolerance (%) – Ig. E-mediated CMA – Non-Ig. E–mediated CMA Tolerance by CMA Mechanism Subgroup 89 90 * * 80 5 6* 5. 6 70 56 60 50 40 30 37. 5 20 8. 3 10 0 6 mo 12 mo Ig. E-mediated CMA Nutramigen® 6 mo 12 mo Non-Ig. E–mediated CMA Nutramigen + LGG *P <0. 05. Berni Canani R, et al. J Allergy Clin Immunol. 2012; 129(2): 580 -582. 56

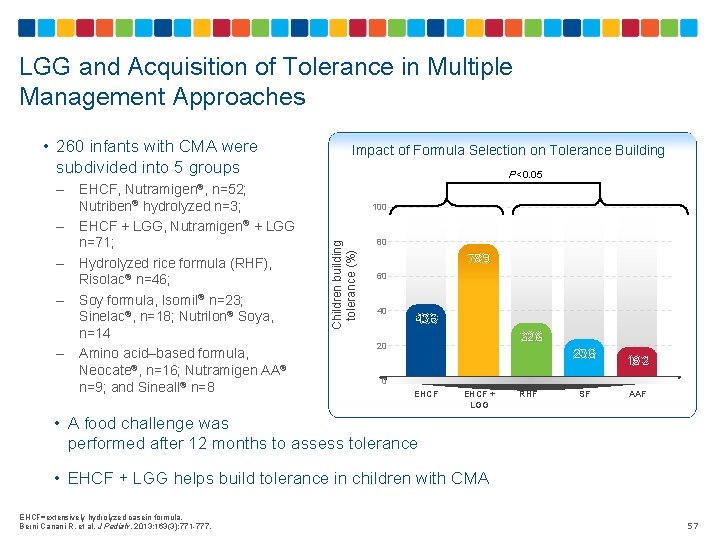
LGG and Acquisition of Tolerance in Multiple Management Approaches • 260 infants with CMA were subdivided into 5 groups – – P<0. 05 EHCF, Nutramigen®, n=52; Nutriben® hydrolyzed n=3; EHCF + LGG, Nutramigen® + LGG n=71; Hydrolyzed rice formula (RHF), Risolac® n=46; Soy formula, Isomil® n=23; Sinelac®, n=18; Nutrilon® Soya, n=14 Amino acid–based formula, Neocate®, n=16; Nutramigen AA® n=9; and Sineall® n=8 100 Children building tolerance (%) – Impact of Formula Selection on Tolerance Building 80 78. 9 60 40 43. 6 32. 6 20 23. 6 18. 2 0 EHCF + LGG RHF SF AAF • A food challenge was performed after 12 months to assess tolerance • EHCF + LGG helps build tolerance in children with CMA EHCF=extensively hydrolyzed casein formula. Berni Canani R, et al. J Pediatr. 2013; 163(3): 771 -777. 57

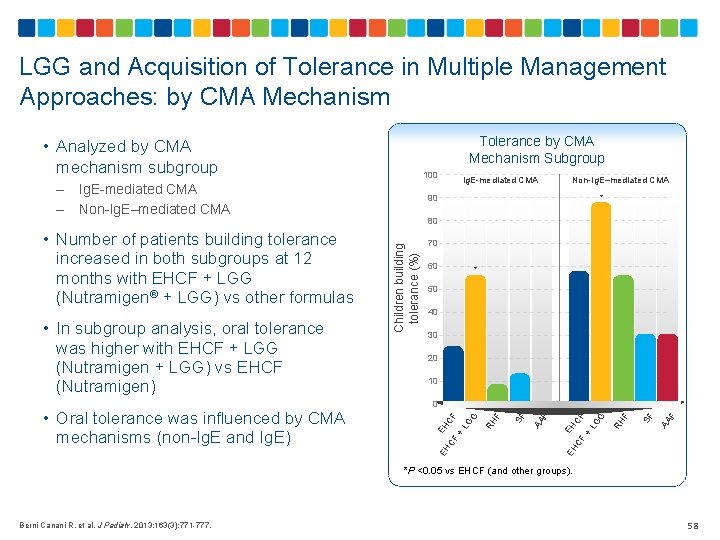
LGG and Acquisition of Tolerance in Multiple Management Approaches: by CMA Mechanism Tolerance by CMA Mechanism Subgroup • Analyzed by CMA mechanism subgroup Ig. E-mediated CMA Non-Ig. E–mediated CMA 70 60 * 50 40 30 20 10 F AA SF F RH EH CF CF + L G G EH F AA SF F 0 EH • Oral tolerance was influenced by CMA mechanisms (non-Ig. E and Ig. E) 80 RH • In subgroup analysis, oral tolerance was higher with EHCF + LGG (Nutramigen + LGG) vs EHCF (Nutramigen) Non-Ig. E–mediated CMA * EH CF CF + L G G • Number of patients building tolerance increased in both subgroups at 12 months with EHCF + LGG (Nutramigen® + LGG) vs other formulas Ig. E-mediated CMA 90 Children building tolerance (%) – – 100 *P <0. 05 vs EHCF (and other groups). Berni Canani R, et al. J Pediatr. 2013; 163(3): 771 -777. 58

Reduced Incidence of Allergic Manifestations In a 3 -year RCT, extensively hydrolyzed caseinbased formula with Lactobacillus rhamnosus GG reduced future manifestations of allergy (atopic dermatitis, urticaria, rhinoconjunctivitis, asthma) in children with Ig. E-mediated CMA by ~50% (vs extensively hydrolyzed casein-based formula without Lactobacillus rhamnosus GG) 59

Summary – Key Points Extensively hydrolyzed casein-based formula with Lactobacillus rhamnosus GG (LGG) achieves faster resolution of CMA-associated proctocolitis compared to the same formula without LGG Extensively hydrolyzed casein formula with Lactobacillus rhamnosus GG (LGG) enhances acquisition of tolerance to cow’s milk protein compared to the same formula without LGG as well as other types of formula (hydrolyzed rice formula, soy formula, and amino acid–based formula) without LGG Extensively hydrolyzed casein based formula with Lactobacillus rhamnosus GG reduced future manifestations of allergy (atopic dermatitis, urticaria, rhinoconjunctivitis, asthma) in children with Ig. E-mediated CMA by ~50% 60
 Cadbury dairy milk
Cadbury dairy milk Sensory evaluation of milk and milk products
Sensory evaluation of milk and milk products Milk for toddlers with milk allergynon dairy
Milk for toddlers with milk allergynon dairy Human milk vs cow milk
Human milk vs cow milk Contamination of milk and milk products
Contamination of milk and milk products Difference between cow milk and human milk
Difference between cow milk and human milk Disaccharide found in milk
Disaccharide found in milk Explain skin analysis techniques and why they are important
Explain skin analysis techniques and why they are important Colostrum alpha lipid
Colostrum alpha lipid Social development in infancy
Social development in infancy Infancy period
Infancy period Socioemotional development in infancy
Socioemotional development in infancy Chapter 7 human growth and development
Chapter 7 human growth and development Ap psych schema
Ap psych schema Infancy and childhood psychology
Infancy and childhood psychology Stage of growth and development
Stage of growth and development Social development in late childhood
Social development in late childhood Early middle childhood
Early middle childhood Infancy and childhood physical development
Infancy and childhood physical development Chapter 10 infancy and childhood
Chapter 10 infancy and childhood Infancy
Infancy Personality development in infancy
Personality development in infancy Chapter 10 infancy and childhood
Chapter 10 infancy and childhood Infancy physical changes
Infancy physical changes Chapter 10 infancy and childhood review worksheet answers
Chapter 10 infancy and childhood review worksheet answers Infants display a wide range of emotional expressions
Infants display a wide range of emotional expressions Catastrophic epilepsy infancy
Catastrophic epilepsy infancy Intellectual in health and social care
Intellectual in health and social care Lesson quiz 3-2 infancy and childhood
Lesson quiz 3-2 infancy and childhood Stages of socialization
Stages of socialization Infancy childhood adolescence adulthood old age
Infancy childhood adolescence adulthood old age Pies in infancy
Pies in infancy Problems of infancy
Problems of infancy New emotions that appear toward the second year
New emotions that appear toward the second year Infancy
Infancy Module 46 infancy and childhood physical development
Module 46 infancy and childhood physical development Messianic prophecies fulfilled in the infancy narratives
Messianic prophecies fulfilled in the infancy narratives Module 47 infancy and childhood cognitive development
Module 47 infancy and childhood cognitive development Chapter 5 cognitive development in infancy and toddlerhood
Chapter 5 cognitive development in infancy and toddlerhood What are the five basic functions of the management process
What are the five basic functions of the management process New approaches to organizing hr
New approaches to organizing hr Trends shaping hrm
Trends shaping hrm Develop new approaches to public governance and engagement
Develop new approaches to public governance and engagement New approaches to sme financing
New approaches to sme financing You have 2 cows
You have 2 cows Rombergs test
Rombergs test Ciwa and cows
Ciwa and cows Mydiatrics
Mydiatrics Cows score
Cows score Cow
Cow Cow male reproductive system
Cow male reproductive system Abdecario
Abdecario Alexander luria
Alexander luria What is codominance
What is codominance Click clack moo cows that type comprehension questions
Click clack moo cows that type comprehension questions Parts of a cows stomach
Parts of a cows stomach Where is a cows blind spot
Where is a cows blind spot