Lewis Dot Diagrams Structures Lewis Dot Diagrams Since










- Slides: 19

Lewis Dot Diagrams / Structures

Lewis Dot Diagrams • Since only valence electrons are part of the chemical reactions, it is useful to show only the valence electrons that are reactive! • We use dots to represent electrons – Like in Bohr models • Except we now only show the VALENCE electrons only and no need for shells

Example - 1 • Draw the Lewis dot diagram of the following – Na – Mg –O

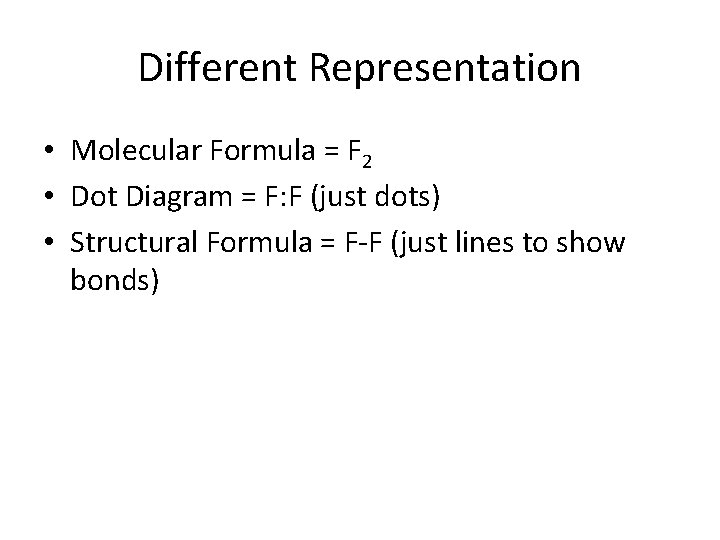
Different Representation • Molecular Formula = F 2 • Dot Diagram = F: F (just dots) • Structural Formula = F-F (just lines to show bonds)

Ionic Lewis Structures • It is the same as Bohr models • The giving away of electrons and the accepting of electrons – So you have to put charges

Example - 2 • Draw the Lewis Structures of the following – Mg. Cl 2 – Mg. O

Practice - 1 • Page 183 - #85

Covalent Lewis Structures • Bonding of 2 or more non-metals • Non-metallic elements when forming covalent bonds will obtain electrons by sharing until they have a stable arrangement of e- such as noble gases • Still must obey the octet rule – Will have to play around sometimes until octet rule is achieved • But there are strategies to drawing

Example - 3 • Draw the Lewis Structure of the following – F 2 – O 2 – H 2

Ideal Number of Bonds • • H, F, Cl, I = 1 bond O, S, Se = 2 bonds N, P, As = 3 bonds C, Si = 4 bonds

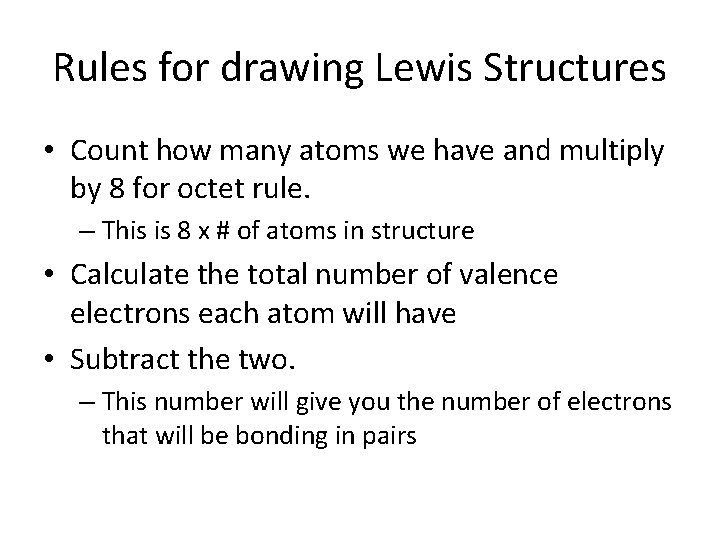
Rules for drawing Lewis Structures • Count how many atoms we have and multiply by 8 for octet rule. – This is 8 x # of atoms in structure • Calculate the total number of valence electrons each atom will have • Subtract the two. – This number will give you the number of electrons that will be bonding in pairs

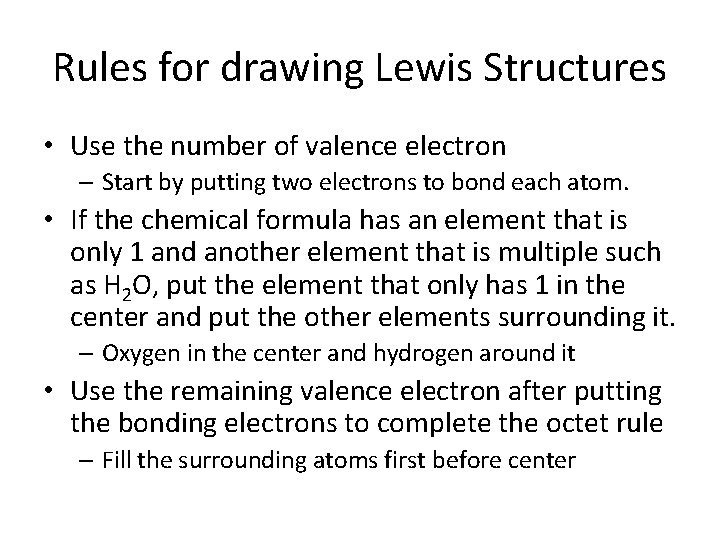
Rules for drawing Lewis Structures • Use the number of valence electron – Start by putting two electrons to bond each atom. • If the chemical formula has an element that is only 1 and another element that is multiple such as H 2 O, put the element that only has 1 in the center and put the other elements surrounding it. – Oxygen in the center and hydrogen around it • Use the remaining valence electron after putting the bonding electrons to complete the octet rule – Fill the surrounding atoms first before center

Rules for drawing Lewis Structures • If any valence electrons remain, fill the central atom • If the central atom doesn’t satisfy the octet rule. – You can rearrange the neighbouring atoms lone pairs/unbonded electrons to make another bond. • Put dashes for any bonds to make it neater • If our molecule is an ion, we add or remove electrons – If it is an ion, we put brackets around it and put the charge in the top right corner

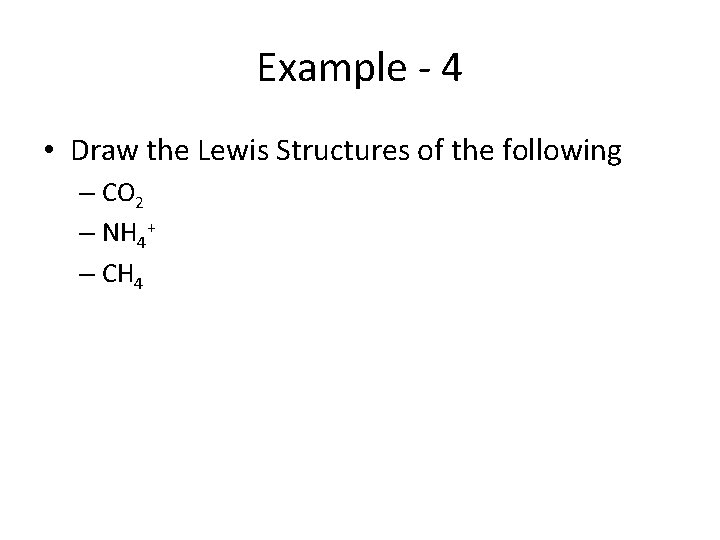
Example - 4 • Draw the Lewis Structures of the following – CO 2 – NH 4+ – CH 4

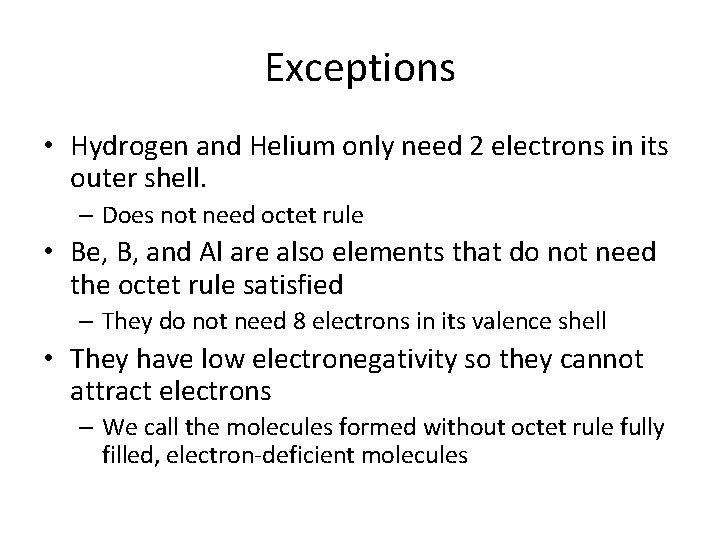
Exceptions • Hydrogen and Helium only need 2 electrons in its outer shell. – Does not need octet rule • Be, B, and Al are also elements that do not need the octet rule satisfied – They do not need 8 electrons in its valence shell • They have low electronegativity so they cannot attract electrons – We call the molecules formed without octet rule fully filled, electron-deficient molecules

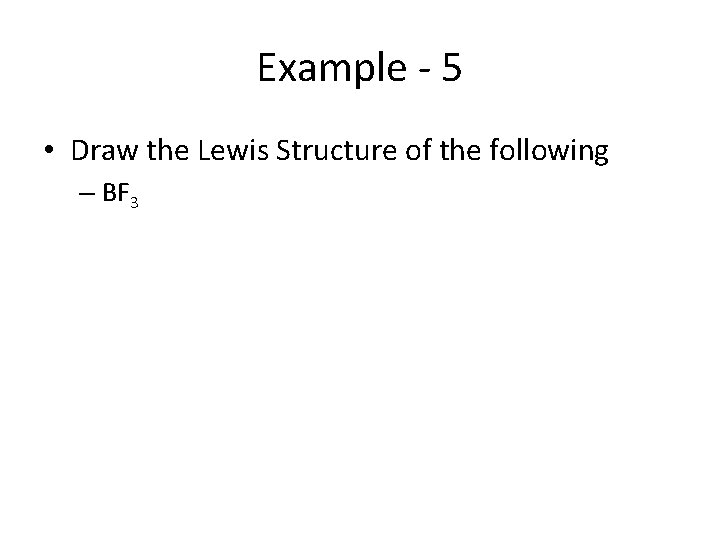
Example - 5 • Draw the Lewis Structure of the following – BF 3

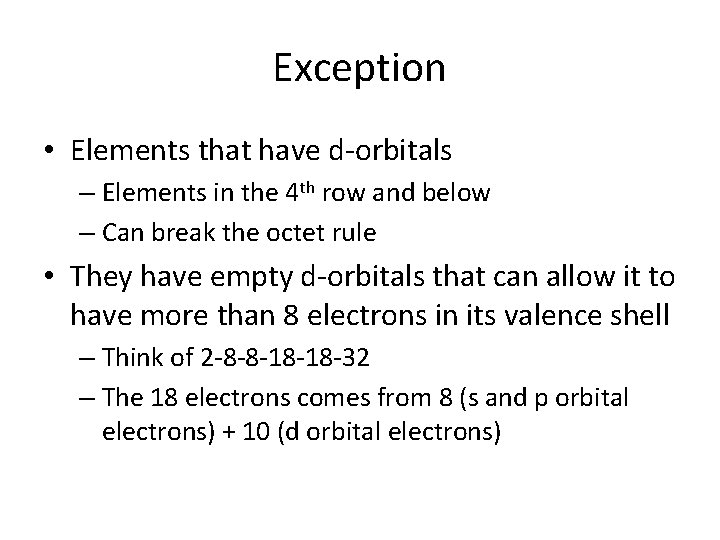
Exception • Elements that have d-orbitals – Elements in the 4 th row and below – Can break the octet rule • They have empty d-orbitals that can allow it to have more than 8 electrons in its valence shell – Think of 2 -8 -8 -18 -18 -32 – The 18 electrons comes from 8 (s and p orbital electrons) + 10 (d orbital electrons)

Example - 6 • Draw the Lewis Structure of the following – PCl 5

Practice - 2 • Page 188 - #86