Medical Nutrition Therapy for Refeeding Syndrome Rachel Hammerling
























- Slides: 33

Medical Nutrition Therapy for Refeeding Syndrome Rachel Hammerling Concordia College, Moorhead MN

Objectives • Be able to describe refeeding syndrome (RFS) • Be able to describe the pathophysiology of starvation • Identify the main pathophysiologic features of RFS • Be able to identify signs & symptoms • Identify recommended treatment & standards of care • Be able to explain ethical issues involved with treatment & care

Discovery of RFS • Observed & described after WWII • Victims of starvation experienced cardiac and/or neurologic dysfunction – After being reintroduced to food • Today, rarely see patients who are severely malnourished, as WWII victims were, in the 1 st week – Neurologic signs & symptoms develop later

What is RFS? • Potentially fatal shifts in fluids & electrolytes • May occur in malnourished patients receiving artificial refeeding – Enterally or parenterally • Complex syndrome – Sodium & fluid imbalance – Changes in glucose, protein, fat metabolism – Thiamine deficiency – Hypokalemia – Hypomagnesaemia

Understanding Starvation • Glucose = main fuel – Shifts to protein & fat • Insulin ↓ due to ↓ availability of glucose • Catabolism of protein → loss of cellular & muscle mass → atrophy of vital organs & internal organs • Respiratory & cardiac function ↓ due to muscular wasting & fluid/electrolyte imbalances • Body is now surviving by slowly consuming itself

How common is RFS? • True incidence is unknown • Study of 10, 197 patients, incidence of hypophosphatemia = 43 % – Malnutrition one of strongest risk factors • Parenteral patients = 100% incidence of hypophosphatemia

Pathogenesis • Electrolytes & minerals involved 1) 2) 3) 4) Phosphorus Potassium Magnesium Glucose


Main Pathophysiologic Features • • • Disturbances of body-fluid distribution Abnormal glucose & lipid metabolisms Thiamine deficiency Hypophosphatemia Hypomagnesemia Hypokalemia

Disturbances of Body-Fluid Distribution • Can influence body functions: • CHO refeeding – ↓ water & sodium excretion, resulting in weight gain 1) Cardiac failure 2) Dehydration or • Protein & fat refeeding fluid overload – Result in weight loss & 3) Hypotension urinary sodium excretion 4) Pre-renal failure – Hypernatremia along 5) Sudden death with azotemia & metabolic acidosis

Abnormal Glucose & Lipid Metabolisms • Glucose – Suppress gluconeogenesis → reduced AA usage • Less-negative N balance – Hyperglycemia • Glucose → fat (Lipogenesis) – Hypertriglyceridemia, fatty liver, & abnormal liver function tests

Thiamine Deficiency • Can result in Wernicke’s encephalopathy or Korsakov’s syndrome, associated with: – Ocular disturbance – Confusion – Ataxia • loss of ability to coordinate muscular movement – Coma – Short-term memory loss – Confabulation • Confusion of imagination with memory

Hypophosphatemia • Predominant feature of RFS • Impaired cellular-energy pathways – Adenosine triphosphate – 2, 3 -diphosphoglycerate • Impaired skeletal-muscle function – Including weakness & myopathy • Seizures & perturbed mental state • Impaired blood clotting processes & hemolysis also can occur

Hypomagnesemia • Most cases not clinically significant • Severe cases: – Cardiac arrhythmias – Abdominal discomfort – Anorexia – Tremors, seizures, & confusion – Weakness

Hypokalemia • Features are numerous: – Cardiac arrhythmias – Hypotension – Cardiac arrest – Weakness – Paralysis – Confusion – Respiratory Depression

Signs & Symptoms • Electrolyte imbalance – Hypokalemia – Hypophosphatemia – Hypomagnesemia • REMEMBER: Even an overweight or obese patient can be malnourished & a victim for RFS

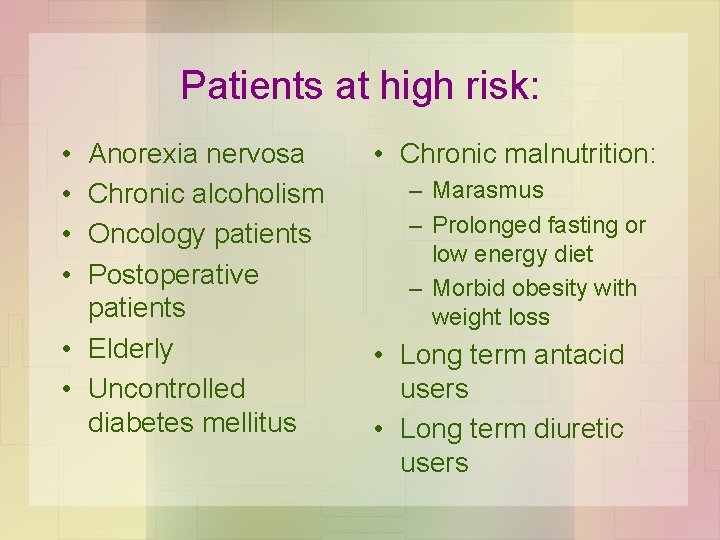
Identifying Patients at High Risk of Refeeding Problems • NICE Guidelines (National Institute for Health & Clinical Excellence) • Either patient has 1 or more: – – BMI <16 Unintentional weight loss >15% in past 3 -6 mo Little/no nutritional intake for 10 days Low levels of potassium, phosphate, or magnesium before feeding • Or patient has 2 or more: – – BMI <18. 5 Unintentional weight loss >10% in past 3 -6 mo Little/no nutritional intake for >5 days History of alcohol misuse or drugs

Patients at high risk: • • Anorexia nervosa Chronic alcoholism Oncology patients Postoperative patients • Elderly • Uncontrolled diabetes mellitus • Chronic malnutrition: – Marasmus – Prolonged fasting or low energy diet – Morbid obesity with weight loss • Long term antacid users • Long term diuretic users

Gastrointestinal Fistula patients • Usually reveals chronic malnutrition – Due to damaged Gl tract & severe abdominal sepsis • High risk for RFS • Be aware of condition & treat the same – Diarrhea commonly occurs & can be treated by enteral nutrition

Intervention: Objectives 1) Gradually correct starvation – Use less than full levels of calorie & fluid requirements 2) Advance calories & volume – Monitor cardiac & respiratory side effects 3) Correct vitamin & mineral deficiencies – Especially with symptoms

Intervention: Objectives Cont. 4) Nutrition support in patients at risk should be increased slowly – Assuring adequate amounts of vitamins & minerals 5) Organ function, fluid balance, & serum electrolytes – Monitor daily during 1 st week & less frequently after

Intervention: Objectives Cont. 6) Monitor for neurological, hematological, & metabolic complications – Of hypokalemia, hypophosphatemia, & hyperglycemia 7) Prevent sudden death

Intervention: Food & Nutrition • • Begin 20 kcal/kg for 1 st 3 days Progress to 25 kcal/kg Gradually ↑ by 7 th day Protein start slow, ↑ gradually – To protect & restore lean body mass • Restrict CHO to 150 -200 g/day – To prevent rapid insulin surge • CHO in PN – Initiate at 2 mg/kg/min – Fat calories should make up the difference

Intervention: Food & Nutrition • Maintain fluid balance – Adjust when edema exists • Adjust for sodium & potassium – Depending on lab values until normal • Supplements – Thiamin – Other vitamins & minerals as needed

Common Drugs Used • Replacement of phosphorus, potassium, & magnesium • Insulin – Used to correct hyperglycemia levels – Monitor blood glucose levels during refeeding

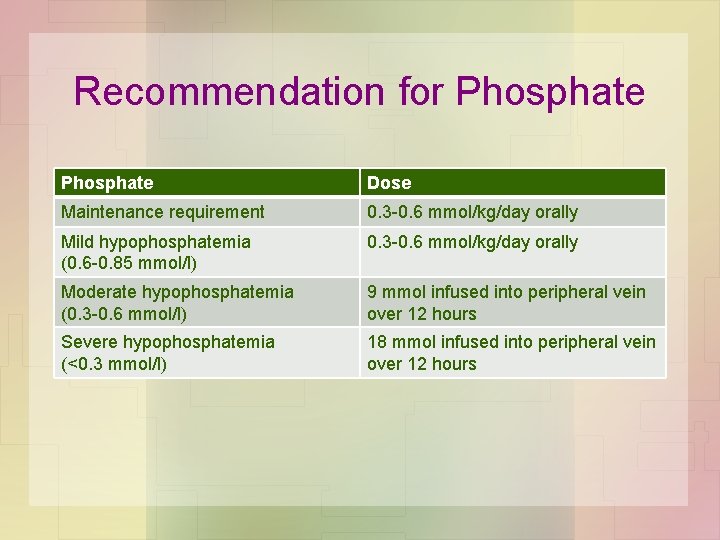
Recommendation for Phosphate Dose Maintenance requirement 0. 3 -0. 6 mmol/kg/day orally Mild hypophosphatemia (0. 6 -0. 85 mmol/l) 0. 3 -0. 6 mmol/kg/day orally Moderate hypophosphatemia (0. 3 -0. 6 mmol/l) 9 mmol infused into peripheral vein over 12 hours Severe hypophosphatemia (<0. 3 mmol/l) 18 mmol infused into peripheral vein over 12 hours

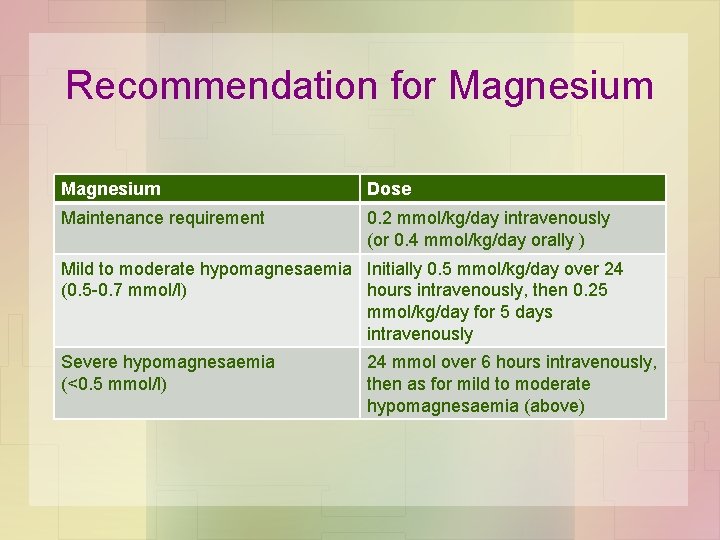
Recommendation for Magnesium Dose Maintenance requirement 0. 2 mmol/kg/day intravenously (or 0. 4 mmol/kg/day orally ) Mild to moderate hypomagnesaemia Initially 0. 5 mmol/kg/day over 24 (0. 5 -0. 7 mmol/l) hours intravenously, then 0. 25 mmol/kg/day for 5 days intravenously Severe hypomagnesaemia (<0. 5 mmol/l) 24 mmol over 6 hours intravenously, then as for mild to moderate hypomagnesaemia (above)

Intervention: Nutrition Education, Counseling, & Care Management • Focus on adequate nutrient intake • Consider referral if food insecurity is a concern • Offer guidelines according to discharge intervention plan • Physician may suggest long-term medication use or therapies

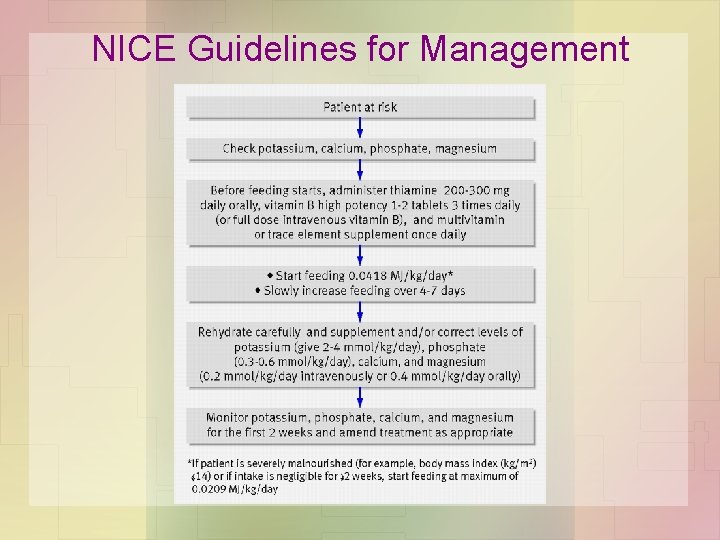
NICE Guidelines for Management

Ethical Issues with RFS • Roles between dietitian, counselor, nurse, doctor, and other professionals • Working with anorexia patients, oncology patients or older patients • Ethnic & religious differences – Muslim patients – Non-English speaking patients


Summary Points • RFS is caused by rapid refeeding after a period of undernutrition • Characterized by hypophosphatemia • Patients at high risk: undernourished, little or no energy intake for > 10 days • Start refeeding at low levels • Correction of electrolyte & fluid imbalances before feeding IS NOT necessary

References Crook, M. A. , Hally, V. , & Panteli, J. V. (2001). The importance of the refeeding syndrome. Nutrition (Burbank, Los Angeles County, Calif. ), 17(7 -8), 632 -637. De Silva, A. , Smith, T. , & Stroud, M. (2008). Attitudes to NICE guidance on refeeding syndrome. BMJ (Clinical Research Ed. ), 337, a 680. Escott-Stump, S. (2008). Nutrition and diagnosis-related care: sixth ed. (Baltimore, Maryland), 578580. Fan, C. , Li, J. (2003). Refeeding syndrome in patients with gastrointestinal fistula. Nutrition (Burbank, Los Angeles County, Calif. ), 24(6), 604 -606. Gariballa, S. (2008). Refeeding syndrome: A potentially fatal condition but remains underdiagnosed and undertreated. Nutrition, 24(6), 604 -606. Khardori, R. (2005). Refeeding syndrome and hypophosphatemia. Journal of Intensive Care Medicine, 20(3), 174 -175. Mehanna, H. M. , Moledina, J. , & Travis, J. (2008). Refeeding syndrome: What it is, and how to prevent and treat it. BMJ (Clinical Research Ed. ), 336(7659), 1495 -1498. Nelms, M. , Sucher, K. , & Long, S. (2007). Nutrition therapy and pathophysiology (Belmont, Calif. ). 166 -167, 194 -195. Walker, R. (2006). Alcohol and the liver. Sports Line, 28(6), 21 -22. Yantis, M. A. , & Velander, R. (2008). How to recognize and respond to refeeding syndrome. Nursing, 38(5).
 Refeeding syndrome electrolytes
Refeeding syndrome electrolytes Refeeding syndrome electrolytes
Refeeding syndrome electrolytes Refeeding syndrome
Refeeding syndrome Freddy santoso
Freddy santoso Hammerling experiment
Hammerling experiment Cariotipo masculino normal
Cariotipo masculino normal Medical nutrition therapy for stroke
Medical nutrition therapy for stroke Medical nutrition therapy for hypertension
Medical nutrition therapy for hypertension Small bowel obstruction
Small bowel obstruction Anne pohju
Anne pohju Nutrition fundamentals of nursing
Nutrition fundamentals of nursing Psychodynamic and humanistic therapies have in common
Psychodynamic and humanistic therapies have in common Bioness integrated therapy system price
Bioness integrated therapy system price What are the major humanistic therapies
What are the major humanistic therapies Medical family therapy
Medical family therapy Iso 22301 utbildning
Iso 22301 utbildning Typiska drag för en novell
Typiska drag för en novell Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Ekologiskt fotavtryck
Ekologiskt fotavtryck Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Adressändring ideell förening
Adressändring ideell förening Vilotidsbok
Vilotidsbok Anatomi organ reproduksi
Anatomi organ reproduksi Densitet vatten
Densitet vatten Datorkunskap för nybörjare
Datorkunskap för nybörjare Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Debattinlägg mall
Debattinlägg mall Autokratiskt ledarskap
Autokratiskt ledarskap Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Arkimedes princip formel
Arkimedes princip formel Offentlig förvaltning
Offentlig förvaltning Kyssande vind
Kyssande vind























































