LIMIT TEST Dr MEENU Assistant Professor Shri Guru























- Slides: 34

LIMIT TEST Dr. MEENU Assistant Professor, Shri Guru Ram Rai Institute of Technology & Sciences 2016

Impurities • • Definition: A compound is said to be impure if it is having foreign matter, i. e. impurities. Chemical purity implies freedom from foreign matter. It means that a pure chemical refers to that compound which is having no foreign matter, i. e. impurities. Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Source of impurities: 1. 2. 3. 4. 5. 6. 7. 8. 9. Raw material employed in manufacturing Reagent used in manufacturing process Method or process used in Chemical method used in manufacture Atmospheric contamination during manufacturing process Defect in manufacturing process Manufacturing hazards Storage conditions Accidental substitution with spurious or useless materials Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Pharmacopoeia • • • A description of the article Tests for identity Physical constants Quantitative assay Limit test Storage conditions official = obey the requirement Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Pharmacopoeia • British pharmacopoeia (BP) • European pharmacopoeia (Eu. Ph) • United state pharmacopoeia (USP) Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Limit test • They are quantitative or semi-quantitative test designed to identify or control small quantities of impurity , these test should be specific and sensitive Nessler cylinder Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Limit test Type: 1. Comparison method 2. Quantitative determination 3. Test in which there is no visible reaction Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Limit test General principle • If the sample is lighter than the standard solution then it is within the pharmacopeial limit (accepted) • If the sample is darker/heavier than the standard solution then it is above the pharmacopeial limit (rejected) Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Limit test NESSLER cylinder ( appendix VII A 127) clear glasses with normal capacity 50 ml, the overall height is about 15 cm, the external height to the 50 ml mark 11. 0 to 12. 4 cm , the thickness of the wall 1. 0 to 1. 5 mm and the thickness of the base 1. 0 to 3. 0 mm the external heights to the 50 mark of cylinders used to test must not differ by more than 1 mm Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Limit test General precaution 1. The liquid used must be clean and filtered if necessary 2. The Nessler cylinder must be made of colorless glass and of the same inner diameter 3. Detecting opalescence or color development must be performed in daylight 4. When comparing turbidity it should be done against black background 5. When comparing color it should be done against white background Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Limit test Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

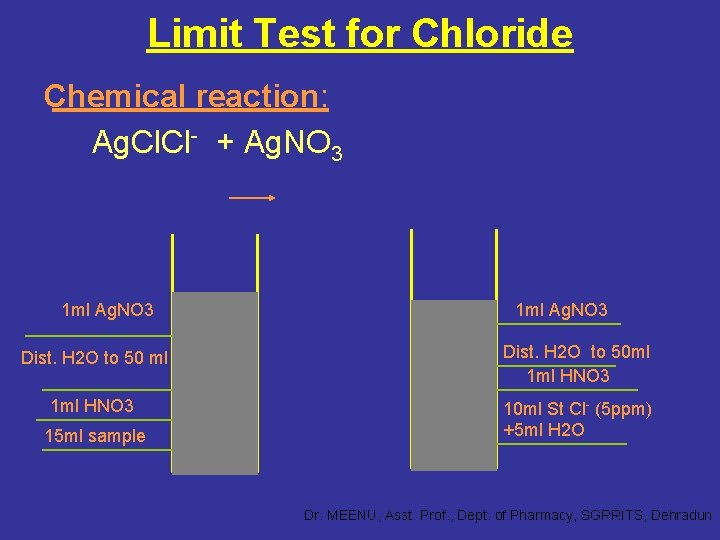
Limit Test for Chloride Chemical reaction: Ag. Cl- + Ag. NO 3 1 ml Ag. NO 3 Dist. H 2 O to 50 ml Dist. H 2 O to 50 ml 1 ml HNO 3 10 ml St Cl- (5 ppm) +5 ml H 2 O 15 ml sample Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Principle: • This test, which is mainly used to control chloride impurity in inorganic substance, depends upon the simple reaction between silver nitrate and soluble chlorides to give insoluble silver chloride in the presence of dilute nitric acid. • The insoluble silver chloride makes the solution opalescent and the extent of opalescence depending upon the amount of chloride present in the substance, is compared with a standard opalescence produced in a standard solution having a known amount of chloride by adding silver nitrate and same volume of dilute nitric acid as used in the test solution. Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

• If the opalescence produced in the test is less intense than that of standard opalescence, the sample passes the limit test for chloride and vice versa. Note: Dilute nitric acid in used to prevent the precipitation of other acid radicals with silver nitrate solution. It acts by providing common i. e. nitrate. Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

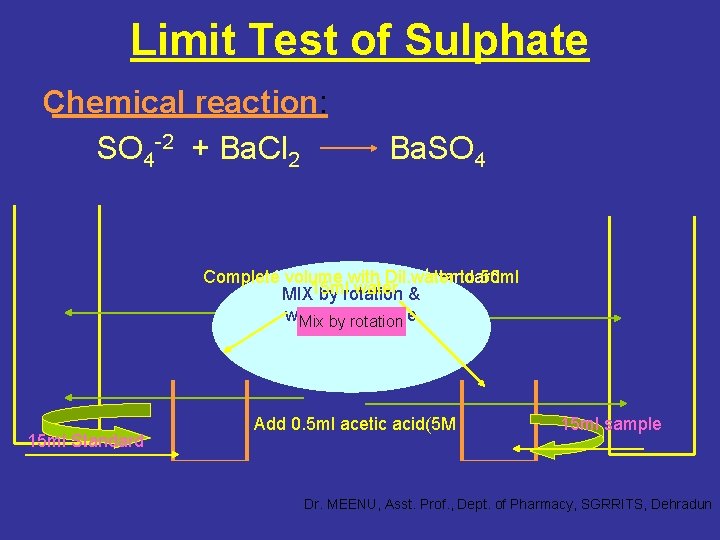
Limit Test of Sulphate Chemical reaction: SO 4 -2 + Ba. Cl 2 Ba. SO 4 Complete 1. 5 ml volume ethanolic with Dil. water SO 4 standard to 50 ml 15 ml water MIX by rotation & wait minute Mixfor by 1 rotation 1 ml 25%Ba. Cl 2 15 ml Standard Add 0. 5 ml acetic acid(5 M 15 ml sample Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Principle: • It is based upon the reaction between barium chloride and soluble sulphate in presence of dil. Hydrocloric acid. • The comparison of turbidity is done with the standard turbidity obtained from a known amount of sulphate and same volume of dilute HCl in the both solutions. • In I. P. barium chloride solution is replaced with barium chloride reagent to enhance the reactivity of test. Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

• The opalescence produced in the test is compared with that of standard opalescence obtained from a standard sulphate solution containing known amount of sulphate produced in the same manner. • If the test opalescence is less intense than that of standard opalescence, the sample passes the limit test for sulphate and vice versa. Note: The solubilities of barium sulphate precipitates are very much affected by the concentration of the acid. The acidity of the solution is controlled by using acetic acid. Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

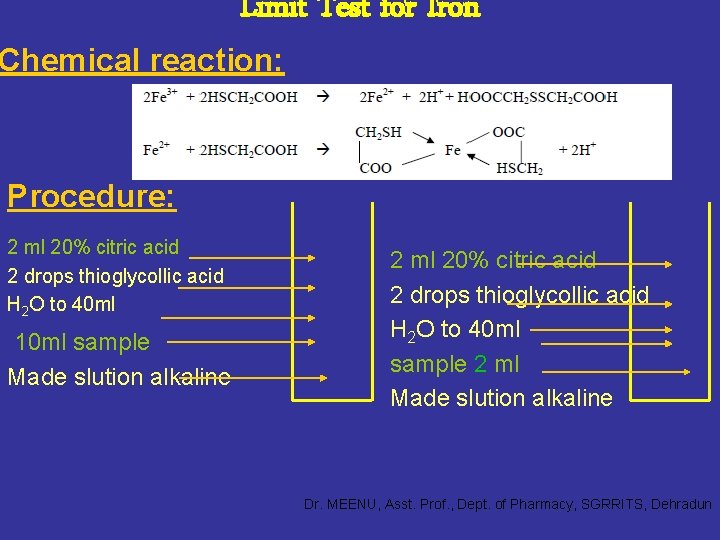
Limit Test for Iron Chemical reaction: Procedure: 2 ml 20% citric acid 2 drops thioglycollic acid H 2 O to 40 ml 10 ml sample Made slution alkaline 2 ml 20% citric acid 2 drops thioglycollic acid H 2 O to 40 ml sample 2 ml Made slution alkaline Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Principle: • The limit test for iron is based on the formation of pale pink to deep reddish purple colour by reaction of iron with thioglycollic acid in the presence of citric acid in a solution made alkaline with ammonia solution. • The colour is due to the formation of the coordination compound, ferrous thioglycolate Fe(HSCH 2 COO)2. • The original state of oxidation of the iron is immaterial since thioglycollic acid also reduces ferric iron to the ferrous state. Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

The colour produced is compared with the standard colour containing a definite quantity of iron by viewing transversely through the Nessler cylinders. NOTE: Citric acid is added in the official process to prevent the precipitation of iron by ammonia. Iron gets precipitated in the form of Fe(OH)2 and Fe(OH)3 depending upon the oxidation state of the iron in the presence of ammonia. • Citric acid makes soluble complex with iron and ammonia will not be able precipitate iron and it will just provide alkaline medium. Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

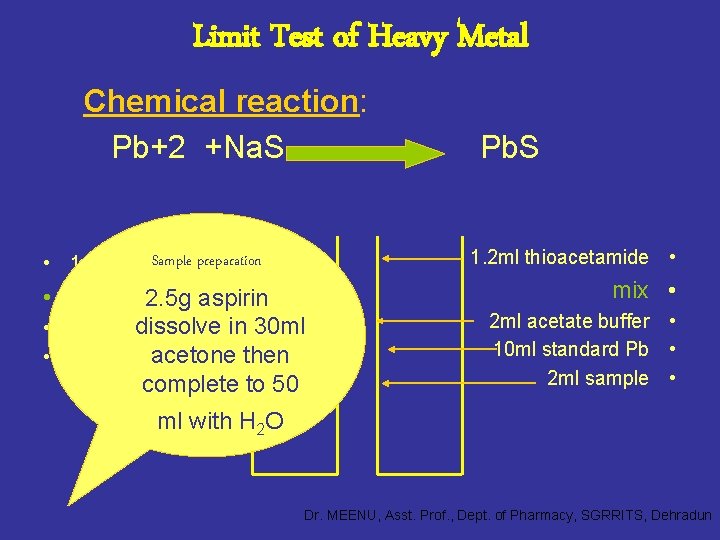
Limit Test of Heavy Metal Chemical reaction: Pb+2 +Na. S 1. 2 ml thioacetamide • Sample preparation • 1. 2 ml thioacetamide • mix Pb. S 2. 5 g aspirin dissolve • 2 ml acetate bufferin 30 ml acetone then • 12 ml sample complete to 50 mix • 2 ml acetate buffer • 10 ml standard Pb • 2 ml sample • ml with H 2 O Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Principle: • Method A, B and C are based upon the reaction of the heavy metal ion with hydrogen sulphide (in method A and B) or sodium sulphide (in method C) leading to the formation of heavy metal sulphides. • The metal sulphides remain distributed in a colloidal state and give rise to a brownish colouration. • The colour produced in the test solution is compared with that of standard solution containing a definite amount of the lead. Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

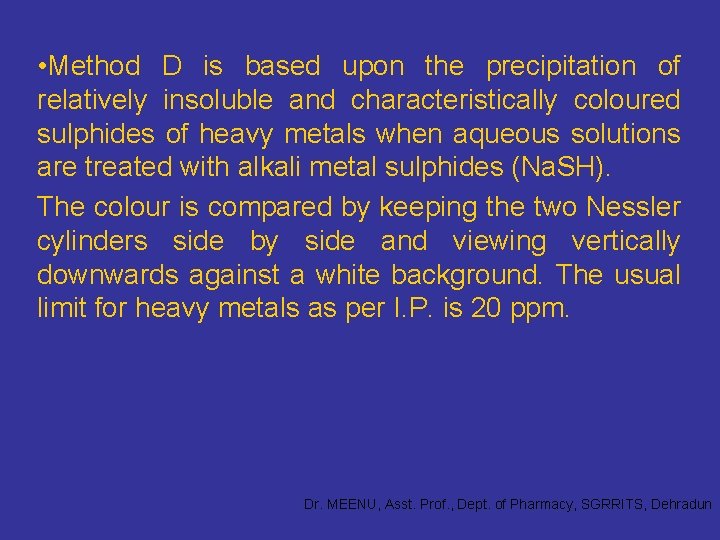
• Method D is based upon the precipitation of relatively insoluble and characteristically coloured sulphides of heavy metals when aqueous solutions are treated with alkali metal sulphides (Na. SH). The colour is compared by keeping the two Nessler cylinders side by side and viewing vertically downwards against a white background. The usual limit for heavy metals as per I. P. is 20 ppm. Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

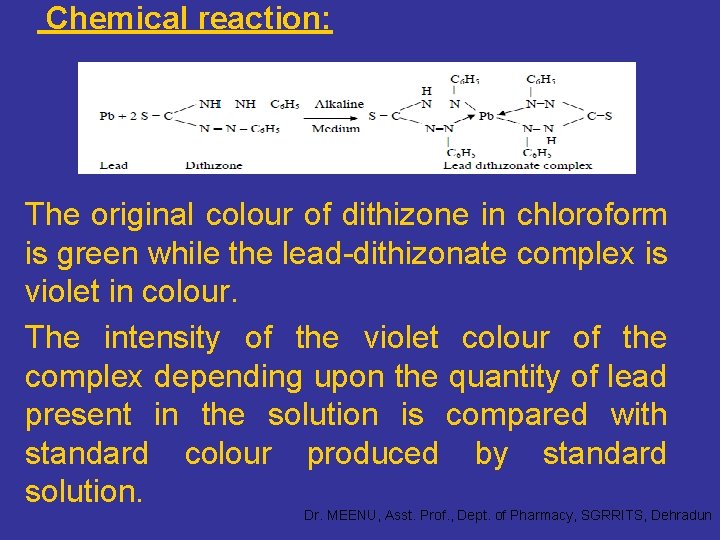
Limit Test of Lead Principle: The limit test for lead is based on the reaction between lead and diphenylthiocarbazone (dithizone) in an alkaline medium to form lead– dithizonate complex. • The lead present as an impurity in the substance is separated by extracting an alkaline solution with dithizone extraction solution. • The interference by other metal ions is eliminated by adjusting the optimum p. H for the extraction by using reagents like ammonium citrate, potassium cyanide and hydroxylamine hydrochloride. Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Chemical reaction: The original colour of dithizone in chloroform is green while the lead-dithizonate complex is violet in colour. The intensity of the violet colour of the complex depending upon the quantity of lead present in the solution is compared with standard colour produced by standard solution. Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

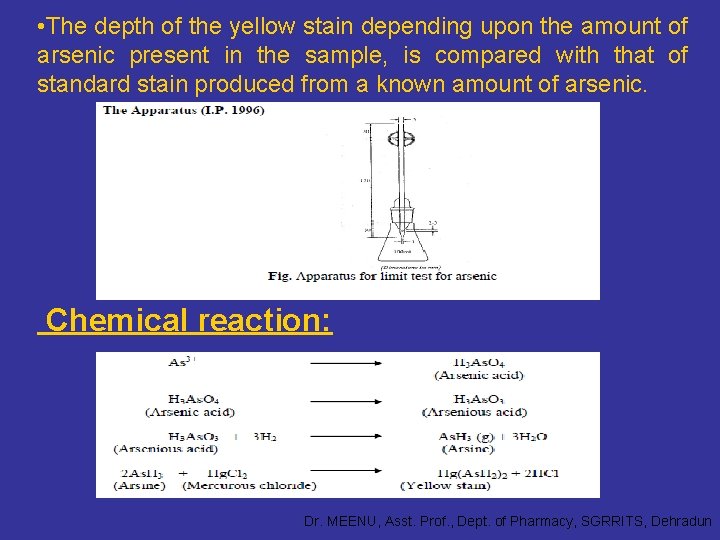
Limit Test of Arsenic Principle: The limit test for arsenic is based on the reduction of the arsenic in the arsenious state to the arsine gas (As. H 3) with zinc and hydrochloric acid. The arsine gas stains the mercuric chloride paper yellow. The sample is dissolved in acid whereby the arsenic present as impurity in the sample gets converted to arsenic acid. The arsenic acid is reduced to arsenious acid by reducing agents like stannous acid, potassium iodine etc. The nascent hydrogen formed during the reaction further reduced arsenious acid to the arsine gas. The arsine gas reacts with mercuric chloride paper to produce a yellow stain. Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

• The depth of the yellow stain depending upon the amount of arsenic present in the sample, is compared with that of standard stain produced from a known amount of arsenic. Chemical reaction: Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Non Aqueous Acid-Base Titration Main reasons for non aqueous titration : • Solubility: many organic compound (acid or base) sparingly soluble in water but readily soluble in organic solvent • Too weak acid or base : they didn’t give sharp end point in aqueous titration but can titrated accurately in suitable non aqueous solvent Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

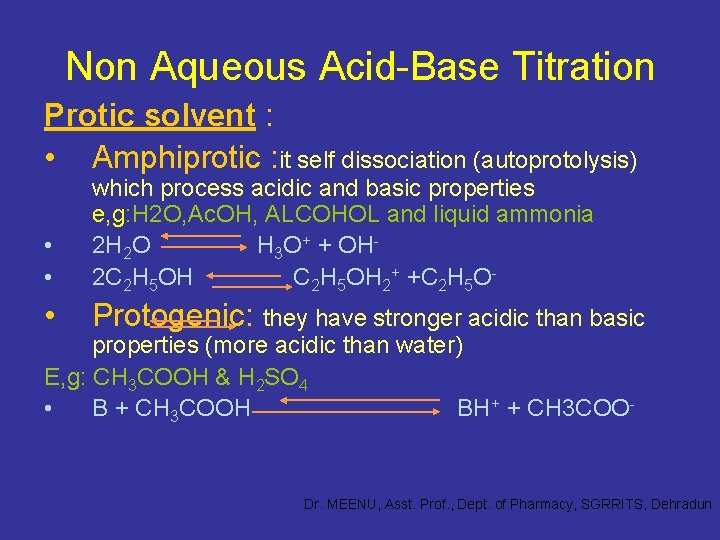
Non Aqueous Acid-Base Titration Protic solvent : • Amphiprotic : it self dissociation (autoprotolysis) • • which process acidic and basic properties e, g: H 2 O, Ac. OH, ALCOHOL and liquid ammonia 2 H 2 O H 3 O+ + OH 2 C 2 H 5 OH 2+ +C 2 H 5 O- • Protogenic: they have stronger acidic than basic properties (more acidic than water) E, g: CH 3 COOH & H 2 SO 4 • B + CH 3 COOH BH+ + CH 3 COO- Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

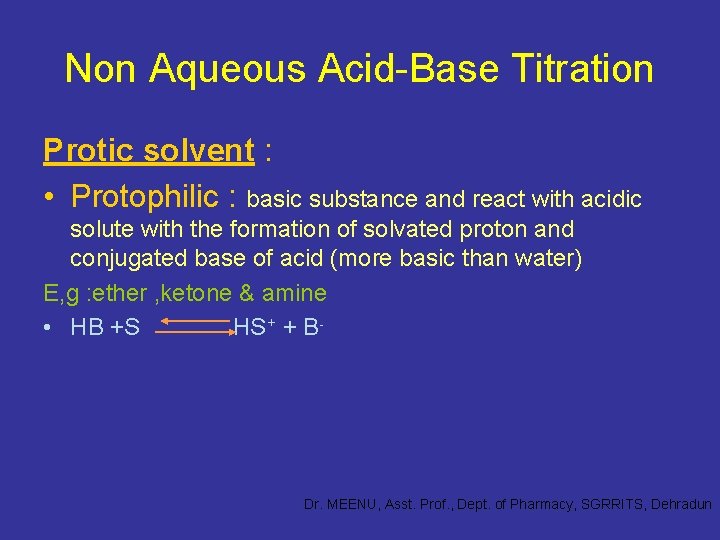
Non Aqueous Acid-Base Titration Protic solvent : • Protophilic : basic substance and react with acidic solute with the formation of solvated proton and conjugated base of acid (more basic than water) E, g : ether , ketone & amine • HB +S HS+ + B- Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Non Aqueous Acid-Base Titration Advantage of These Titrant • Excellent potentiometric curve using ordinary glass or calomel electrode • The salt formed from this titration are soluble in the solvent commonly used Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Safety • Crystal violet may cause cancer. Severe eye irritant. Harmful by inhalation, ingestion and through skin contact. • Perchloric acid It is very corrosive to skin and eyes, It can also ignite or explode upon contact with organic material such as cloth or wood also Causes hypothyroidism digestive and respiratory tract burns& it is Corrosive to metal • glacial acetic acid This material is strongly corrosive and causes serious burns. Very harmful if swallowed. Lachrymator Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

References: 1. Indian Pharmacopoeia, 1996, Vol. I & II. 2. United States Pharmacopoeia, USP 29 and National Formulary, NF 24, Asian ed. , 2006 3. A. H. Beckett, & J. B. Stenlake, Practical Pharmaceutical Chemistry, 5 th ed. , Part One, CBS Publishers and Distributors, 2004 4. L. M. Atherden, Bentley and Driver’s Textbook of Pharmaceutical Chemistry, 8 th ed. , Oxford University Press, 2005 5. Holleman-Wiberg, Inorganic Chemistry, Academic Press, 2001 6. J. D. Lee, Concise Inorganic Chemistry, 5 th ed. , Blackwell Publishing, 2006 7. Remington’s Pharmaceutical Sciences, Indian ed. , 21 st ed. , Vol. I & Vol. – II, Mack Publishing Co. 8. J. H. Block, E. Roche, T. O. Soine, & C. O. Wilson, Inorganic Medicinal and Pharmaceutical Chemistry, Lea and Febiger. 9. F. A. Cotton, & G. , Wilkinson, Basic Inorganic Chemistry, Wiley 10. L. A. Discher, Modern Inorganic Pharmaceutical Chemistry. 11. Vogel, s qualitative inorganic analysis, revised by G. Svehla, 7 th ed. , Pearson Education Publisher Dr. MEENU, Asst. Prof. , Dept. of Pharmacy, SGRRITS, Dehradun

Thank you