Making the Implicit Explicit in the Teaching of



![Fading Path 3 S Determine target [A]/[HA] Determine target PH Path 2 Path 1 Fading Path 3 S Determine target [A]/[HA] Determine target PH Path 2 Path 1](https://slidetodoc.com/presentation_image/c85e0936306357b56df5dc7967244ee8/image-7.jpg)


- Slides: 37

Making the Implicit Explicit in the Teaching of Chemical Equilibrium David Yaron, Michael Karabinos, Jodi Davenport, Jordi Cuadros Department of Chemistry, Carnegie Mellon University Gaea Leinhardt, Jim Greeno, Karen Evans Learning Research and Development Center, University of Pittsburgh GRC 2007 http: //www. chemcollective. org 1

Overview of Projects • Chemcollective (www. chemcollective. org) – NSF CCLI and NSDL – Digital library of virtual labs and scenario based learning activities – Tutors and supported problem solving – Community building and support • Open Learning Initiative (www. cmu. edu/oli) – William and Flora Hewlett Foundation – Full enactment of instruction (based on chemcollective activities) • Pittsburgh Science of Learning Center (www. learnlab. org) – NSF SLC http: //www. chemcollective. org – Fundamental studies to advance theory of learning GRC 2007 2

Overview Analysis of the domain Initial problem analysis and selection of procedure Supporting Implementation of computation practicethe nature of practice or procedure Changing Reflection on problem solving efforts GRC 2007 Use technology to provide hints and feedback. http: //www. chemcollective. org 3

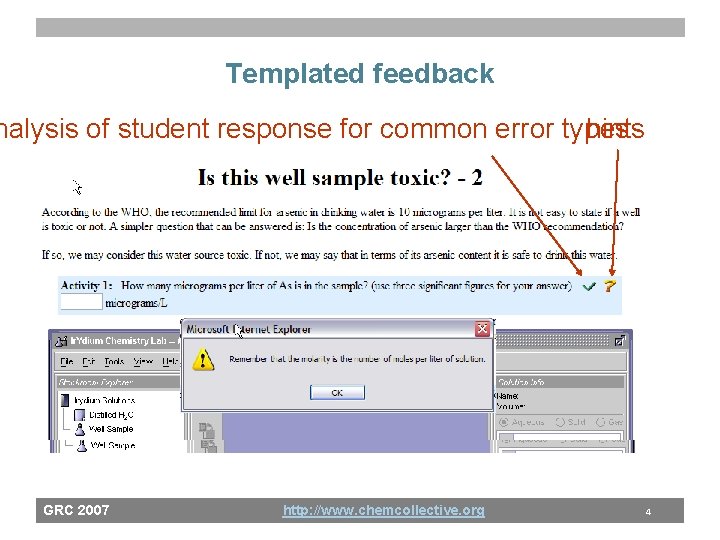
Templated feedback nalysis of student response for common error types hints GRC 2007 http: //www. chemcollective. org 4

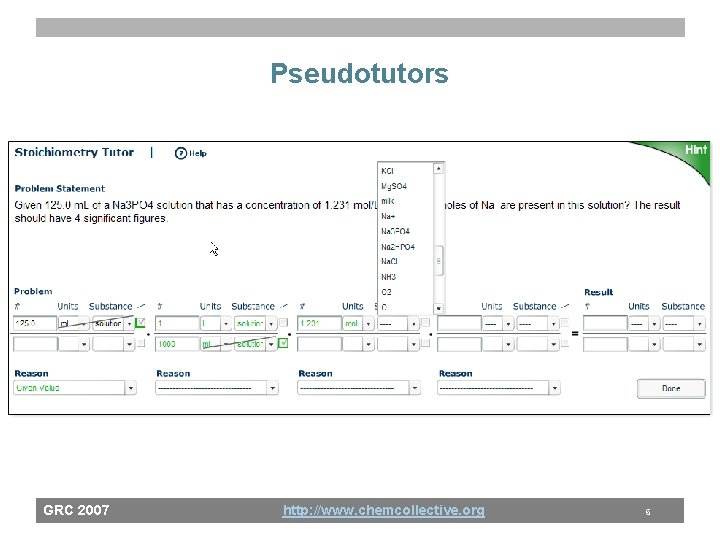
Pseudotutors GRC 2007 http: //www. chemcollective. org 5

Pseudotutors GRC 2007 http: //www. chemcollective. org 6
![Fading Path 3 S Determine target AHA Determine target PH Path 2 Path 1 Fading Path 3 S Determine target [A]/[HA] Determine target PH Path 2 Path 1](https://slidetodoc.com/presentation_image/c85e0936306357b56df5dc7967244ee8/image-7.jpg)
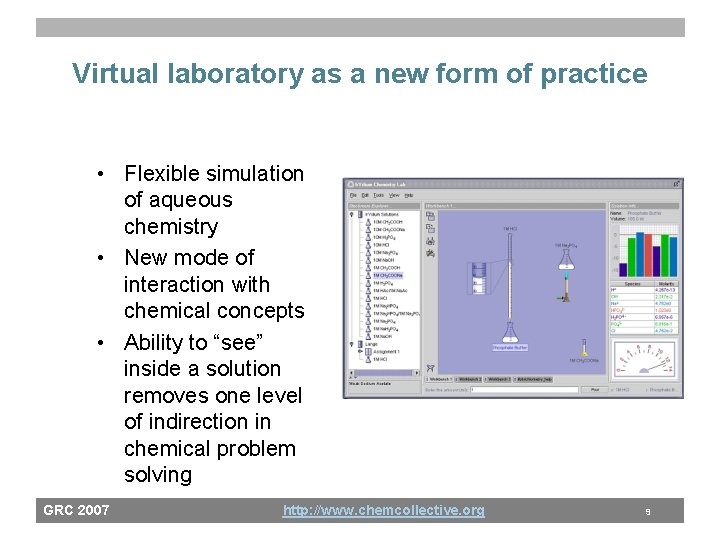
Fading Path 3 S Determine target [A]/[HA] Determine target PH Path 2 Path 1 Construct solution with target [A]/[HA] F Determine solutions and volumes mixed. Schematic representation of scaffolding for design of a buffer solution. Ovals represent episodes (pseudotutors or templated feedback). Support is added/faded by switching paths. GRC 2007 http: //www. chemcollective. org 7

Overview Initial problem analysis and selection of procedure Implementation of computation or procedure Reflection on problem solving efforts GRC 2007 Can the problem solving be more connected to underlying chemical concepts. Goal should be fluency with concepts, not procedures. Use technology to fundamentally change the nature of practice. http: //www. chemcollective. org 8

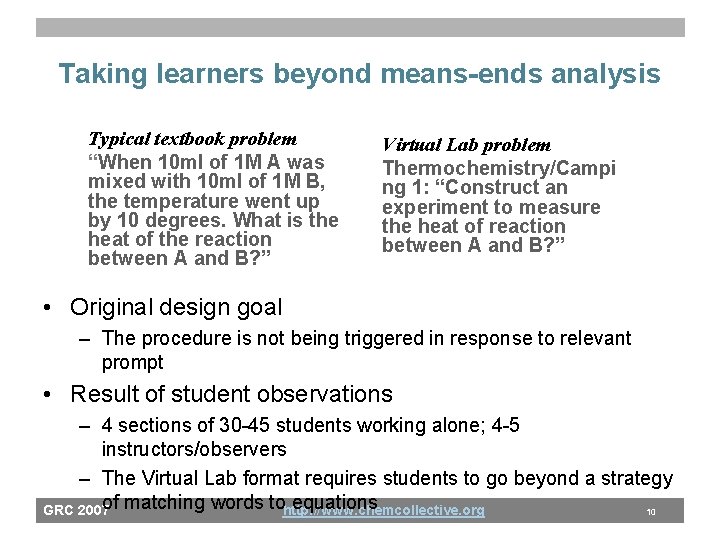
Virtual laboratory as a new form of practice • Flexible simulation of aqueous chemistry • New mode of interaction with chemical concepts • Ability to “see” inside a solution removes one level of indirection in chemical problem solving GRC 2007 http: //www. chemcollective. org 9

Taking learners beyond means-ends analysis Typical textbook problem “When 10 ml of 1 M A was mixed with 10 ml of 1 M B, the temperature went up by 10 degrees. What is the heat of the reaction between A and B? ” Virtual Lab problem Thermochemistry/Campi ng 1: “Construct an experiment to measure the heat of reaction between A and B? ” • Original design goal – The procedure is not being triggered in response to relevant prompt • Result of student observations – 4 sections of 30 -45 students working alone; 4 -5 instructors/observers – The Virtual Lab format requires students to go beyond a strategy of matching words tohttp: //www. chemcollective. org equations GRC 2007 10

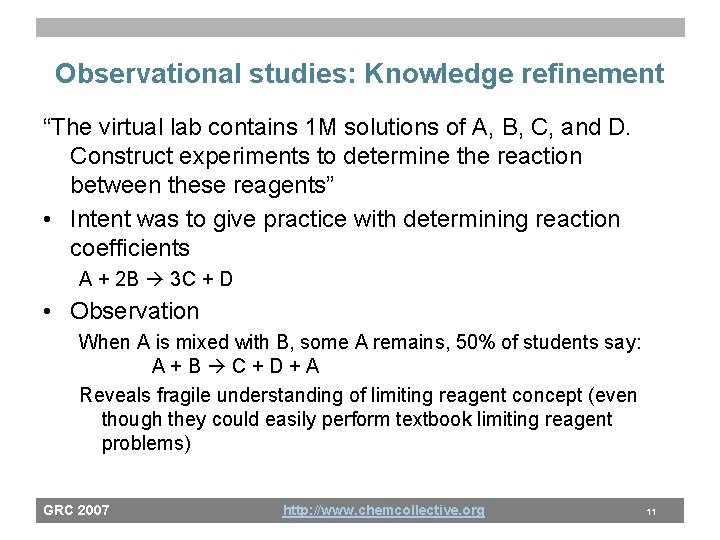
Observational studies: Knowledge refinement “The virtual lab contains 1 M solutions of A, B, C, and D. Construct experiments to determine the reaction between these reagents” • Intent was to give practice with determining reaction coefficients A + 2 B 3 C + D • Observation When A is mixed with B, some A remains, 50% of students say: A+B C+D+A Reveals fragile understanding of limiting reagent concept (even though they could easily perform textbook limiting reagent problems) GRC 2007 http: //www. chemcollective. org 11

Learning in a large lecture course • Study at Carnegie Mellon – Second semester intro course, 150 students • Information used – Pretest – 9 homework activities (virtual labs with templated feedback) – 3 hour exams – 2 pop exams (practice exam given 5 days before hour exam) – Final exam GRC 2007 http: //www. chemcollective. org 12

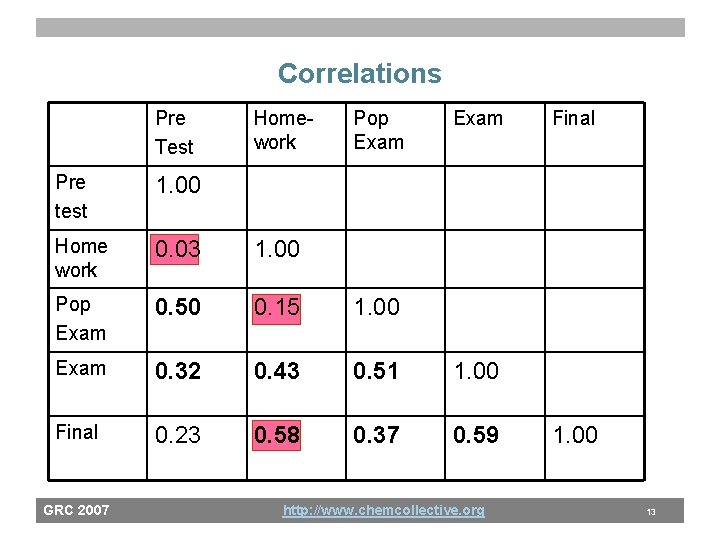
Correlations Pre Test Homework Pop Exam Pre test 1. 00 Home work 0. 03 1. 00 Pop Exam 0. 50 0. 15 1. 00 Exam 0. 32 0. 43 0. 51 1. 00 Final 0. 23 0. 58 0. 37 0. 59 GRC 2007 http: //www. chemcollective. org Final 1. 00 13

Regression and structural equation model • Linear regression accounts for 48% of the variance in the final grades • Influence of homework accounts for half of the model predictions • Structural equation model supports conclusions drawn from the regression GRC 2007 http: //www. chemcollective. org 14

Assessment within online stoichiometry course • Study design – Treatment (20): Online course including a scenario, tutors and virtual lab homework – Control (20): Paper and pencil, worked examples and practice – Assessment was traditional problem solving of quantitative stoichiometry problems, and some qualitative questions • Preliminary results – Biggest predictor of learning in online course is number of engagements with the virtual lab GRC 2007 http: //www. chemcollective. org 15

Overview Initial problem analysis and selection of procedure Implementation of computation or procedure Reflection on problem solving efforts GRC 2007 What overall structure are we trying to convey? An important role we, as chemists, can play is reconceptualizing the domain, i. e. what should we teach, and how. Goal of a high AP score is different than goal of robust learning of chemical concepts. http: //www. chemcollective. org 16

Results from other domains • Expert blind spot – Ability to rank difficulty of math problems is worst for teachers of that subject • Geometry – Sub-goal structure of proofs was implicit knowledge (Anderson, Koedinger, Greeno. . ) • Statistics – Students could carry out statistical analysis procedures, but could not select appropriate procedures (Lovett) GRC 2007 http: //www. chemcollective. org 17

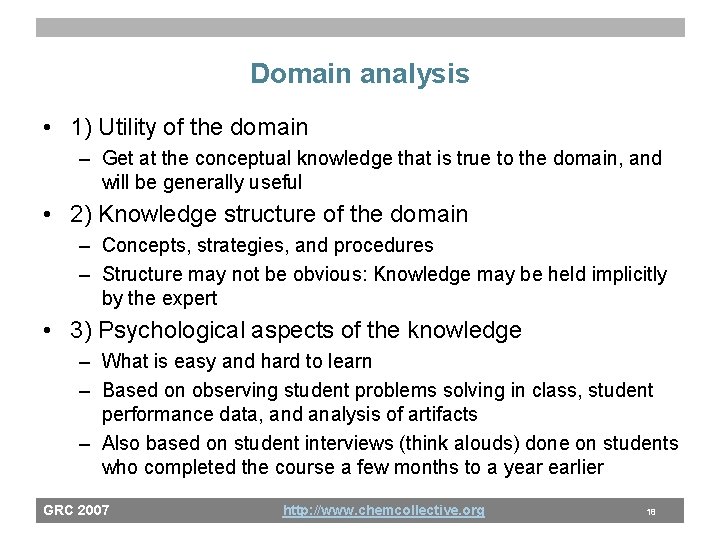
Domain analysis • 1) Utility of the domain – Get at the conceptual knowledge that is true to the domain, and will be generally useful • 2) Knowledge structure of the domain – Concepts, strategies, and procedures – Structure may not be obvious: Knowledge may be held implicitly by the expert • 3) Psychological aspects of the knowledge – What is easy and hard to learn – Based on observing student problems solving in class, student performance data, and analysis of artifacts – Also based on student interviews (think alouds) done on students who completed the course a few months to a year earlier GRC 2007 http: //www. chemcollective. org 18

Domain analysis for chemical literacy • Focused only on “Utility of the domain” • Standards should go beyond expert opinions of what to teach • Evidence of the domain as practiced – Nobel prizes for past 50 years (1952 -2002) – NY Times Science Times for 2002 (54 reports) – Scientific American News Bites for 2002 (32 reports) • Evidence of the domain as taught – CA state content standards – Best selling textbooks GRC 2007 http: //www. chemcollective. org 19

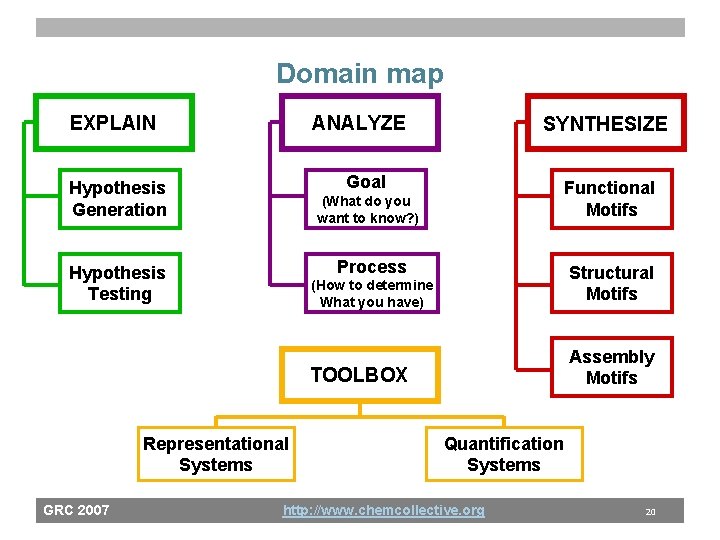
Domain map EXPLAIN ANALYZE Goal Hypothesis Generation Functional Motifs (What do you want to know? ) Process Hypothesis Testing Representational Systems GRC 2007 SYNTHESIZE (How to determine What you have) Structural Motifs TOOLBOX Assembly Motifs Quantification Systems http: //www. chemcollective. org 20

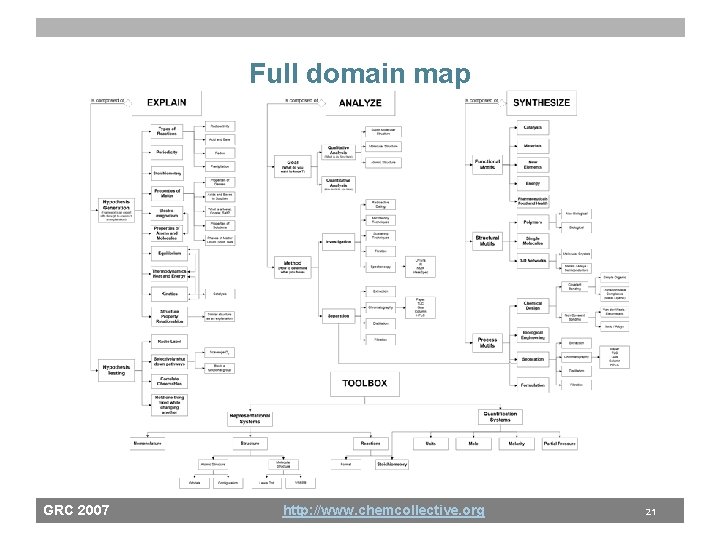
Full domain map GRC 2007 http: //www. chemcollective. org 21

Domain analysis Middle school through high school: Big concepts • Structure – Relation to properties • Functional groups • Emergent properties (bonding pattern molecular interactions - 3 d structure) • Transformation – Physical transformations and chemical reactions • Energy and motion – Heat – Molecular motion Materials themes: Water, gold and plastic GRC 2007 http: //www. chemcollective. org 22

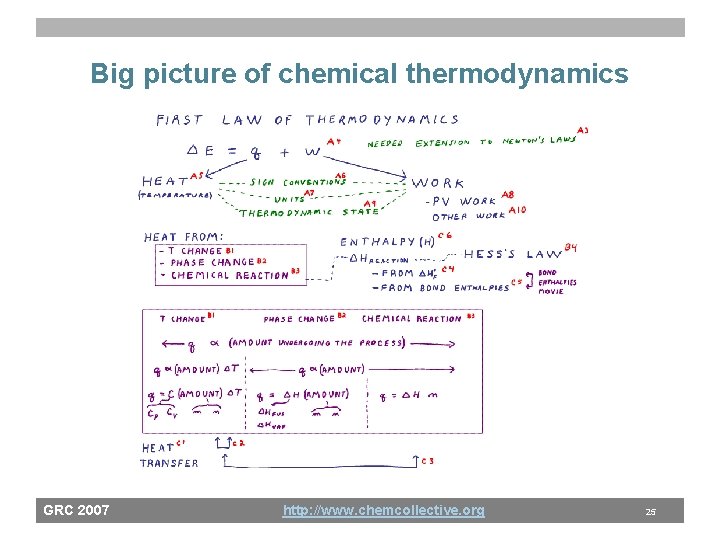
Domain analysis: Chemical thermodynamics 1) Utility of domain – Heat transfer and energy flow in systems is important – “Camping” scenario, of heating meals ready-to-eat 2) Knowledge structure of the domain – Heat flow from system 1 system 2 – Three processes that generate or absorb heat • Heat/cool • Phase change • Chemical reaction 3) Psychological aspects of the knowledge – Student observations suggest difficulty is correlated with “visibility” of the heat source/drain: Hardest is heat from a chemical reaction. GRC 2007 http: //www. chemcollective. org 23

Chemical thermodynamics instruction • Use a structured dialogue to expose a general strategy to solving heat-exchange problems. – Traditional instruction leaves this as “implicit knowledge” • Structured dialogue for heat exchange – What is the source of the heat? • How do you describe that effect: (q=m Cv DT, q=n DH, . . ) – What is the drain of the heat? • How do you describe that effect: (q=m Cv DT, q=n DH, . . ) GRC 2007 http: //www. chemcollective. org 24

Big picture of chemical thermodynamics GRC 2007 http: //www. chemcollective. org 25

Chemical equilibrium / Acid-base chemistry 1) Utility of the domain – How is this knowledge used in organic chemistry and molecular biology 1) Compare p. H to p. Ka to determine ionization state 2) Buffers used to control p. H (qualitative not quantitative) 3) Titration as an analytical technique – Current instruction 1: Almost a footnote (in the indicators section) 2 -3: Coverage may not be sufficiently qualitative GRC 2007 http: //www. chemcollective. org 26

Chemical equilibrium / Acid-base chemistry 2) Knowledge structure – Flexibility with “progress of reaction” is required in problem analysis – General strategy can be constructed based on • First, determine concentration of “majority species” • Second, determine concentration of “minority species” 3) Psychological aspects of the knowledge – Le. Chatlier (especially with addition/removal of a species) is most retained concept – Broad confusion regarding “progress of reaction” • Q vs. K • Meaning of “initial” vs. “equilibrium” state GRC 2007 http: //www. chemcollective. org 27

Some features of the instruction • Sequencing – Le. Chatlier’s principle plays role of “prior knowledge” – Human respiration is scenario to which to attach “initial” vs. “equilibrium” state • Blood entering lungs and muscles experiences a new initial state • Blood leaving lungs and muscles has reached new equilibrium • Progress of Reaction – Concept of progress of reaction (and Q) introduced before K – Visualizations used • General strategy for equilibrium problem analysis http: //www. chemcollective. org – Majority vs. Minority Species GRC 2007 28

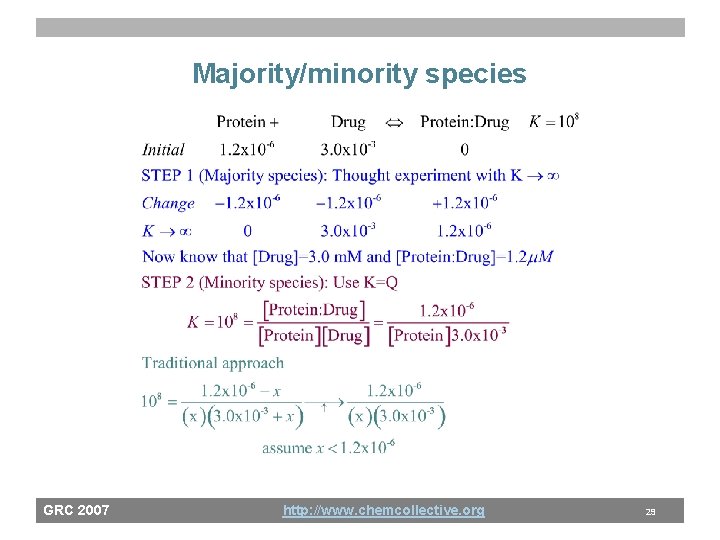
Majority/minority species GRC 2007 http: //www. chemcollective. org 29

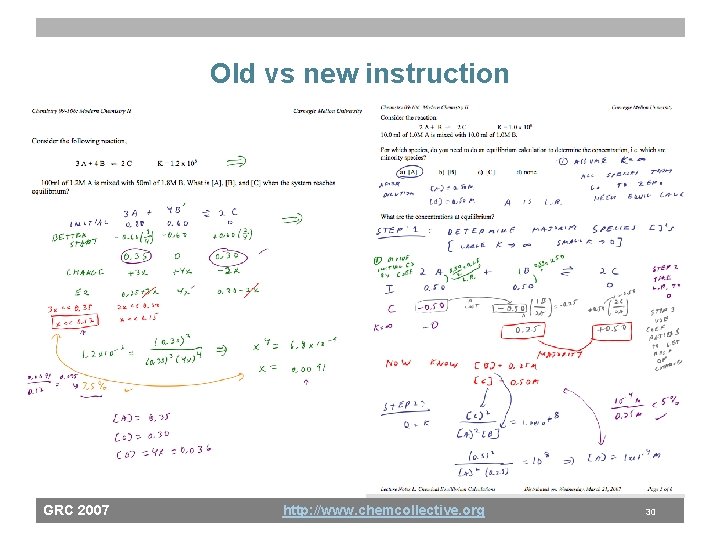
Old vs new instruction GRC 2007 http: //www. chemcollective. org 30

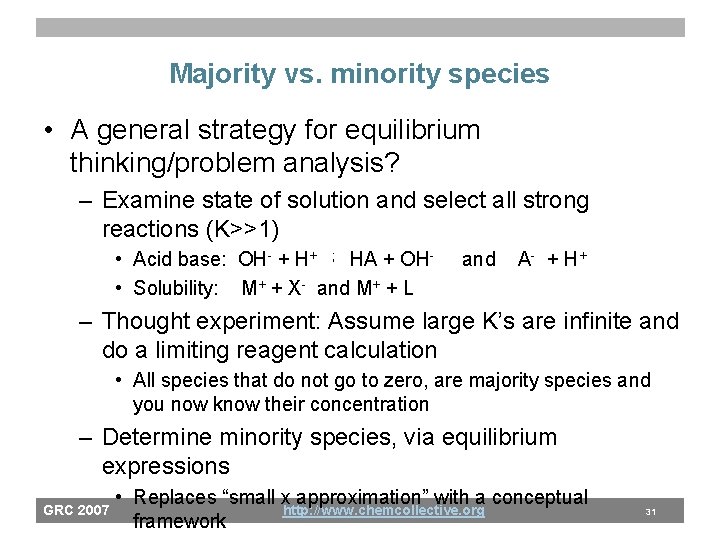
Majority vs. minority species • A general strategy for equilibrium thinking/problem analysis? – Examine state of solution and select all strong reactions (K>>1) • Acid base: OH- + H+ ; HA + OH • Solubility: M+ + X- and M+ + L and A - + H+ – Thought experiment: Assume large K’s are infinite and do a limiting reagent calculation • All species that do not go to zero, are majority species and you now know their concentration – Determine minority species, via equilibrium expressions GRC 2007 • Replaces “small x approximation” with a conceptual http: //www. chemcollective. org framework 31

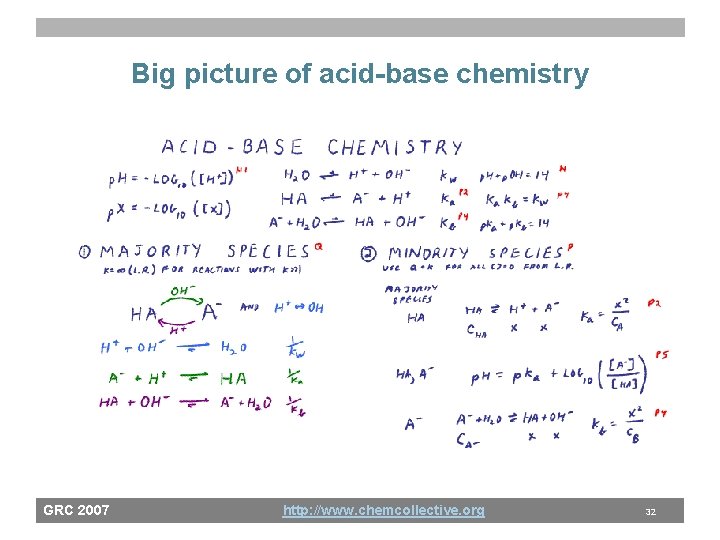
Big picture of acid-base chemistry GRC 2007 http: //www. chemcollective. org 32

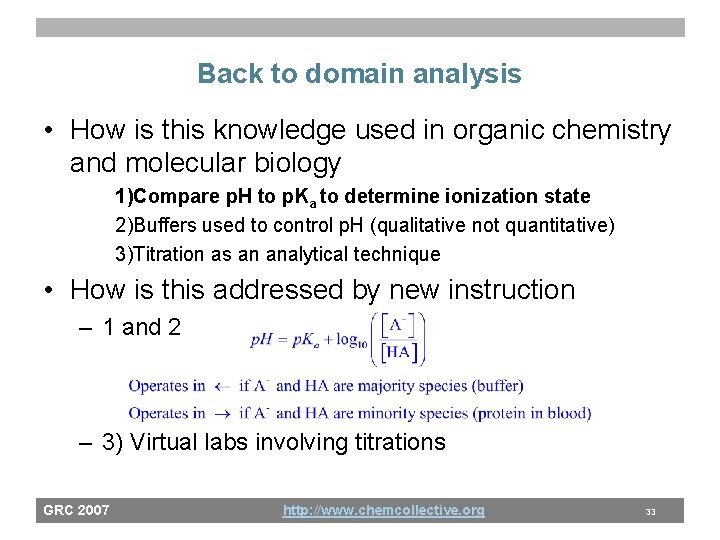
Back to domain analysis • How is this knowledge used in organic chemistry and molecular biology 1)Compare p. H to p. Ka to determine ionization state 2)Buffers used to control p. H (qualitative not quantitative) 3)Titration as an analytical technique • How is this addressed by new instruction – 1 and 2 – 3) Virtual labs involving titrations GRC 2007 http: //www. chemcollective. org 33

Development status • Stoichiometry – Full set of tutorials and supported problems (virtual lab and tutors released on Chem. Collective and OLI) • Thermochemistry – Supported problems, based on structured dialogues (virtual labs and tutors): Fully tested and in process of release. • Equilibrium/Acid-Base – Supported problems on buffer design and mechanism (with fading): Fully tested. – Combined instruction/supported problems implementing new strategy: In final development, most has been tested. GRC 2007 http: //www. chemcollective. org 34

Research status • Study of the factors influencing learning in large chemistry classrooms (J. Chem. Ed. , in press) – Online homework activities contribute substantially to learning – Benefits are not correlated with pre-test • Controlled study of online stoichiometry course – Karen Evans’ thesis to be defended this summer, replicate in next academic year – Virtual lab engagement strongest predictor of learning in the course • Expert/novice comparison of problem solving in acid-base chemistry (see Davenport poster) – Results influenced instructional design described here. • Controlled studies of new instructional approaches (see Davenport poster) – Majority/minority instruction improves performance on 2 A+3 B 4 C K = 1. 4 x 10 10 From 22% to 58% correct. (Finer grained analysis underway. ) – Studies on full instructional modules being analyzed, and further studies GRC 2007 http: //www. chemcollective. org 35 planned.

Discussion points • How different is majority/minority strategy from traditional instruction? • What aspects of the chemistry domain most need to be re-conceptualized? • Should we shift emphasis in freshman course towards literacy? GRC 2007 http: //www. chemcollective. org 36

Thanks To • Erin Fried • Jason Chalecki Michael Karabinos • Greg Hamlin Jodi Davenport • Brendt Thomas Donovan Lange • Stephen Ulrich D. Jeff Milton • Jason Mc. Kesson Jordi Cuadros • Aaron Rockoff Rea Freeland • Jon Sung Emma Rehm • Jean Vettel William Mc. Cue • Rohith Ashok David H. Dennis • Joshua Horan Tim Palucka LRDC, University Jef Guarent • Gaea Leinhardt Amani Ahmed • Jim Greeno Giancarlo Dozzi • Karen Evans Katie Chang • Baohui Zhang Carnegie Mellon Funding • • • • GRC 2007 • • NSF: CCLI, NSDL, SLC William and Flora Hewlett Foundation Howard Hughes Medical Institute Dreyfus Foundation of Pittsburgh http: //www. chemcollective. org 37