Catastrophic Epilepsy of Infancy Diagnostic Considerations in Progressive





![Etymology • Ceroid • L. [cera], wax, + G. [eidos], appearance • Compare with Etymology • Ceroid • L. [cera], wax, + G. [eidos], appearance • Compare with](https://slidetodoc.com/presentation_image/672abdb6f2467cebf4944ee51189e1a6/image-17.jpg)












- Slides: 45

Catastrophic Epilepsy of Infancy Diagnostic Considerations in Progressive Myoclonic Epilepsy

Motivation • 12 month old with myoclonic seizures

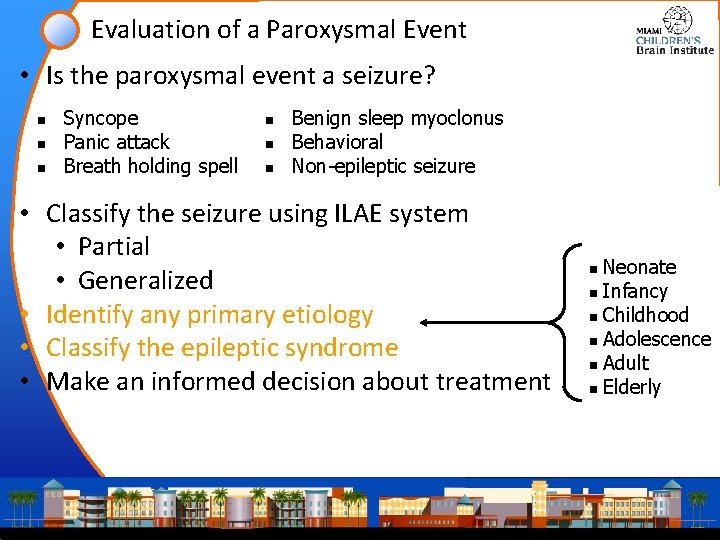
Evaluation of a Paroxysmal Event • Is the paroxysmal event a seizure? n n n Syncope Panic attack Breath holding spell n n n Benign sleep myoclonus Behavioral Non-epileptic seizure • Classify the seizure using ILAE system • Partial • Generalized • Identify any primary etiology • Classify the epileptic syndrome • Make an informed decision about treatment Neonate n Infancy n Childhood n Adolescence n Adult n Elderly n

Epilepsies in infancy • Occur between 0 -18 months of age • Incidence at this time is ~80/100, 000 • Higher than childhood (up to 12 y) • Higher thatn adolescence (up to 18 y) • Developmental tasks • Refinement of motor skills • Development of complex intellectual skills • Development of complex social skills • Impact of epilepsy on these tasks can be divided • Benign • Intermediate • Catastrophic (boundaries of this category are not clear cut)

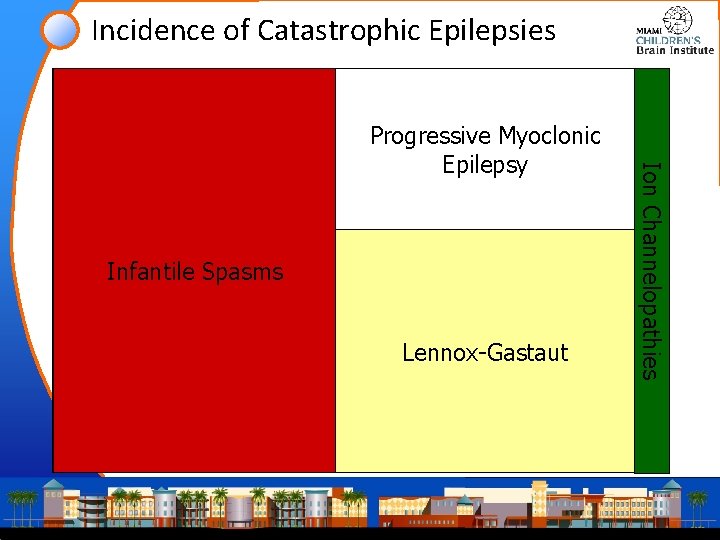
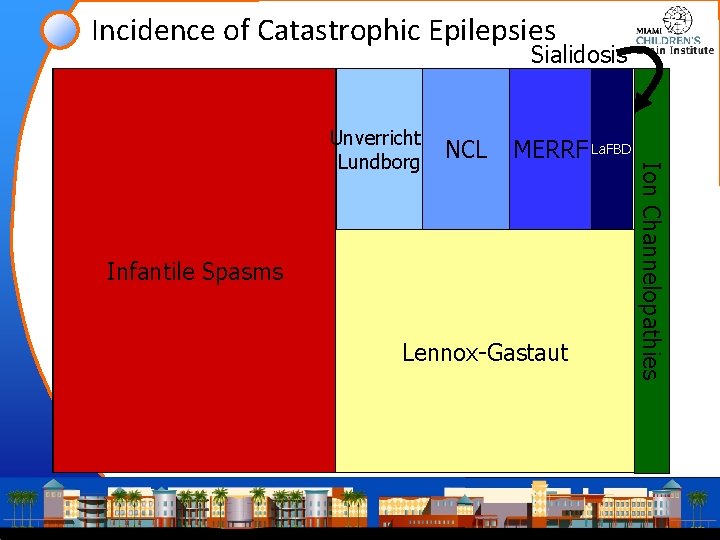
Incidence of Catastrophic Epilepsies Infantile Catastrophic Spasms Epilepsy in Childhood Lennox-Gastaut Ion Channelopathies Progressive Myoclonic Epilepsy

Incidence of Catastrophic Epilepsies Sialidosis Infantile Catastrophic Spasms Epilepsy in Childhood Lennox-Gastaut Ion Channelopathies Progressive Myoclonic. La. FBD Unverricht. NCL MERRF Lundborg Epilepsy

Organization • • • MERRF NCL Unverricht-Lundborg Lafora body disease Sialidosis Dravet syndrome

Myoclonic Epilepsy with Ragged Red Fibers

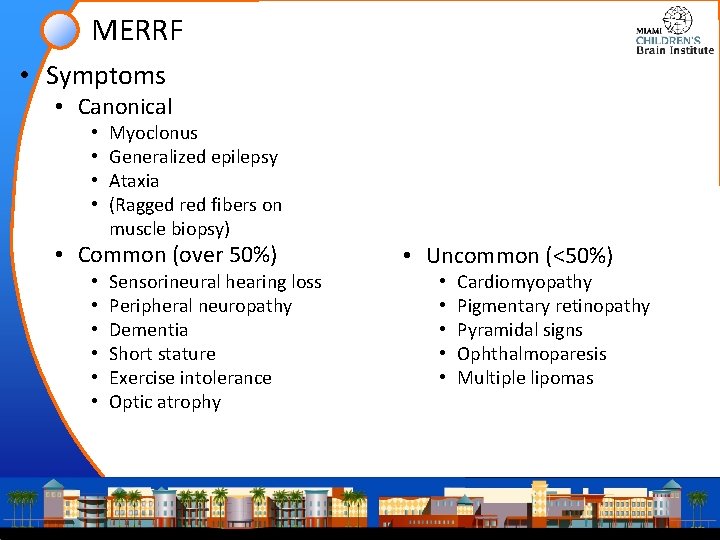
MERRF • Symptoms • Canonical • • Myoclonus Generalized epilepsy Ataxia (Ragged red fibers on muscle biopsy) • Common (over 50%) • • • Sensorineural hearing loss Peripheral neuropathy Dementia Short stature Exercise intolerance Optic atrophy • Uncommon (<50%) • • • Cardiomyopathy Pigmentary retinopathy Pyramidal signs Ophthalmoparesis Multiple lipomas

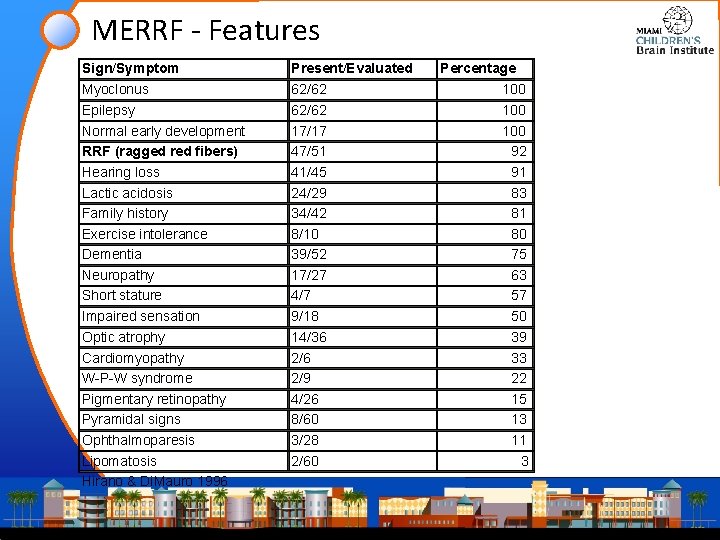
MERRF - Features Sign/Symptom Present/Evaluated Myoclonus Epilepsy Normal early development RRF (ragged red fibers) Hearing loss Lactic acidosis Family history Exercise intolerance Dementia Neuropathy Short stature Impaired sensation Optic atrophy Cardiomyopathy W-P-W syndrome Pigmentary retinopathy Pyramidal signs Ophthalmoparesis Lipomatosis Hirano & Di. Mauro 1996 62/62 17/17 47/51 41/45 24/29 34/42 8/10 39/52 17/27 4/7 9/18 14/36 2/9 4/26 8/60 3/28 2/60 Percentage 100 100 92 91 83 81 80 75 63 57 50 39 33 22 15 13 11 3

MERRF • Onset is usually in childhood (may be younger) • May be confused with Friedreich ataxia (abnormalities of proprioception and pes cavus) • FH short stature • Findings • elevated serum lactate • RRF on muscle biopsy • Pathology • • Neuronal loss/gliosis of dentate nucleus and inferior olivary complex Dropout of Purkinje cells and neurons of the red nucleus Pallor of the posterior columns Degeneration of the gracile and cuneate nuclei • Course: slowly progressive or rapidly downhill.

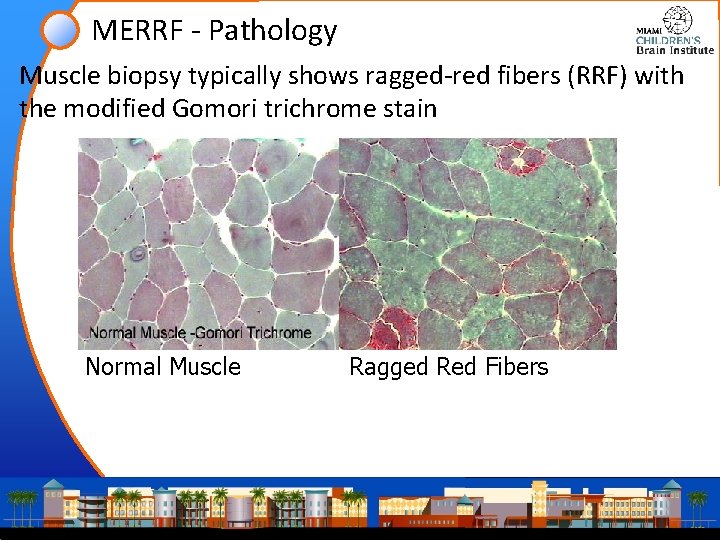
MERRF - Pathology Muscle biopsy typically shows ragged-red fibers (RRF) with the modified Gomori trichrome stain Normal Muscle Ragged Red Fibers

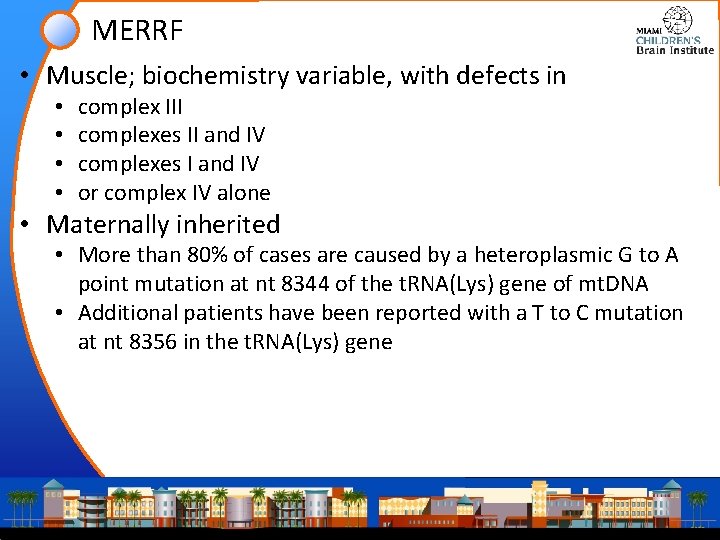
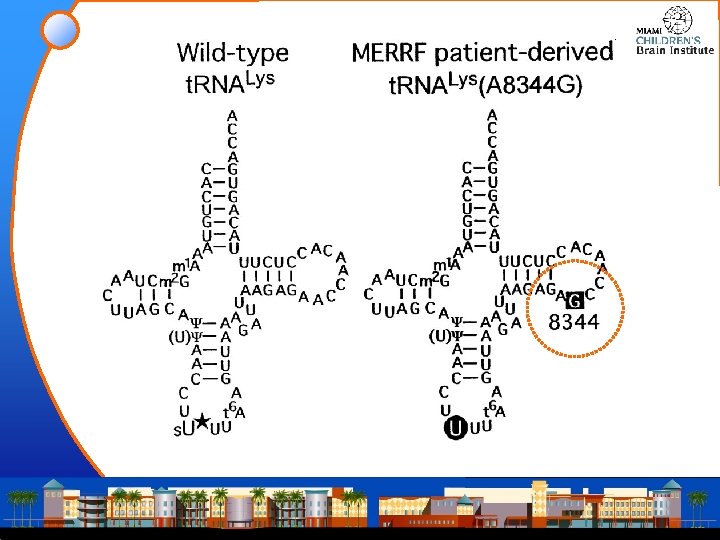
MERRF • Muscle; biochemistry variable, with defects in • • complex III complexes II and IV complexes I and IV or complex IV alone • Maternally inherited • More than 80% of cases are caused by a heteroplasmic G to A point mutation at nt 8344 of the t. RNA(Lys) gene of mt. DNA • Additional patients have been reported with a T to C mutation at nt 8356 in the t. RNA(Lys) gene


MERRF - Treatment • The seizure disorder can be treated with conventional anticonvulsant therapy. No controlled studies have compared the efficacy of different anticonvulsants. • No treatment for the genetic defect is currently available. • Coenzyme Q 10 (100 mg three times a day) • L-carnitine (1000 mg 3 times a day) Are often used in hopes of improving mitochondrial function.

Neuronal Ceroid Lipofuscinosis
![Etymology Ceroid L cera wax G eidos appearance Compare with Etymology • Ceroid • L. [cera], wax, + G. [eidos], appearance • Compare with](https://slidetodoc.com/presentation_image/672abdb6f2467cebf4944ee51189e1a6/image-17.jpg)
Etymology • Ceroid • L. [cera], wax, + G. [eidos], appearance • Compare with “cerumen” • Lipofuscinosis • • • The product of peroxidation of unsaturated fatty acids and symptomatic, perhaps, of membrane damage rather than being deleterious in its own right G. [lipos] “fat” L. [fuscare] “to make dark” Compare with “obfuscation” (~“brown”) L. [-osis] “abnormal condition” or “a state of disease” • Ceroid and Lipofuscins are not the same • “Ceroid is acid fast, fat insolvent, and probably a type of lipofuscin, although differing from true lipofuscins by failing to stain with Schmorl ferric-ferricyanide reduction stain”

Neuronal ceroid lipofuscinosis • Most common neurodegenerative disease in children (three autosomal recessive disorders) • Characterized by • Accumulation of autofluorescent substance within lysosomes of tissue (especially neurons) • Epilepsy • Progressive epileptic encephalopathy • Vision loss • Pathologic findings • Light microscopy - ceroid • EM - Granular osmophilic deposits, curvilinear profiles, fingerprint bodies • Individual genes mutated in six forms have been identified


NCL • Infantile type (Haltia-Santavuori) • Begins end of the 1 st year • Death by ≈10 years • Late infantile type (Jansky-Bielschowsky) • most common type of NCL • Presentation: myoclonic seizures beginning between 2 and 4 years in a previously normal child • May live to 5 th decade INCL • Juvenile • Adult • Northern epilepsy variant LINCL JNCL ANCL

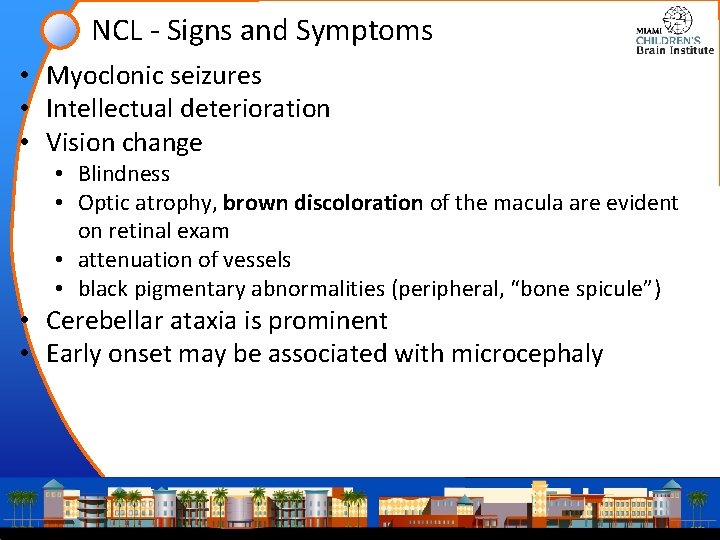
NCL - Signs and Symptoms • Myoclonic seizures • Intellectual deterioration • Vision change • Blindness • Optic atrophy, brown discoloration of the macula are evident on retinal exam • attenuation of vessels • black pigmentary abnormalities (peripheral, “bone spicule”) • Cerebellar ataxia is prominent • Early onset may be associated with microcephaly

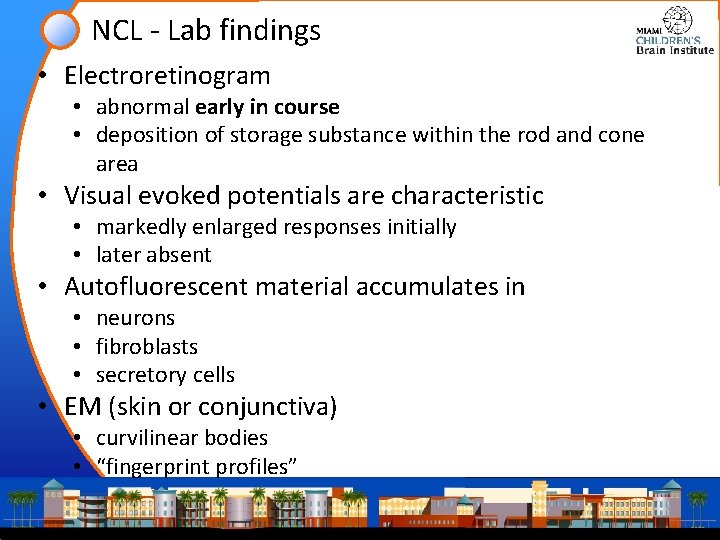
NCL - Lab findings • Electroretinogram • abnormal early in course • deposition of storage substance within the rod and cone area • Visual evoked potentials are characteristic • markedly enlarged responses initially • later absent • Autofluorescent material accumulates in • neurons • fibroblasts • secretory cells • EM (skin or conjunctiva) • curvilinear bodies • “fingerprint profiles”

NCL - Genetics and Pathophysiology • Infantile type: • • • gene: palmitoyl protein thioesterase (PPT) a. k. a. PPT; CLN 1; INCL; PPT 1 chromosome 1 p 32 lysosomal enzyme palmitoyl-protein thioesterase-1 Failure of synaptic fusion and vesicle recycling • Late infantile type: • • • gene: TPP 1 (sedolisin family of serine proteases) lysosomal cleavage of N-terminal tripeptides from substrates weaker endopeptidase activity Synthesized as inactive state, and activated by acidification Failure to degrade specific neuropeptides and a subunit of ATP synthase in the lysosome

Unverricht-Lundborg

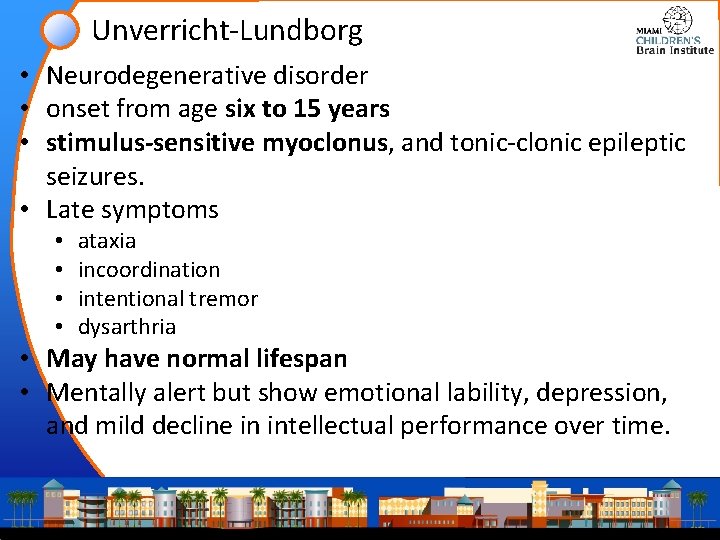
Unverricht-Lundborg • Neurodegenerative disorder • onset from age six to 15 years • stimulus-sensitive myoclonus, and tonic-clonic epileptic seizures. • Late symptoms • • ataxia incoordination intentional tremor dysarthria • May have normal lifespan • Mentally alert but show emotional lability, depression, and mild decline in intellectual performance over time.

Lafora Body Disease

Lafora body disease • Presents between 10 and 18 years • Epilepsy • Generalized tonic-clonic seizures • Myoclonic jerks appear later, but become more frequent and pronounced • Mental deterioration is evident within 1 year of onset • Other neurological signs • cerebellar signs • extrapyramidal signs • EEG • polyspike-wave discharges • occipital predominance • progressive slowing and disorganized background

Lafora Body Disease - Brain Lafora bodies in the brain. Dense intraneuronal inclusions. H&E Stain Lafora bodies are present throughout the nervous system, particularly in the dentate nucleus, red nucleus, substantia nigra, and hippocampus.

Sialidosis

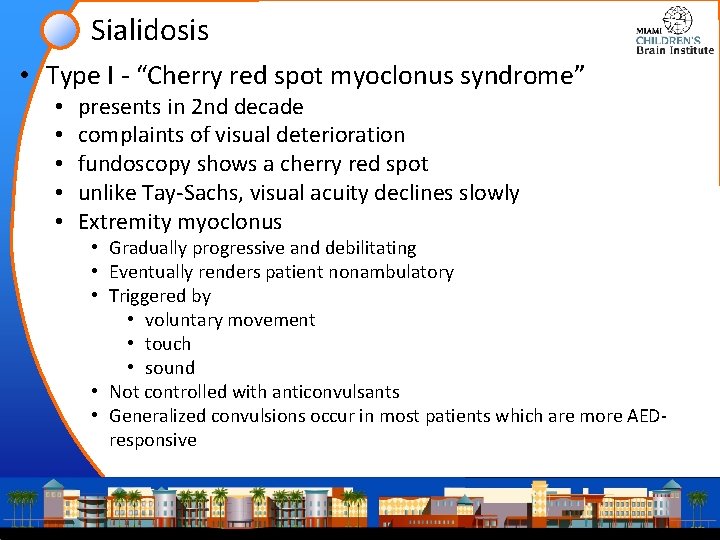
Sialidosis • Type I - “Cherry red spot myoclonus syndrome” • • • presents in 2 nd decade complaints of visual deterioration fundoscopy shows a cherry red spot unlike Tay-Sachs, visual acuity declines slowly Extremity myoclonus • Gradually progressive and debilitating • Eventually renders patient nonambulatory • Triggered by • voluntary movement • touch • sound • Not controlled with anticonvulsants • Generalized convulsions occur in most patients which are more AEDresponsive

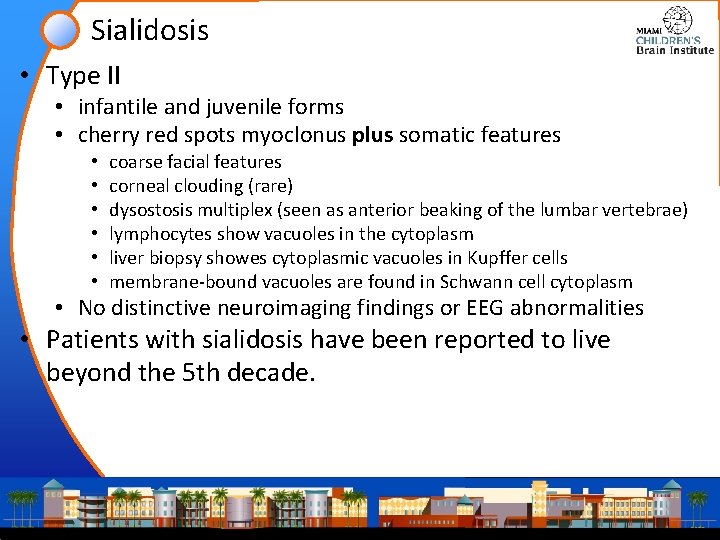
Sialidosis • Type II • infantile and juvenile forms • cherry red spots myoclonus plus somatic features • • • coarse facial features corneal clouding (rare) dysostosis multiplex (seen as anterior beaking of the lumbar vertebrae) lymphocytes show vacuoles in the cytoplasm liver biopsy showes cytoplasmic vacuoles in Kupffer cells membrane-bound vacuoles are found in Schwann cell cytoplasm • No distinctive neuroimaging findings or EEG abnormalities • Patients with sialidosis have been reported to live beyond the 5 th decade.

Sialidosis Pathophysiology • Progressive lysosomal storage of sialidated glycopeptides and oligosaccharides caused by a deficiency of the enzyme neuraminidase sialic acid (N-acetylneuraminic acid, NANA) • => Accumulation and excretion of sialic acid (N-acetylneuraminic acid) covalently linked ('bound') to a variety of oligosaccharides and/or glycoproteins. • Distinct from the sialurias where there is storage and excretion of “free” sialic acid • Neuraminidase activity in sialuria is normal or elevated.

Dravet Syndrome

Dravet syndrome • Severe Myoclonic Epilepsy of Infancy (SMEI) • Multiple mutations • Allelic with “generalized epilepsy with febrile seizures plus” (GEFS+)

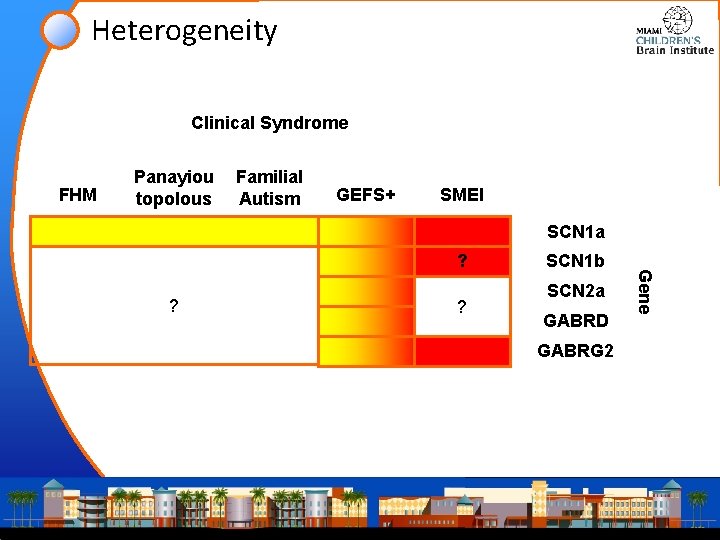
Heterogeneity Clinical Syndrome FHM Panayiou topolous Familial Autism GEFS+ SMEI SCN 1 a ? ? SCN 1 b SCN 2 a GABRD GABRG 2 Gene ?

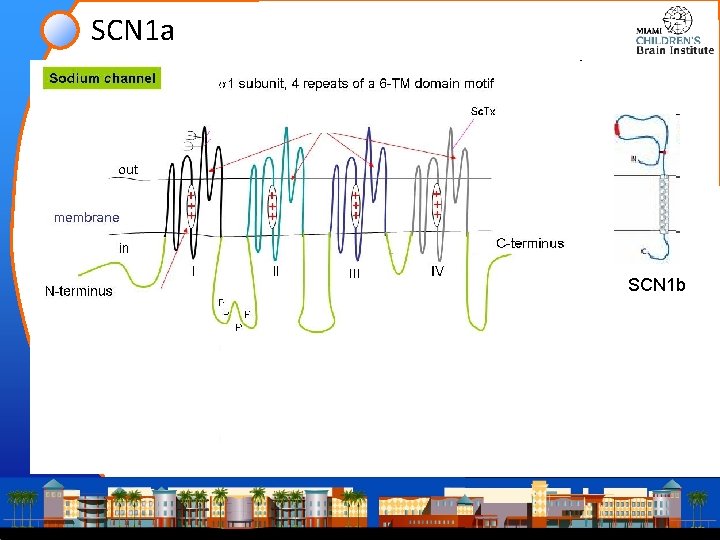
SCN 1 a SCN 1 b

Red flags for SCN 1 a mutation • Febrile seizures that • Start before age 1 year • Persist beyond 5 -6 years • Are prolonged; lasting >30 minutes • Febrile seizures that evolve into epilepsy • Seizures that are provoked by a hot bath or rapid temperature fluctuation • Seizures following vaccination • Family history of epilepsy, especially with heterogeneous seizure types

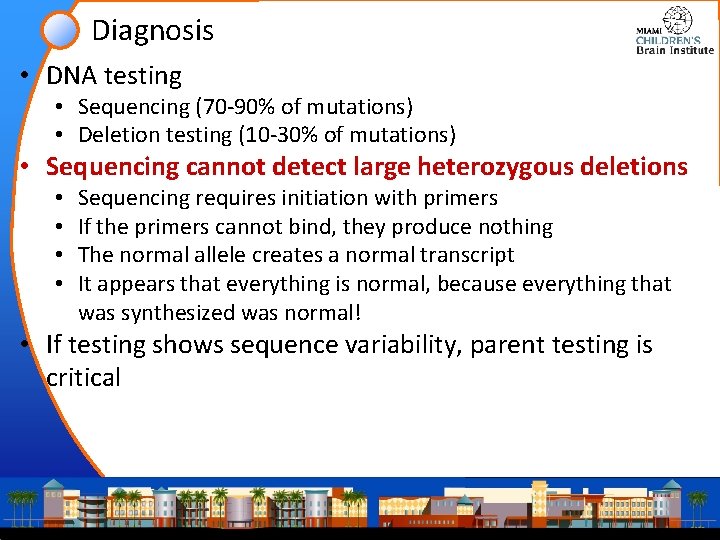
Diagnosis • DNA testing • Sequencing (70 -90% of mutations) • Deletion testing (10 -30% of mutations) • Sequencing cannot detect large heterozygous deletions • • Sequencing requires initiation with primers If the primers cannot bind, they produce nothing The normal allele creates a normal transcript It appears that everything is normal, because everything that was synthesized was normal! • If testing shows sequence variability, parent testing is critical

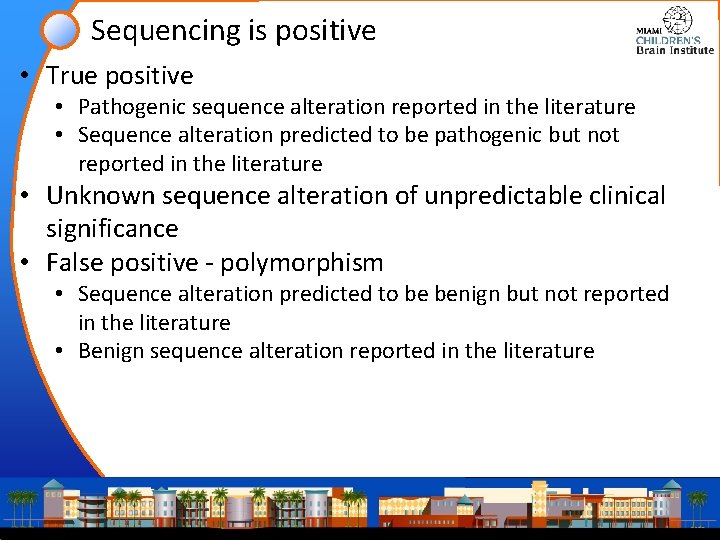
Sequencing is positive • True positive • Pathogenic sequence alteration reported in the literature • Sequence alteration predicted to be pathogenic but not reported in the literature • Unknown sequence alteration of unpredictable clinical significance • False positive - polymorphism • Sequence alteration predicted to be benign but not reported in the literature • Benign sequence alteration reported in the literature

Sequencing is negative • True negative • Patient does not have a mutation in the tested gene (e. g. , etiology is not genetic, or is caused by a different gene) • False positive • Patient has a sequence alteration that cannot be detected by sequence analysis • a large deletion • splice site deletion • Patient has a sequence alteration in a region of the gene (e. g. , an intron or regulatory region) not covered by the laboratory's test

Gene “normal” Correct conclusion true negative Wrong conclusion not detected splice site? large deletion? upstream regulatory region? previously reported stop codon (nonsense) unreported missense intron? known genetic polymorphism parent testing Uncertain Gene “abnormal”

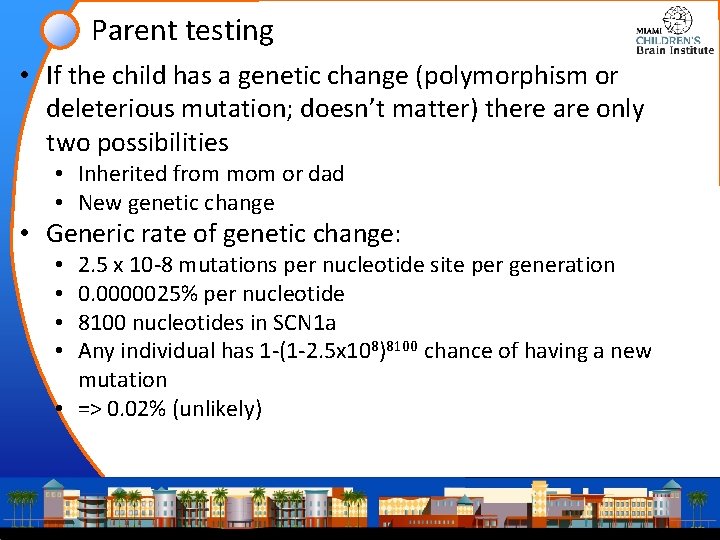
Parent testing • If the child has a genetic change (polymorphism or deleterious mutation; doesn’t matter) there are only two possibilities • Inherited from mom or dad • New genetic change • Generic rate of genetic change: 2. 5 x 10 -8 mutations per nucleotide site per generation 0. 0000025% per nucleotide 8100 nucleotides in SCN 1 a Any individual has 1 -(1 -2. 5 x 108)8100 chance of having a new mutation • => 0. 02% (unlikely) • •

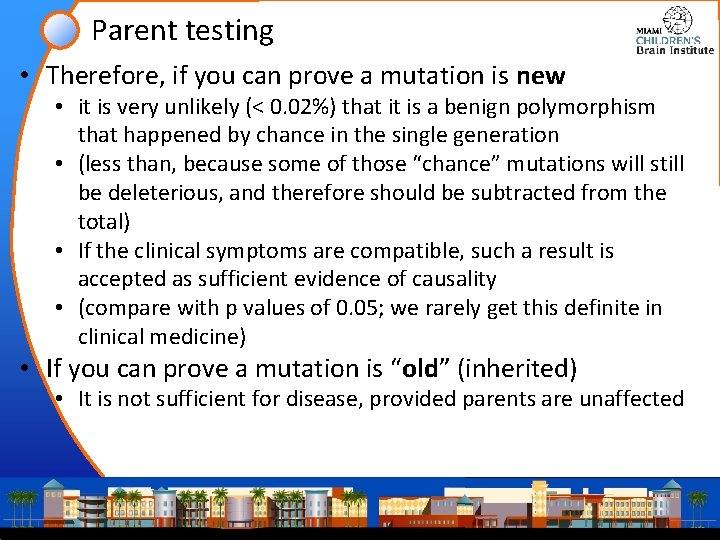
Parent testing • Therefore, if you can prove a mutation is new • it is very unlikely (< 0. 02%) that it is a benign polymorphism that happened by chance in the single generation • (less than, because some of those “chance” mutations will still be deleterious, and therefore should be subtracted from the total) • If the clinical symptoms are compatible, such a result is accepted as sufficient evidence of causality • (compare with p values of 0. 05; we rarely get this definite in clinical medicine) • If you can prove a mutation is “old” (inherited) • It is not sufficient for disease, provided parents are unaffected

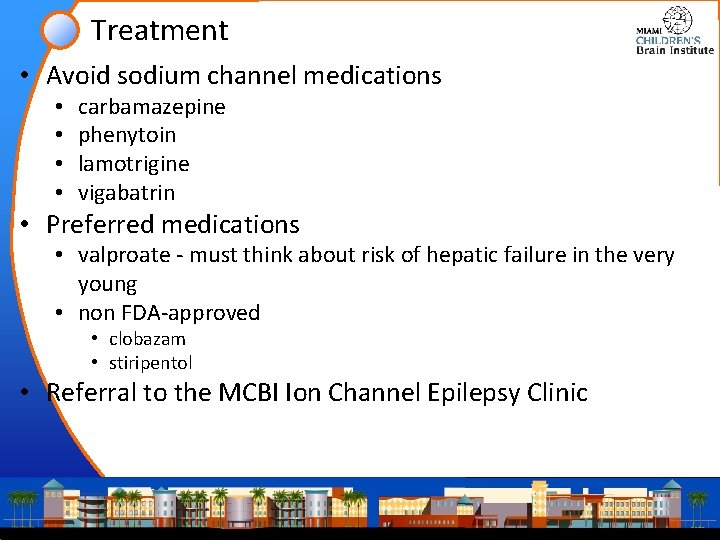
Treatment • Avoid sodium channel medications • • carbamazepine phenytoin lamotrigine vigabatrin • Preferred medications • valproate - must think about risk of hepatic failure in the very young • non FDA-approved • clobazam • stiripentol • Referral to the MCBI Ion Channel Epilepsy Clinic

Thank You
 Catastrophic epilepsy infancy
Catastrophic epilepsy infancy Facilitative emotions examples
Facilitative emotions examples Catastrophic risk protection endorsement
Catastrophic risk protection endorsement Catastrophic event
Catastrophic event What are some types of catastrophic events
What are some types of catastrophic events Past perfect continuous tense wh questions
Past perfect continuous tense wh questions Passive present progressive
Passive present progressive Past simple past continuous past perfect exercises
Past simple past continuous past perfect exercises Growth and development stages
Growth and development stages Internally programmed growth of a child
Internally programmed growth of a child Infancy childhood adolescence adulthood old age
Infancy childhood adolescence adulthood old age Emotional development in infancy
Emotional development in infancy Chapter 10 infancy and childhood
Chapter 10 infancy and childhood Chapter 7 human growth and development
Chapter 7 human growth and development Module 47 infancy and childhood cognitive development
Module 47 infancy and childhood cognitive development What is intellectual development in health and social care
What is intellectual development in health and social care Chapter 10 infancy and childhood
Chapter 10 infancy and childhood Social development in infancy
Social development in infancy Pies in infancy
Pies in infancy Age
Age Infancy physical changes
Infancy physical changes Module 47 infancy and childhood cognitive development
Module 47 infancy and childhood cognitive development Social development in late childhood
Social development in late childhood Chapter 5 cognitive development in infancy and toddlerhood
Chapter 5 cognitive development in infancy and toddlerhood Lesson quiz 3-2 infancy and childhood
Lesson quiz 3-2 infancy and childhood Infancy
Infancy Infancy period
Infancy period Module 46 infancy and childhood physical development
Module 46 infancy and childhood physical development Chapter 10 infancy and childhood review worksheet answers
Chapter 10 infancy and childhood review worksheet answers Infancy and childhood psychology
Infancy and childhood psychology Early middle childhood
Early middle childhood The cases of anna, isabelle, and genie
The cases of anna, isabelle, and genie Problems of infancy
Problems of infancy Personality development in infancy
Personality development in infancy Socioemotional development in infancy
Socioemotional development in infancy Messianic prophecies fulfilled in the infancy narratives
Messianic prophecies fulfilled in the infancy narratives Infant personality development
Infant personality development Acclimatisation pdhpe
Acclimatisation pdhpe Collaboration design considerations
Collaboration design considerations Tax considerations for setting up a new business
Tax considerations for setting up a new business Azure landing zone considerations
Azure landing zone considerations Design considerations for mobile computing
Design considerations for mobile computing Ethical considerations examples
Ethical considerations examples Retromylohyoid muscle
Retromylohyoid muscle Database design considerations
Database design considerations Writing strategies and ethical considerations
Writing strategies and ethical considerations