Lewis Dot Structures Aka Dot Diagrams Electron Dot






- Slides: 12

Lewis Dot Structures Aka Dot Diagrams Electron Dot Diagrams

Valence electrons are the electrons on the outermost energy level. 6 valence electrons on this atom. Image from Wikipedia

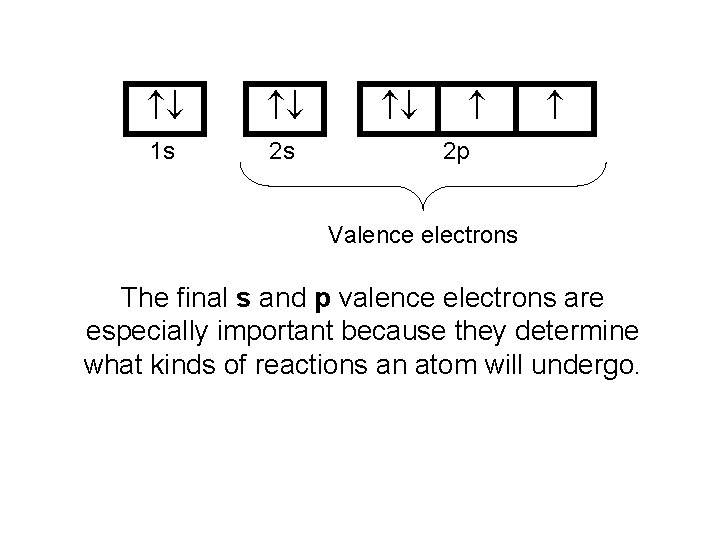
1 s 2 s 2 p Valence electrons The final s and p valence electrons are especially important because they determine what kinds of reactions an atom will undergo.

If you write the Shorthand Configuration for an element, the s and p electrons on the highest energy level are the valence electrons. Example: Tin, Sn: va len ce ele ctr o ns 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 2

Lewis Dot Structures show only the Valence electrons for an element. • A Dot Diagram consists of two parts: – the element symbol – dots that represent the S and P block electrons Sn Dots may only go on the sides of the element symbol

Rules for Writing Lewis Dot Diagrams: • Only use s and p valence electrons • If there are d or f valence electrons in between the s and p sublevels, they are left out of the dot diagram. 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 2

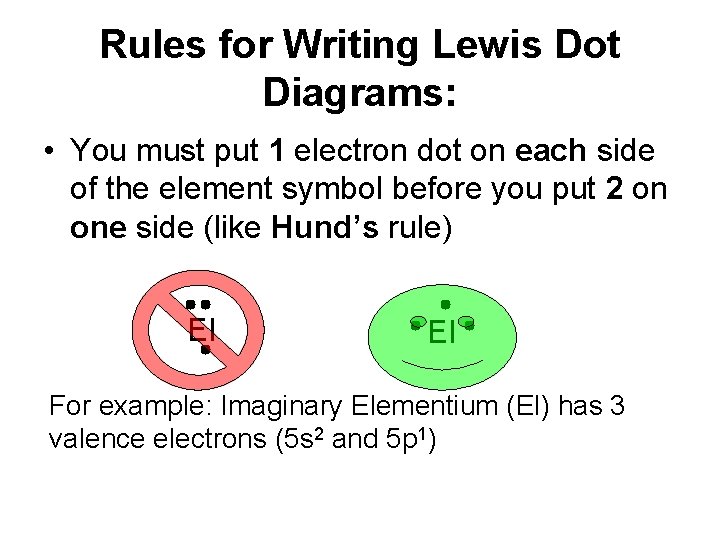
Rules for Writing Lewis Dot Diagrams: • You must put 1 electron dot on each side of the element symbol before you put 2 on one side (like Hund’s rule) El El For example: Imaginary Elementium (El) has 3 valence electrons (5 s 2 and 5 p 1)

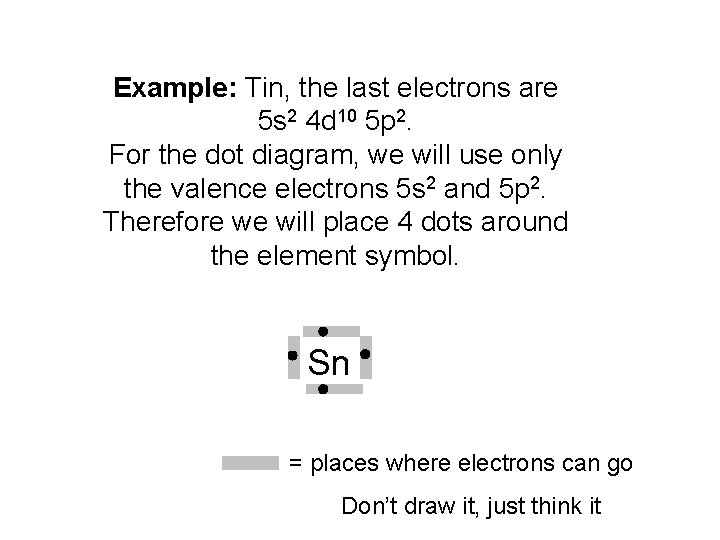
Example: Tin, the last electrons are 5 s 2 4 d 10 5 p 2. For the dot diagram, we will use only the valence electrons 5 s 2 and 5 p 2. Therefore we will place 4 dots around the element symbol. Sn = places where electrons can go Don’t draw it, just think it

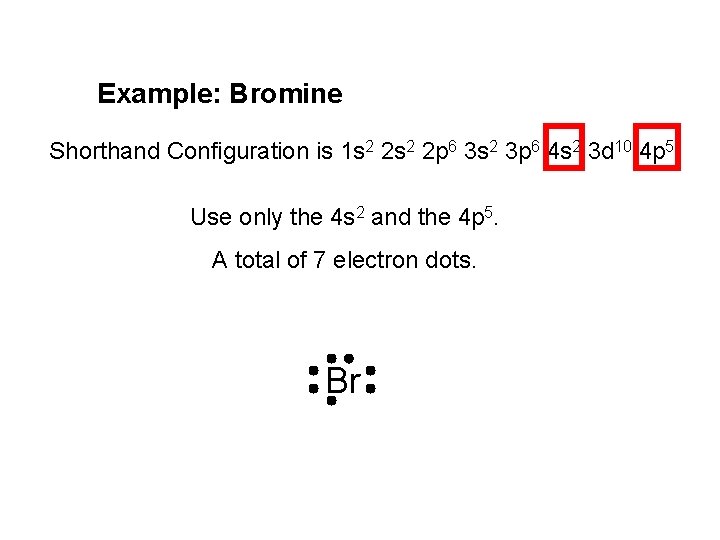
Example: Bromine Shorthand Configuration is 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5. Use only the 4 s 2 and the 4 p 5. A total of 7 electron dots. Br

Practice problems • Neon • Sodium • Aluminum

Answers to Practice Ne Na Al No, it does not matter which side has no electron dots as long as there are the correct number of sides without dots.

Assignment • Complete the Dot Diagram Periodic Table Assignment. • Answer all questions. • Be sure to label your Dot Diagram Periodic Table when the instructions say to. • You will turn them in next week (Tuesday)