Lewis Dot Structures EQ How are Lewis Dot










- Slides: 26

Lewis Dot Structures EQ: How are Lewis Dot Structures drawn to model the sharing or transfer of electrons in chemical compounds?

What will you be doing? ? ? � Draw a Lewis Dot Diagram for an element given its location on the periodic table. � Show cations and anions are formed during ionic bonding using Lewis Dot Diagrams for the elements involved.

Review � What � How � What is a valence electron? many valence electrons does sulfur have? is the octet rule? does sulfur need to do in order to achieve the octet rule?

Review � For the following: ◦ Determine the bond type ◦ State whether it’s an electrolyte or nonelectrolyte 1. Sodium and oxygen 2. Sulfur and fluorine 3. Carbon and nitrogen

Ionic vs. Covalent � What types of elements make up an ionic bond? � What types of elements make up a covalent bond?

Lewis Dot Structures � Model used to represent valence electrons in elements and compounds � Used to predict molecular shape � MUST FOLLOW HUND’S RULE � Define rule): Hund’s Rule (seats on a school bus

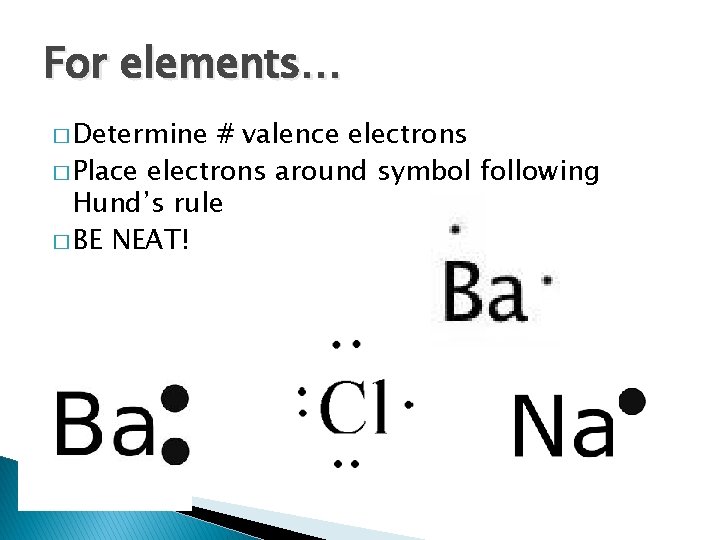
For elements… � Determine # valence electrons � Place electrons around symbol following Hund’s rule � BE NEAT!

Example � Sulfur � How many valence electrons?

You try! � Carbon � Boron � Argon

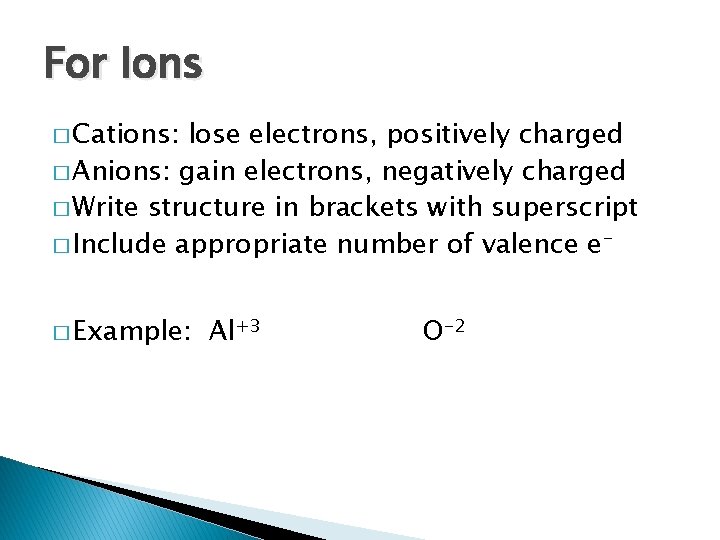
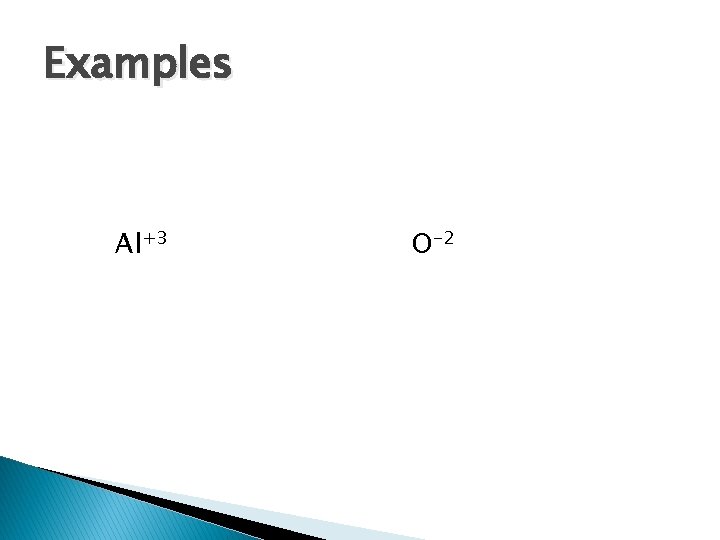
For Ions � Cations: lose electrons, positively charged � Anions: gain electrons, negatively charged � Write structure in brackets with superscript � Include appropriate number of valence e� Example: Al+3 O-2

Examples Al+3 O-2

You try! Ca 2+ Cl-

For Ionic Compounds � What type of elements? � Draw as ions in brackets � Place Opposite charges together to show ionic bond Example: Na. Cl Mg. Br 2

You try! Ca. O KI

PRACTICE! � COMPLETE PAGE THE WORKSHEET ON THE NEXT

Review � On the white boards…. � What is an ionic bond? � Give me 2 elements that would form an ionic bond

Review � What � Give is a covalent bond? me two elements that would form a covalent bond

REVIEW � What difference in electronegativity would make a bond polar covalent?

REVIEW � Draw � Is the Lewis Dot structure for Na. O 2 this compound an electrolyte or nonelectrolyte?

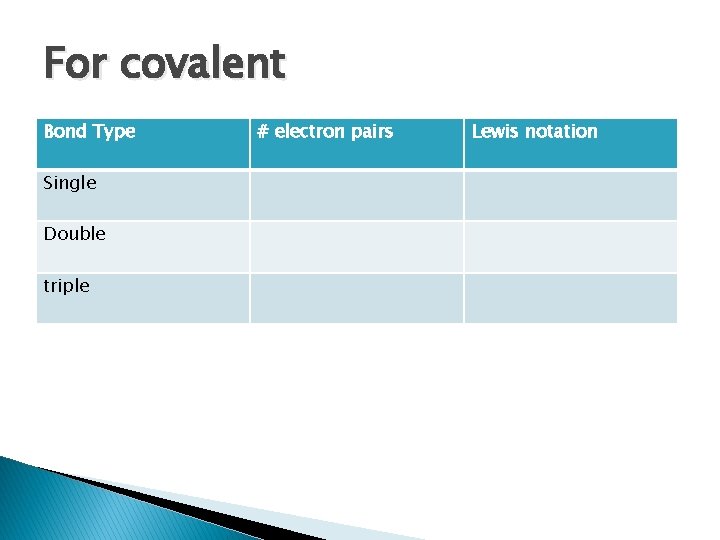
For covalent Bond Type Single Double triple # electron pairs Lewis notation

Steps for drawing Covalent Lewis Dot Structure � 1. add up the total # of valence electrons � 2. Put central atom in the middle, with other atoms connected with a single bond ◦ Remember, each line represents 2 electrons � 3. add remaining electrons to outer atoms, then central atoms. a. If not enough electrons, form double/triple bonds. b. If electrons are left over, place as lone pairs on central atom.

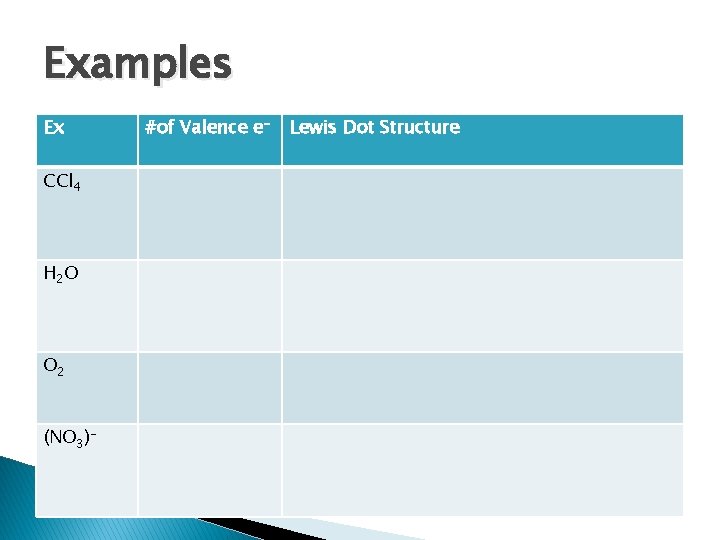
Examples Ex CCl 4 H 2 O O 2 (NO 3)- #of Valence e- Lewis Dot Structure

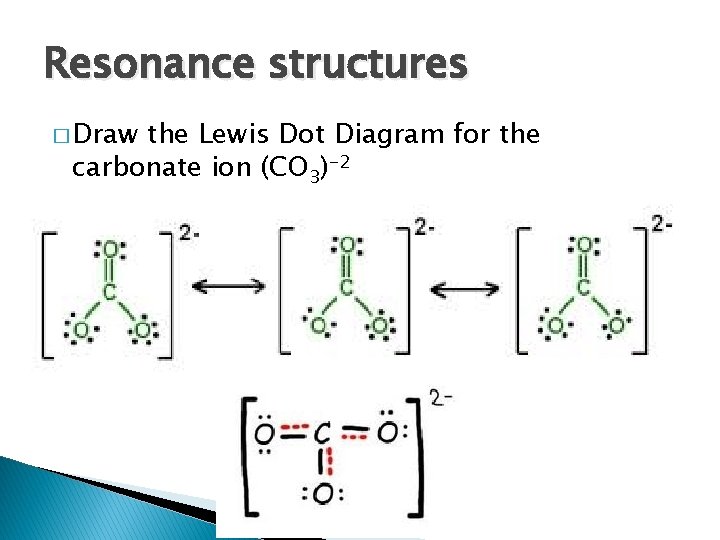
Resonance structures � Draw the Lewis Dot Diagram for the carbonate ion (CO 3)-2

Resonance Structures � Occurs when a double bond or triple bond can occur in multiple places � Draw all possible structures with a double headed arrow connecting them � In class, represent with an “R”

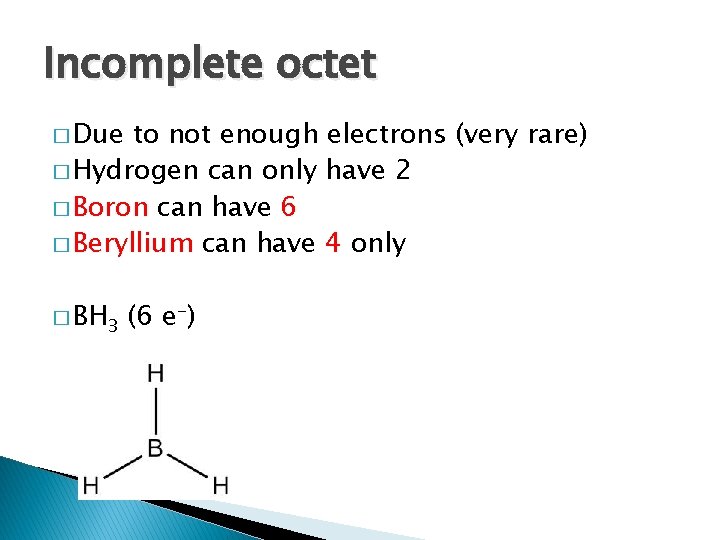
Incomplete octet � Due to not enough electrons (very rare) � Hydrogen can only have 2 � Boron can have 6 � Beryllium can have 4 only � BH 3 (6 e-)

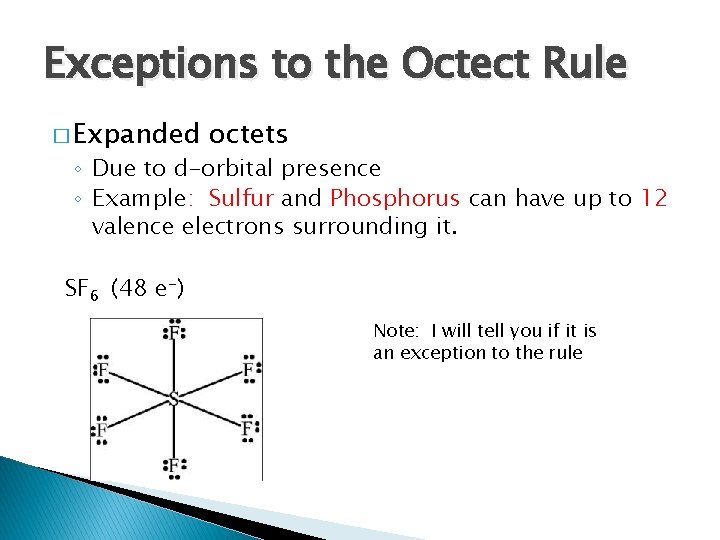
Exceptions to the Octect Rule � Expanded octets ◦ Due to d-orbital presence ◦ Example: Sulfur and Phosphorus can have up to 12 valence electrons surrounding it. SF 6 (48 e-) Note: I will tell you if it is an exception to the rule