METHADONE MELLAR DAVIS WAEL LASHEEN DECLAN WALSH METHADONE





























- Slides: 81

METHADONE MELLAR DAVIS, WAEL LASHEEN, DECLAN WALSH

METHADONE § SYNTHETIC PSEUDOPIPERDINE DEVELOPED OVER 50 YEARS AGO § DISTINCTLY DIFFERENT FROM ALKALOID OPIOIDS (MORPHINE) (CODEINE) AND SYNTHETIC THEBAINE DERIVATIVES (OXYCODONE) 2

METHADONE R (L) AND S (D) ENANTIOMER § R ENANTIOMER BINDS WITH SIMILAR AFFINITY TO MU RECEPTORS AS MORPHINE (KM 3. 5 NM AND 1. 4 NM RESPECTIVELY) § BOTH R AND S ENANTIOMERS BIND TO N-METHYL -D-ASPARTATE RECEPTORS § TWICE THE INTRINSIC EFFICACY OF MORPHINE 3

METHADONE § DELTA OPIOID RECEPTOR AGONIST (R AND S) § SEROTONIN AND NOREPINEPHRINE REUPTAKE INHIBITOR (R AND S) § HIGH DOSES BLOCK POTASSIUM CHANNELS 4

ABSORPTION § ABSORPTION RAPID AND COMPLETE (47 - 91%) § DRUG LEVELS CAN BE MEASURED 30 MINUTES AFTER ORAL DOSING, PEAK CONCENTRATIONS OCCUR AT 2. 5 HOURS § INTESTINAL CYP 3 A 4 AND P-GLYCOPROTEIN MAY REDUCE ABSORPTION § NOT A MAJOR FACTOR IN THE LARGE INTERINDIVIDUAL DIFFERENCES IN KINETICS 5

ABSORPTION § PKA IS 9. 2 (BETTER ABSORBED IN AN ALKALINE ENVIRONMENT) § REDUCED ACIDITY (OMEPRAZOLE) INCREASES ABSORPTION § NON-SATURABLE KINETICS § PRESYSTEMIC CLEARANCE (ABSORPTION AND BIOAVAILABILITY) IS 21% § UNALTERED BY DIET 6

RECTAL METHADONE § SIMILAR ABSORPTION AND BIOAVAILABILITY AS ORAL METHADONE § MICROENEMAS > HYDROGENATED OIL BASE SUPPOSITORIES 7

SUBLINGUAL METHADONE § ABSORPTION IS 34% (51% FENTANYL AND 18% MORPHINE) § BUFFERING THE PH TO 8. 5 DOUBLES ABSORPTION (75%) 8

METABOLISM § BIEXPONENTIAL KINETICS § EXTRACTION RATIO 0. 08 - 0. 16 § DEMETHYLATED TO AN INACTIVE METABOLITE (EDDP) BY CYP 3 A 4 § INDUCTION OF CYP 3 A 4 BY METHADONE WITH CHRONIC DOSING 9

CYTOCHROME ENZYMES § CYP 3 A 4 > CYP 2 D 6, CYP 1 A 2, CYP 2 C 9, CYP 2 C 19 § ULTRARAPID METABOLIZERS HAVE HALF THE METHADONE DRUG LEVELS AS POOR METABOLIZERS (HOMOZYGOTE CYP 2 D 6 MUTATIONS) 10

METHADONE CLEARANCE § METHADONE CLEARANCE CAN VARY BETWEEN INDIVIDUALS 100 -FOLD (0. 023 - 2. 1 LITERS PER MINUTE) WITH A MEAN OF 0. 095 LITERS PER MINUTE 11

CAUSES OF INTERINDIVIDUAL DIFFERENCES IN METHADONE § MU OPIOID RECEPTOR GENETICS § P-GLYCOPROTEIN ACTIVITY § CYP 3 A 4 BASAL AND INDUCTION ACTIVITY § CYP 2 D 6, CYP 1 A 2, CYP 2 C 9, CYP 2 C 19 § GENOTYPE OF ALPHA 1 ACID GLYCOPROTEIN § CO-MEDICATIONS 12

ROUTES § ORAL § SUBLINGUAL (1: 1) § RECTAL (1: 1) § SUBCUTANEOUS (2: 1) § INTRAVENOUS (2: 1) 13

SAFE COMBINATIONS § RIFAMBUTIN (FOR RIFAMPICIN) § FAMOTIDINE (FOR CIMETIDINE) § MIRTAZAPINE (FOR SSRI) § HALOPERIDOL OR OLANZAPINE (FOR RESPERIDONE) § VALPROIC ACID, GABAPENTIN (FOR PHENOBARBITOL, PHENYTOIN, CARBAMAZEPINE) 14

METHADONE TOXICITY § SIMILAR TO OTHER OPIOIDS § REDUCED CONSTIPATION COMPARED TO MORPHINE § TORSADES DE POINTES AND PROLONGED QTC WITH INCREASED RISK PARTICULAR WITH PARENTERAL 15

DEATH FROM METHADONE § MORE COMMON WITH INITIAL THERAPY § DEATHS AT STEADY STATE ARE RELATED TO: ü INTERFERING CO-MEDICATION ü ILLICIT DRUG TAKING (DIAZEPAM, ALCOHOL, COCAINE, CANNABIS, OTHER OPIOIDS) 16

METHADONE AND CANCER PAIN § THE ORIGINAL MANUFACTURER’S RECOMMENDATION OF 2. 5 - 10 MG EVERY 3 - 4 HOURS IS EXCESSIVE. § EQUIANALGESIA TABLES THAT PUT EQUIVALENTS NEAR UNITY WITH MORPHINE ARE DANGEROUS. 17

METHADONE AND CANCER PAIN § METHODS OF OPIOID ROTATION INVOLVE A “STOP-START” STRATEGY ü A Q 3 -HOUR AS NEEDED SCHEDULE ü LINEAR RATIO BASED UPON MORPHINE EQUIVALENTS EVERY 8 HOURS ATC 18

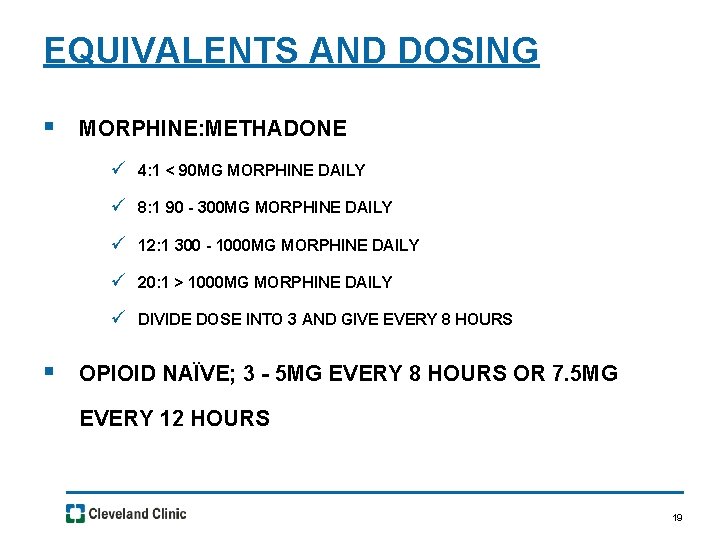
EQUIVALENTS AND DOSING § MORPHINE: METHADONE ü 4: 1 < 90 MG MORPHINE DAILY ü 8: 1 90 - 300 MG MORPHINE DAILY ü 12: 1 300 - 1000 MG MORPHINE DAILY ü 20: 1 > 1000 MG MORPHINE DAILY ü DIVIDE DOSE INTO 3 AND GIVE EVERY 8 HOURS § OPIOID NAÏVE; 3 - 5 MG EVERY 8 HOURS OR 7. 5 MG EVERY 12 HOURS 19

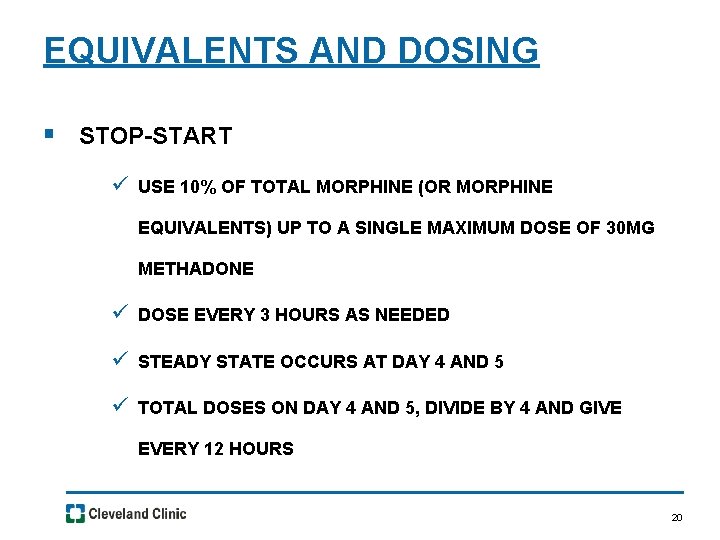
EQUIVALENTS AND DOSING § STOP-START ü USE 10% OF TOTAL MORPHINE (OR MORPHINE EQUIVALENTS) UP TO A SINGLE MAXIMUM DOSE OF 30 MG METHADONE ü DOSE EVERY 3 HOURS AS NEEDED ü STEADY STATE OCCURS AT DAY 4 AND 5 ü TOTAL DOSES ON DAY 4 AND 5, DIVIDE BY 4 AND GIVE EVERY 12 HOURS 20

METHADONE DOSING § SHOULD BE DONE BY SOMEONE WITH EXPERIENCE § DO NOT ADD BENZODIAZEPINES DURING TITRATION, AVOID ALCOHOL § USE ACETOMINOPHEN IF PAIN RECURS BEFORE THREE HOURS 21

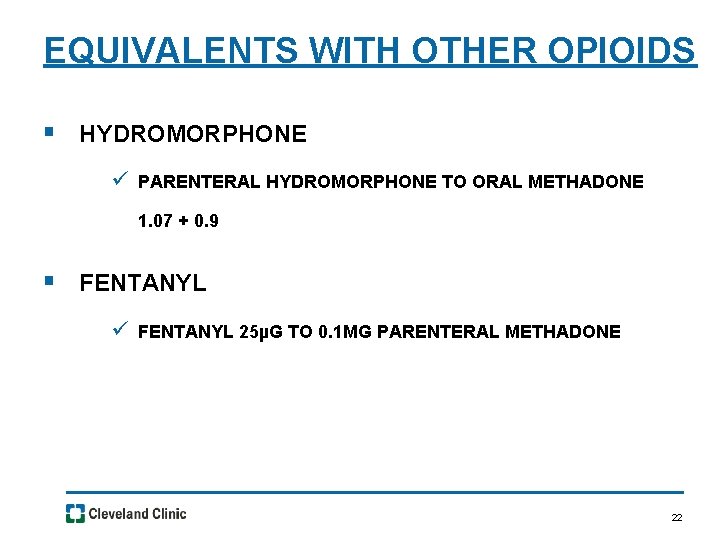
EQUIVALENTS WITH OTHER OPIOIDS § HYDROMORPHONE ü PARENTERAL HYDROMORPHONE TO ORAL METHADONE 1. 07 + 0. 9 § FENTANYL ü FENTANYL 25µG TO 0. 1 MG PARENTERAL METHADONE 22

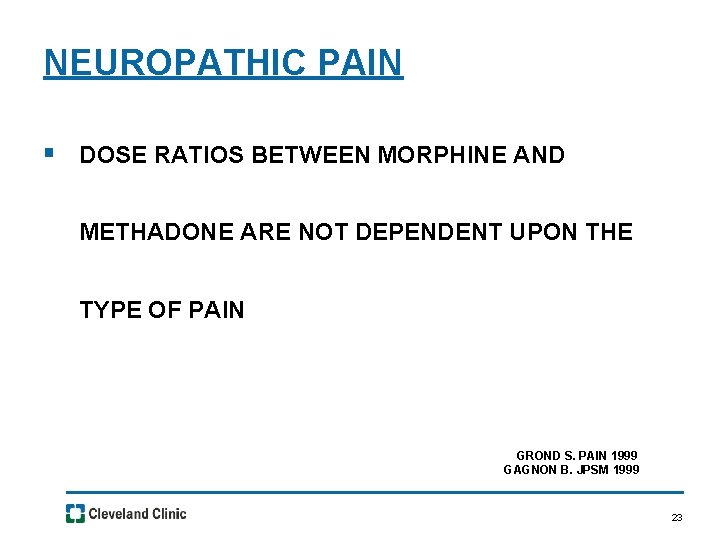
NEUROPATHIC PAIN § DOSE RATIOS BETWEEN MORPHINE AND METHADONE ARE NOT DEPENDENT UPON THE TYPE OF PAIN GROND S. PAIN 1999 GAGNON B. JPSM 1999 23

CANDIDATES FOR METHADONE § REFRACTORY PAIN § PATIENTS ON HIGH DOSE OPIOIDS WITH BURDENSOME COSTS § PATIENTS WITH LIMITED FINANCES § HOSPICES § NEUROPATHIC PAIN § CHEAP SUSTAINED RELEASE OPIOID Ripamonte C. Pain 1997 24

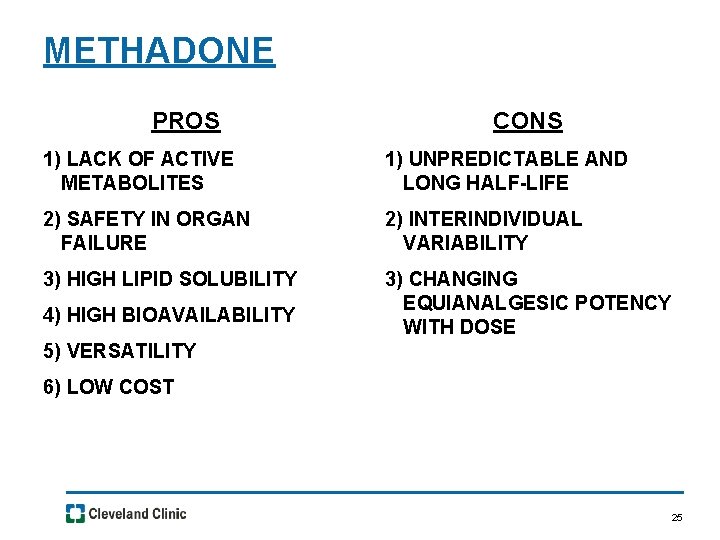
METHADONE PROS CONS 1) LACK OF ACTIVE METABOLITES 1) UNPREDICTABLE AND LONG HALF-LIFE 2) SAFETY IN ORGAN FAILURE 2) INTERINDIVIDUAL VARIABILITY 3) HIGH LIPID SOLUBILITY 3) CHANGING EQUIANALGESIC POTENCY WITH DOSE 4) HIGH BIOAVAILABILITY 5) VERSATILITY 6) LOW COST 25

SUMMARY § METHADONE IS UNIQUE PHARMACOLOGICALLY § MULITPLE RECEPTOR AGONIST, NMDA ANTAGONISTS AND MONOAMINE REUPTAKE INHIBITORS § RELATIVELY SAFE IN ORGAN FAILURE § DOSING SCHEMES ARE DIFFERENT THAN WITH OTHER OPIOIDS 26

27

METHADONE AND CARDIAC TOXICITY 28

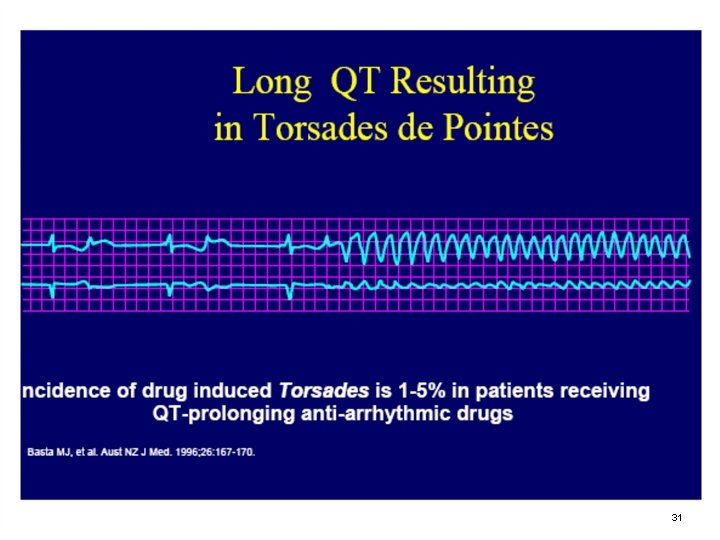
INTRODUCTION § METHADONE HAS BEEN ASSOCIATED WITH PROLONGED QTC AND TORSADES DE POINTES (TDP) § UNIQUE BLOCK OF IONIC CURRENT THROUGH SPECIFIC TYPE CARDIAC K+ CHANNELS § CARDIAC K+ CHANNELS ARE DERIVED FROM HUMAN ETHER-A-GO-GO-RELATED GENE (HERG) 29

INTRODUCTION § DELAYED REPOLARIZATION LEADS TO PROLONGED QTC INTERVALS (>500 MSEC) AND VENTRICULAR TACHYCARDIA (TDP) § ALSO INTERLEAD VARIATION BETWEEN QTC INTERVALS ON SURFACE LEADS 30

31

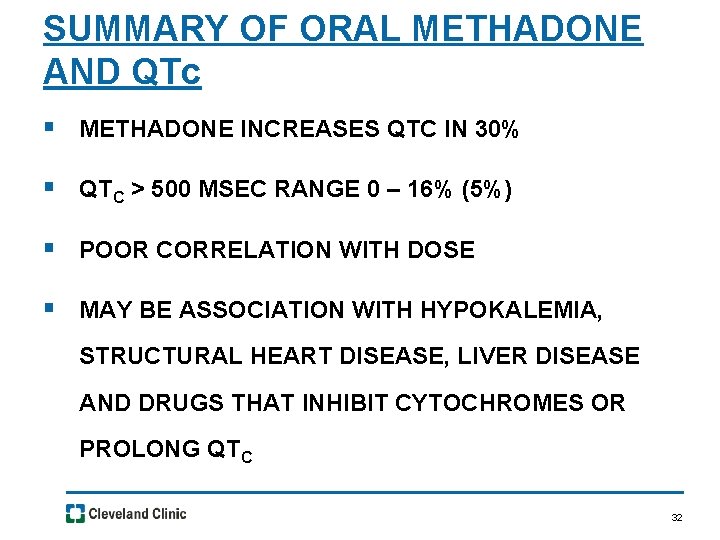
SUMMARY OF ORAL METHADONE AND QTc § METHADONE INCREASES QTC IN 30% § QTC > 500 MSEC RANGE 0 – 16% (5%) § POOR CORRELATION WITH DOSE § MAY BE ASSOCIATION WITH HYPOKALEMIA, STRUCTURAL HEART DISEASE, LIVER DISEASE AND DRUGS THAT INHIBIT CYTOCHROMES OR PROLONG QTC 32

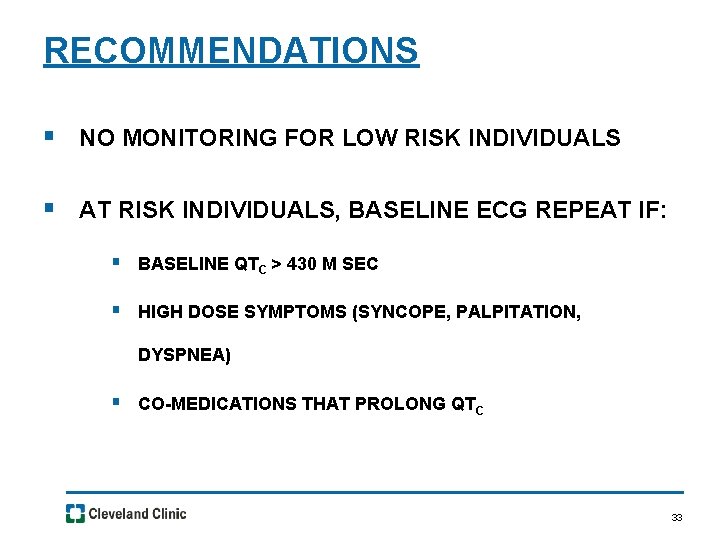
RECOMMENDATIONS § NO MONITORING FOR LOW RISK INDIVIDUALS § AT RISK INDIVIDUALS, BASELINE ECG REPEAT IF: § BASELINE QTC > 430 M SEC § HIGH DOSE SYMPTOMS (SYNCOPE, PALPITATION, DYSPNEA) § CO-MEDICATIONS THAT PROLONG QTC 33

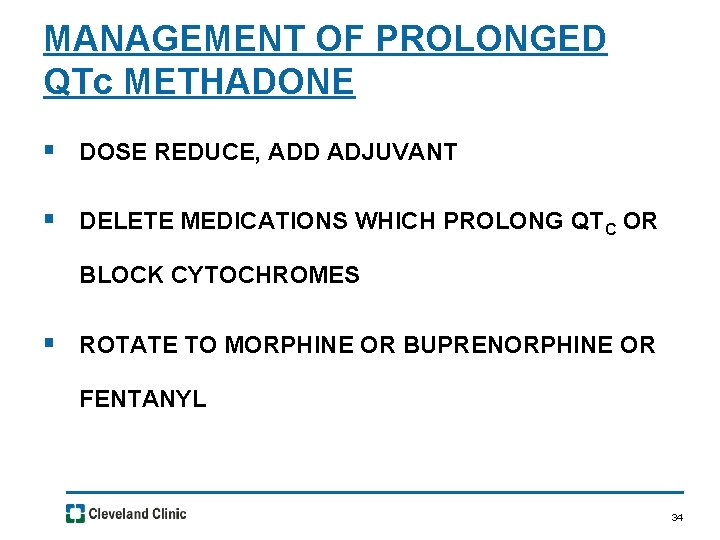
MANAGEMENT OF PROLONGED QTc METHADONE § DOSE REDUCE, ADD ADJUVANT § DELETE MEDICATIONS WHICH PROLONG QTC OR BLOCK CYTOCHROMES § ROTATE TO MORPHINE OR BUPRENORPHINE OR FENTANYL 34

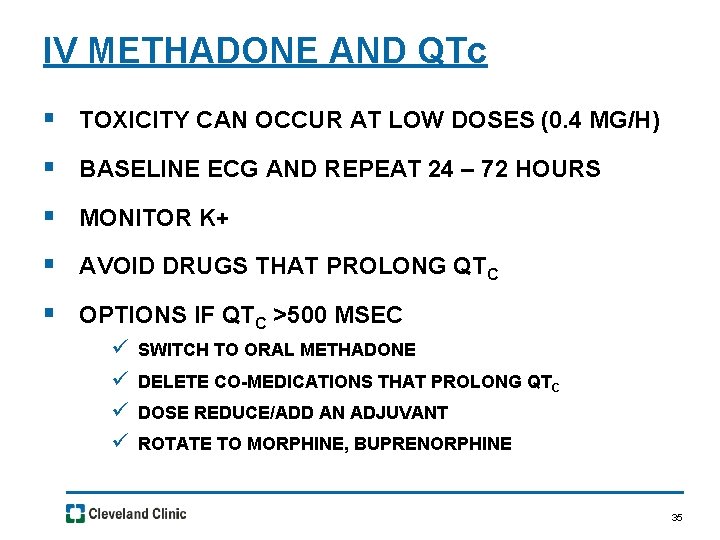
IV METHADONE AND QTc § TOXICITY CAN OCCUR AT LOW DOSES (0. 4 MG/H) § BASELINE ECG AND REPEAT 24 – 72 HOURS § MONITOR K+ § AVOID DRUGS THAT PROLONG QTC § OPTIONS IF QTC >500 MSEC ü ü SWITCH TO ORAL METHADONE DELETE CO-MEDICATIONS THAT PROLONG QTC DOSE REDUCE/ADD AN ADJUVANT ROTATE TO MORPHINE, BUPRENORPHINE 35

FDA BLACK BOX WARNING § DEATHS: UNINTENTIONAL OVERDOSE, DRUG INTERACTIONS, AND CARDIAC TOXICITY (QT PROLONGATION AND TDP) § PHYSICIAN’S NEED TO UNDERSTAND TOXICITY AND UNIQUE METHADONE PROPERTIES § DOSES SHOULD BE CAREFULLY CHOSEN AND SLOWLY TITRATED § CAREFULLY MONITOR WHEN SWITCHING TO METHADONE AND CHANGING DOSE 36

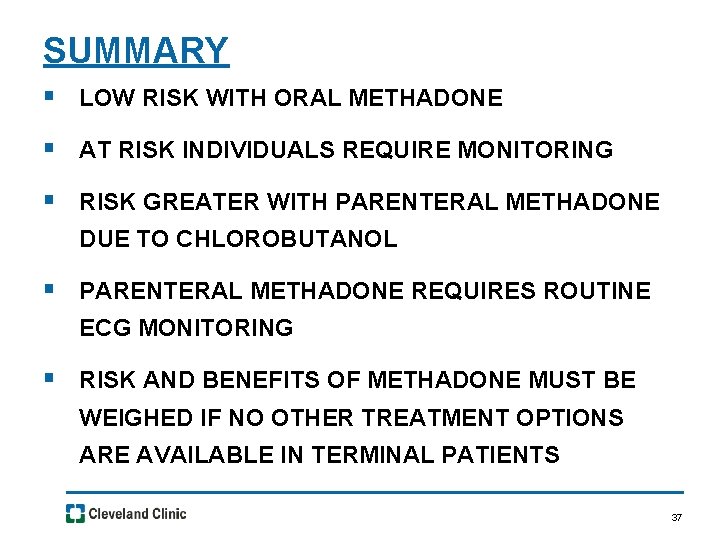
SUMMARY § LOW RISK WITH ORAL METHADONE § AT RISK INDIVIDUALS REQUIRE MONITORING § RISK GREATER WITH PARENTERAL METHADONE DUE TO CHLOROBUTANOL § PARENTERAL METHADONE REQUIRES ROUTINE ECG MONITORING § RISK AND BENEFITS OF METHADONE MUST BE WEIGHED IF NO OTHER TREATMENT OPTIONS ARE AVAILABLE IN TERMINAL PATIENTS 37

PATIENT CONTROLLED ANALGESIA (PCA) MELLAR DAVIS, WAEL LASHEEN, DECLAN WALSH 38

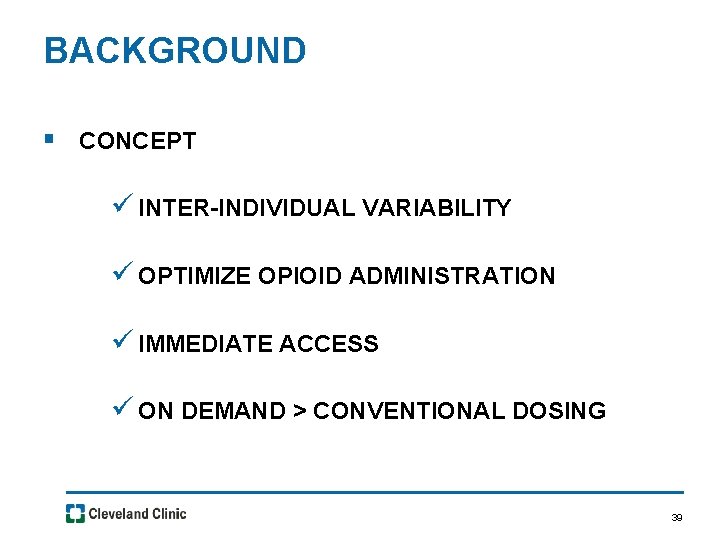
BACKGROUND § CONCEPT ü INTER-INDIVIDUAL VARIABILITY ü OPTIMIZE OPIOID ADMINISTRATION ü IMMEDIATE ACCESS ü ON DEMAND > CONVENTIONAL DOSING 39

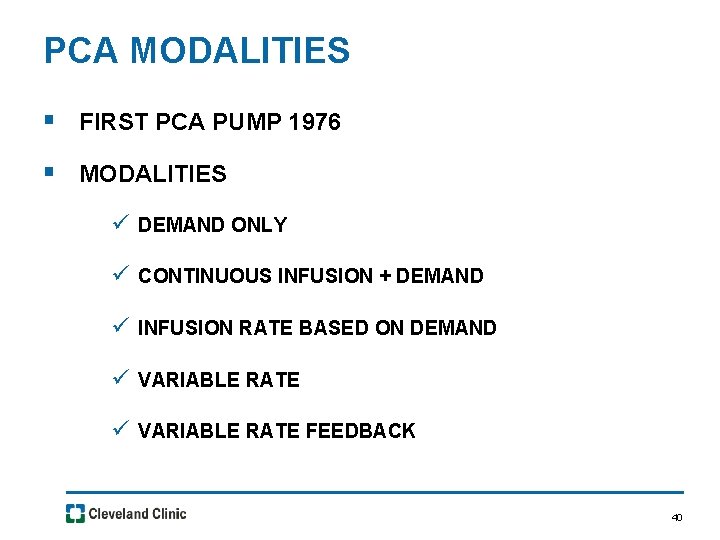
PCA MODALITIES § FIRST PCA PUMP 1976 § MODALITIES ü DEMAND ONLY ü CONTINUOUS INFUSION + DEMAND ü INFUSION RATE BASED ON DEMAND ü VARIABLE RATE FEEDBACK 40

PCA SETUP: DRUG CHOICE § OPIOIDS ü ALL OPIOIDS ü SHORT ACTING ARE SAFER THAN LONG ACTING § NON-OPIOIDS ü MOST COMPATIBLE WITH OPIOIDS • ATROPINE, DEXAMETH, DIAZEPAM, LORAZEPAM, KETEROLAC, HALDOL, LEVOPROME, METOCHLOPRAMIDE ü PHYENYTOIN IS NOT COMPATIBLE WITH OPIOIDS 41

PCA ROUTES OF DELIVERY § INTRAVENOUS § SUBCUTANEOUS § INTRAMUSCULAR § ORAL, NASAL, SUBLINGUAL § SPINAL, VENTRICULAR § OTHERS. . . 42

PCA STRATEGY • • • LOADING DOSE DEMAND DOSE LOCKOUT INTERVAL CONTINUOUS INFUSION DOSE LIMITS 43

PCA ADVANTAGES § PATIENT ü REMOVES DELAY DEMAND / DELIVERY ü PATIENT CONTROL / SECURITY ü DETERMINE PAIN THRESHOLDS § DOSING ü ASSESS ANALGESIC REQUIREMENTS ü INFLUENCES TRADITIONAL DOSING PROTOCOLS § ADAPTABLE ü INTERINDIVIDUAL REQUIREMENTS ü TEMPORAL PAIN PATTERN 44

PATIENT RISK FACTORS § AGE § HEAD INJURY § SLEEP APNEA § OBESITY § RESPIRATORY FAILURE § BENZODIAZEPINES § HYPONATREMIA § RENAL FAILURE 45

COMPLICATIONS § OPERATOR ERRORS § PROGRAMMING ERRORS § ACCIDENTAL BOLUS § INAPPROPRIATE üDOSE üLOCKOUT üDRUG SELECTION § SURROGATE ACTIVATION § PUMP MALFUNCTION 46

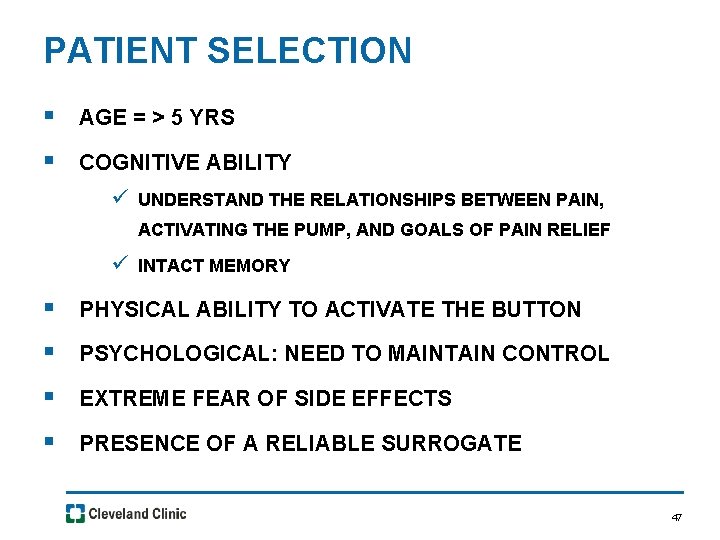
PATIENT SELECTION § AGE = > 5 YRS § COGNITIVE ABILITY ü UNDERSTAND THE RELATIONSHIPS BETWEEN PAIN, ACTIVATING THE PUMP, AND GOALS OF PAIN RELIEF ü INTACT MEMORY § PHYSICAL ABILITY TO ACTIVATE THE BUTTON § PSYCHOLOGICAL: NEED TO MAINTAIN CONTROL § EXTREME FEAR OF SIDE EFFECTS § PRESENCE OF A RELIABLE SURROGATE 47

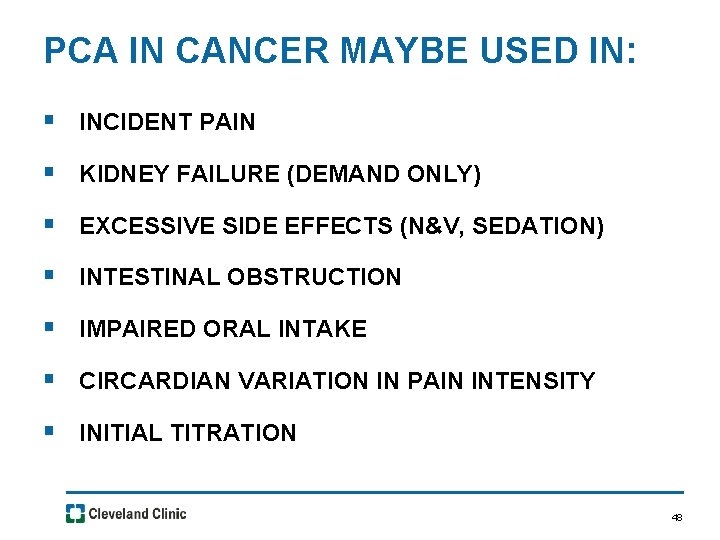
PCA IN CANCER MAYBE USED IN: § INCIDENT PAIN § KIDNEY FAILURE (DEMAND ONLY) § EXCESSIVE SIDE EFFECTS (N&V, SEDATION) § INTESTINAL OBSTRUCTION § IMPAIRED ORAL INTAKE § CIRCARDIAN VARIATION IN PAIN INTENSITY § INITIAL TITRATION 48

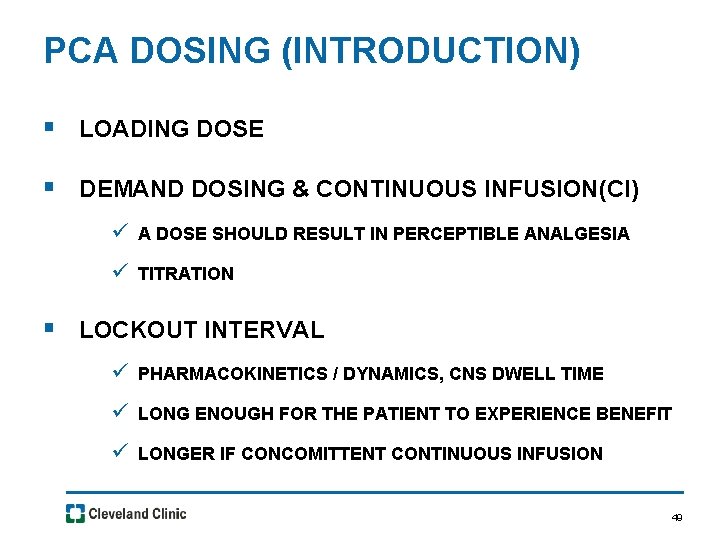
PCA DOSING (INTRODUCTION) § LOADING DOSE § DEMAND DOSING & CONTINUOUS INFUSION(CI) ü A DOSE SHOULD RESULT IN PERCEPTIBLE ANALGESIA ü TITRATION § LOCKOUT INTERVAL ü PHARMACOKINETICS / DYNAMICS, CNS DWELL TIME ü LONG ENOUGH FOR THE PATIENT TO EXPERIENCE BENEFIT ü LONGER IF CONCOMITTENT CONTINUOUS INFUSION 49

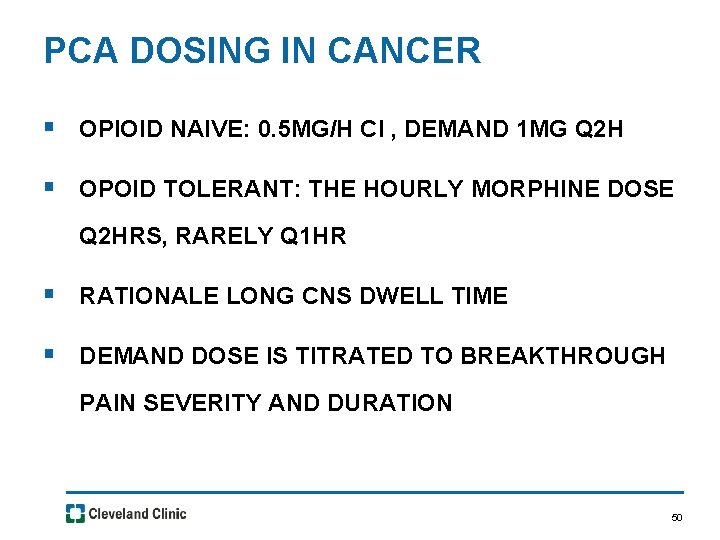
PCA DOSING IN CANCER § OPIOID NAIVE: 0. 5 MG/H CI , DEMAND 1 MG Q 2 H § OPOID TOLERANT: THE HOURLY MORPHINE DOSE Q 2 HRS, RARELY Q 1 HR § RATIONALE LONG CNS DWELL TIME § DEMAND DOSE IS TITRATED TO BREAKTHROUGH PAIN SEVERITY AND DURATION 50

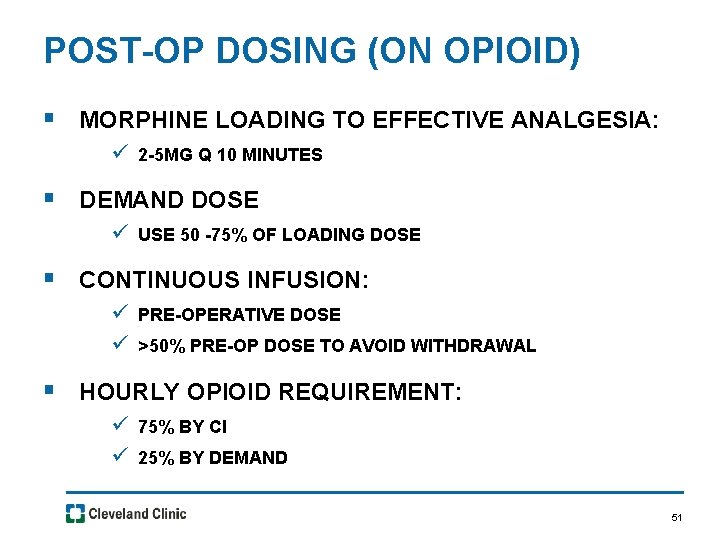
POST-OP DOSING (ON OPIOID) § MORPHINE LOADING TO EFFECTIVE ANALGESIA: ü 2 -5 MG Q 10 MINUTES § DEMAND DOSE ü USE 50 -75% OF LOADING DOSE § CONTINUOUS INFUSION: ü PRE-OPERATIVE DOSE ü >50% PRE-OP DOSE TO AVOID WITHDRAWAL § HOURLY OPIOID REQUIREMENT: ü 75% BY CI ü 25% BY DEMAND 51

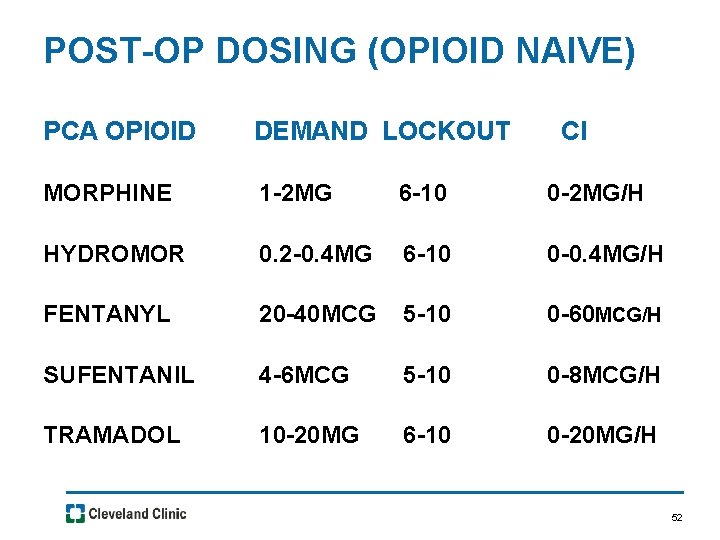
POST-OP DOSING (OPIOID NAIVE) PCA OPIOID DEMAND LOCKOUT CI MORPHINE 1 -2 MG 6 -10 0 -2 MG/H HYDROMOR 0. 2 -0. 4 MG 6 -10 0 -0. 4 MG/H FENTANYL 20 -40 MCG 5 -10 0 -60 MCG/H SUFENTANIL 4 -6 MCG 5 -10 0 -8 MCG/H TRAMADOL 10 -20 MG 6 -10 0 -20 MG/H 52

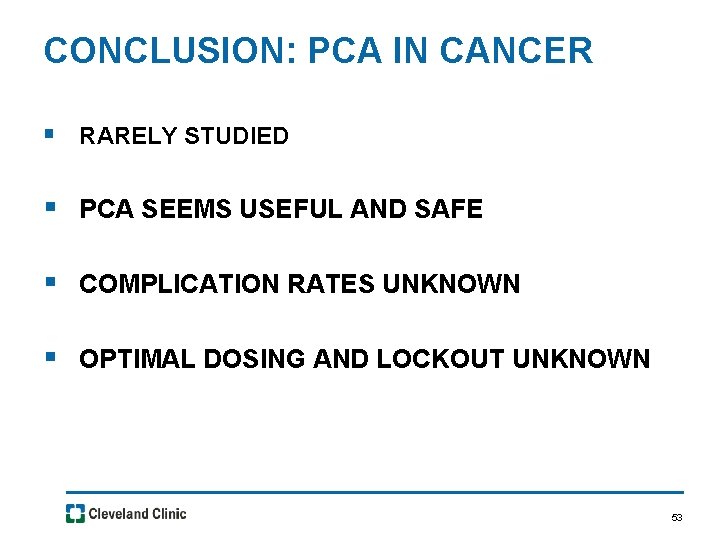
CONCLUSION: PCA IN CANCER § RARELY STUDIED § PCA SEEMS USEFUL AND SAFE § COMPLICATION RATES UNKNOWN § OPTIMAL DOSING AND LOCKOUT UNKNOWN 53

PERIOPERATIVE MANAGEMENT OF CHRONIC PAIN PATIENTS 54

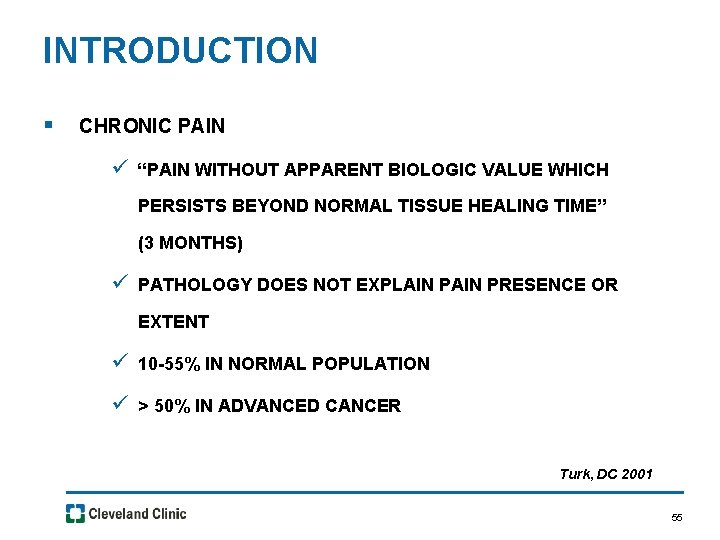
INTRODUCTION § CHRONIC PAIN ü “PAIN WITHOUT APPARENT BIOLOGIC VALUE WHICH PERSISTS BEYOND NORMAL TISSUE HEALING TIME” (3 MONTHS) ü PATHOLOGY DOES NOT EXPLAIN PRESENCE OR EXTENT ü 10 -55% IN NORMAL POPULATION ü > 50% IN ADVANCED CANCER Turk, DC 2001 55

SIGNIFICANCE OF POST-OP PAIN . § MODERATE TO SEVERE PAIN IN 20 -30% § CARDIOPULMONARY COMPLICATIONS § UNEXPECTED ADMISSIONS FROM AMB. SURGERY § PROLONGED CONVALESCENCE 56

POST-OP PAIN IN OPIOID TOLERANT § POORER PAIN CONTROL § INCREASED OPIOID REQUIREMENTS § 3 X EPIDURAL MORPHINE THAN OPIOID-NAÏVE § 4 X MORPHINE BY INTERMITTENT BOLUS § POSTOPERATIVE PCA DOSES > REPLACEMENT 57 .

CAUSES OF INCREASED POSTOPERATIVE PAIN AND OPIOID REQUIREMENTS IN OPIOIDTOLERANT. § PROGRESSIVE CANCER § TOLERANCE § OPIOID-INDUCED HYPERALGESIA ü INCREASED PAIN SENSITIVITY 58

DIFFERENCES IN SIDE EFFECTS BETWEEN OPIOID-NAÏVE AND TOLERANT INDIVIDUALS § ↓ NAUSEA & PRURITUS IN OPIOID-TOLERANT DELEON CASASOLA 1993 RAPP 1995 59

OPTIMIZING PERIOPERATIVE OPIOIDS USE IN OPIOID-TOLERANT § MINIMAL EFFECTIVE OPIOID DOSE IS UNKNOWN § POSTOPERATIVE OPIOID > ANTICIPATED § ADEQUATE OPIOIDS TO AVOID WITHDRAWAL § TRANSITION TO PREOPERATIVE OPIOID DOSES CHALLENGING AND OFTEN DELAYED 60

PLAN PERIOPERATIVE MANAGEMENT § EPIDURALS § REGIONAL BLOCKS § DISCONTINUE NSAIDS 48 HRS BEFORE EPIDURAL § OPIOID DOSE MAINTAINED ON DAY OF SURGERY 61

ACUTE POSTOPERATIVE MANAGEMENT. § EXPECT OPIOID REQUIREMENTS 2 -4 X NAÏVE INDIVIDUALS § START PCA § ORAL ROUTE: 1. 5 X PREOPERATIVE ORAL OPIOID DOSE PLUS DEMAND ONLY FOR RESCUE DOSES 62

ACUTE POSTOPERATIVE MANAGEMENT. § IV ROUTE: CONTINUOUS DOSE TO MATCH PRE-OP OPIOID REQUIREMENT + DEMAND § REGIONAL BLOCK: PROVIDE ½ THE PRE-OP OPIOID DOSE § ADD ADJUNCT (ACETAMINOPHEN, KETOROLAC, KETAMINE, GABAPENTIN) 63

MANAGEMENT POSTOPERATIVE TRANSITION PHASE § USE OPIOID DOSE DURING FIRST 24 -48 HOURS § DELIVER ½ AS LONG-ACTING OPIOID § DELIVER ½ AS RESCUE EVERY 3 -4 HOURS § ADD NSAID, ACETAMINOPHEN AND TAPER OPIOID 64

65

PERIOPERATIVE MANAGEMENT OF ADDICTION: MISCONCEPTIONS § MAINTENANCE OPIOIDS (BUPRENORPHINE , METHADONE) PROVIDES ADEQUATE ANALGESIA POSTOPERATIVE § USE OF SHORT ACTING OPIOIDS IN THE POSTOPERATIVE PERIOD INCREASES RISK OF ADDICTION RELAPSE 66

PERIOPERATIVE MANAGEMENT OF ADDICTION: MISCONCEPTIONS § ADDITIVE EFFECTS OF SHORT ACTING OPIOIDS WITH MAINTENANCE OPIOIDS INCREASES RESPIRATORY DEPRESSION § REPORTING PAIN MAY BE A MANIPULATION TO OBTAIN OPIOID ANALGESICS OR DRUG SEEKING 67

ISSUES PARTICULAR TO ADDICTION § PSEUDO-ADDICTION: ”DRUG SEEKING” DUE TO INADEQUATELY CONTROLLED PAIN § THERAPEUTIC DEPENDENCE: ”DRUG SEEKING” OUT OF FEAR OF EMERGENCE OF WITHDRAWAL § PSEUDO-OPIOID DEPENDENCE: CONTINUED REPORTS OF PAIN TO PREVENT CURRENTLY EFFECTIVE DOSES OF OPIOIDS FROM BEING REDUCED 68

MANAGEMENT § REASSURANCE THAT ADDICTION DOES NOT PREVENT PAIN CONTROL § CONTINUE OPIOID MAINTENANCE IN THE PERIOPERATIVE PERIOD § CONFIRM OPIOID TIMING AND DOSE WITH ADDICTION SPECIALIST § DISCUSS PAIN MANAGEMENT PLANS W/ PATIENT 69

MANAGEMENT § SHORT ACTING OPIOIDS TO TREAT PAIN § REQUIREMENTS MAY BE 3 -4 Fold > OPIOID NAÏVE § MAY REQUIRE SCHEDULED RATHER THAN AS NEEDED SHORT ACTING OPIOIDS § DO NOT STOP MAINTENANCE THERAPY § PCA MAY BE USED BUT SHOULD BE MONITORED 70

MANAGEMENT BUPRENORPHINE MAINTENANCE § CONTINUE BUPRENORPHINE AND ADD SHORT ACTING OPIOIDS § DIVIDE & GIVE BUPRENORPHINE EVERY 6 -8 HRS § DISCONTINUE BUPRENORPHINE AND USE SHORT ACTING OPIOIDS VIA CONTINUOUS AND DEMAND PCA 71

MANAGEMENT BUPRENORPHINE MAINTENANCE § CONVERT TO 20 -40 MG METHADONE DAILY AND USE SHORT ACTING OPIOIDS FOR PAIN § CONVERT BACK TO BUPRENORPHINE AT DISCHARGE 72

REFERENCES § Buvanendran, A. and J. S. Kroin (2007). "Useful adjuvants for postoperative pain management. " Best Pract Res Clin Anaesthesiol 21(1): 31 -49. § Carroll, I. R. , M. S. Angst, et al. (2004). "Management of perioperative pain in patients chronically consuming opioids. " Reg Anesth Pain Med 29(6): 576 -91. § De Leon-Casasola, O. A. , D. P. Myers, et al. (1993). "A comparison of postoperative epidural analgesia between patients with chronic cancer taking high doses of oral opioids versus opioid-naive patients. " Anesth Analg 76(2): 302 -7. § Kopf, A. Banzhaf, et al. (2005). "Perioperative management of the chronic pain patient. " Best Pract Res Clin Anaesthesiol 19(1): 59 -76. § Rapp, S. E. , L. B. Ready, et al. (1995). "Acute pain management in patients with prior opioid consumption: a case-controlled retrospective review. " Pain 61(2): 195 -201. § Tiippana, E. M. , K. Hamunen, et al. (2007). "Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. " Anesth Analg 104(6): 1545 -56. 73

CASE 1 • 48 YEAR OLD FEMALE WITH OVARIAN CANCER AND TOXICITY AS WELL AS FOR RESPONSE TO MORPHINE SR 60 MG TWICE DAILY FOR ABDOMINAL PAIN • PHYSICAL EXAMINATION DEMONSTRATES WASTING, ASCITES , PERIUMBILICAL NODES • MEDICATIONS: SERTRALINE 50 MG, METOPROLOL 25 MG TWICE DAILY AND ORAL STOOL SOFTENERS 74

CASE 1 • ECG QTC 450 MSEC • LABORATORY: CREATININE 1. 8, NORMAL BILIRUBIN 75

CASE 1: TREATMENT • METHADONE SHOULD NOT BE STARTED DUE TO THE QTC INTERVAL • METHADONE SHOULD NOT BE USED DUE TO INTERACTIONS WITH SERTRALINE • METHADONE SHOULD NOT BE STARTED DUE TO THE CREATININE • “STOP-START” STRATEGY MAY BE USED WITH STOPPING MORPHINE AND STARTING METHADONE 10 MG EVERY 3 HOURS AS NEEDED 76

CASE 1 • YOU START METHADONE EVERY 3 HOURS AS NEEDED • 6 DAYS LATER SHE IS TAKING 20 MG PER DAY ON AVERAGE WITH PAIN CONTROL. • SHE IS DISCHARGED HOME ON METHADONE 10 MG TWICE DAILY AND EVERY 3 HOURS AS NEEDED • TWO WEEKS LATER SHE IS ADMITTED WITH NAUSEA AND VOMITING AND IS UNABLE TO TAKE HER ORAL MEDICATIONS. 77

CASE 1 • REPEAT ECG QTC 460 MSEC • IV HYDRATION IS STARTED 78

CASE 1: TREATMENT • STOP METHADONE AND START FENTANYL OR BUPRENORPHINE • START METHADONE IV AT 0. 5 MG PER HOUR WITH 0. 51 MG EVERY 3 HOURS, REPEAT ECG IN 2 -3 DAYS • SWITCH TO RECTAL METHADONE 10 MG EVERY 12 HOURS AND AS NEEDED • START HALOPERIDOL FOR NAUSEA AND OBTAIN A PLAIN X-RAY OF THE ABDOMEN • START ONDANSETRON FOR NAUSEA AND OBTAIN A PLAIN X-RAY OF THE ABDOMEN 79

CASE 2 • 65 YEAR OLD FEMALE WITH BREAST CANCER ON 40 MG METHADONE TWICE DAILY FOR BONE PAIN • SHE SUSTAINS A PATHOLOGIC HIP FRACTURE REQUIRING SURGERY • MEDICATIONS: METHADONE , TEMAZEPAM 15 MG AT NIGHT, PRINIVIL 20 MG DAILY AND LAXATIVES • LABORATORY: NORMAL CREATININE AND BILIRUBIN 80

CASE 2 : TREATMENT • DISCONTINUE METHADONE ON THE DAY OF SURGERY AND USE AS NEEDED HYDROMORPHONE 2 MG HOURLY AS NEEDED • USE METHADONE 7. 5 MG EVERY 3 HOURS AS NEEDED FOR PAIN FOR POST- OP PAIN • CONTINUE METHADONE 40 MG TWICE DAILY IN THE POST- OP PERIOD AND USE HYDROMORPHONE 0. 81 MG EVERY 1 -2 HOURS AS NEEDED BY PCA • START KETOROLAC 15 MG IV Q 6 HOURS POST- OP AND CONTINUE METHADONE 40 MG TWICE DAILY • AVOID COMBINING SHORT ACTING POTENT OPIOIDS AND METHADONE. USE TRAMADOL 100 MG EVERY 6 HOURS WITH METHADONE 81
 Wael ouarda
Wael ouarda Wael is the teacher of n students
Wael is the teacher of n students Exposition of the fly by katherine mansfield
Exposition of the fly by katherine mansfield Ibogaine history
Ibogaine history Methadone clinic durango co
Methadone clinic durango co Sts methadone clinic
Sts methadone clinic Declan hogan chef
Declan hogan chef An angel declan galbraith
An angel declan galbraith Declan wall
Declan wall Declan hogan chef
Declan hogan chef Basic kitchen procedures
Basic kitchen procedures Declan hynes
Declan hynes What is kitchen brigade
What is kitchen brigade B. timothy walsh
B. timothy walsh Dr lorraine walsh
Dr lorraine walsh Dr claire walsh
Dr claire walsh Angka indeks adalah… *
Angka indeks adalah… * D walsh sdsu
D walsh sdsu Bud walsh into the wild
Bud walsh into the wild Sir thomas malory biography
Sir thomas malory biography Indek 2
Indek 2 Pearl sydenstricker buck
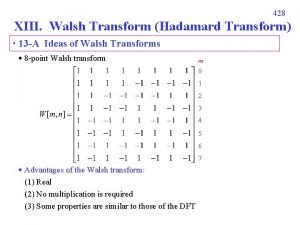
Pearl sydenstricker buck Walsh transform
Walsh transform Languageism
Languageism Walsh consulting engineers
Walsh consulting engineers Iqips
Iqips Psy walsh
Psy walsh Bernadine walsh
Bernadine walsh James walsh brown
James walsh brown Image transforms in digital image processing
Image transforms in digital image processing Olga walsh
Olga walsh Conor walsh harvard
Conor walsh harvard Robyn walsh slp
Robyn walsh slp Walsh v wilkie
Walsh v wilkie Mary g walsh writing center
Mary g walsh writing center Landis walsh
Landis walsh Haar transform matrix for n=8
Haar transform matrix for n=8 Ratonzote
Ratonzote William j. walsh
William j. walsh Davis school district payroll
Davis school district payroll Davis clements
Davis clements Lineas de langer
Lineas de langer Kuali uc davis
Kuali uc davis Davis weather station map
Davis weather station map Ucdavis irb
Ucdavis irb Davis alves
Davis alves Leila davis elementary
Leila davis elementary Mike davis architect
Mike davis architect Forester miller and davis model
Forester miller and davis model Ruth shim uc davis
Ruth shim uc davis William davis primary school
William davis primary school Gerry davis stance
Gerry davis stance Davis and moore 1945
Davis and moore 1945 Unmc omaha
Unmc omaha Evelyn cortez-davis
Evelyn cortez-davis Frs uc davis
Frs uc davis Tawanka elementary school calendar
Tawanka elementary school calendar Kerumunan menurut kingsley davis
Kerumunan menurut kingsley davis Thomas davis model
Thomas davis model Ellis and davey classification
Ellis and davey classification Area of responsibilities
Area of responsibilities Hester davis fall assessment
Hester davis fall assessment Cover letter uc davis
Cover letter uc davis Paulina davis nyls
Paulina davis nyls Rocky davis incision
Rocky davis incision Alvarado skoru
Alvarado skoru Davis model of corporate social responsibility
Davis model of corporate social responsibility Crocker nuclear laboratory
Crocker nuclear laboratory Bailey davis wikipedia
Bailey davis wikipedia Uc davis benefits
Uc davis benefits Is 16 too young to drive a car by robert davis
Is 16 too young to drive a car by robert davis Rocky davis incision
Rocky davis incision Ecs 129 uc davis
Ecs 129 uc davis Inmidazol
Inmidazol Miv uc davis
Miv uc davis Davis slope decline theory
Davis slope decline theory Siss advisor
Siss advisor Uc davis basc advising
Uc davis basc advising Yong ung kai v enting case summary
Yong ung kai v enting case summary Temple os
Temple os Eop program
Eop program Rocky davis incision
Rocky davis incision