IONIC COMPOUNDS LEWIS DOT IONIC COMPOUNDS LEWIS DOT


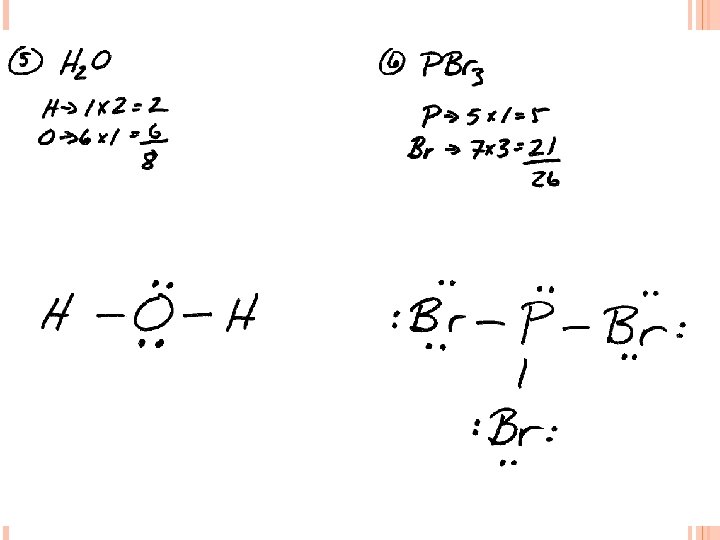
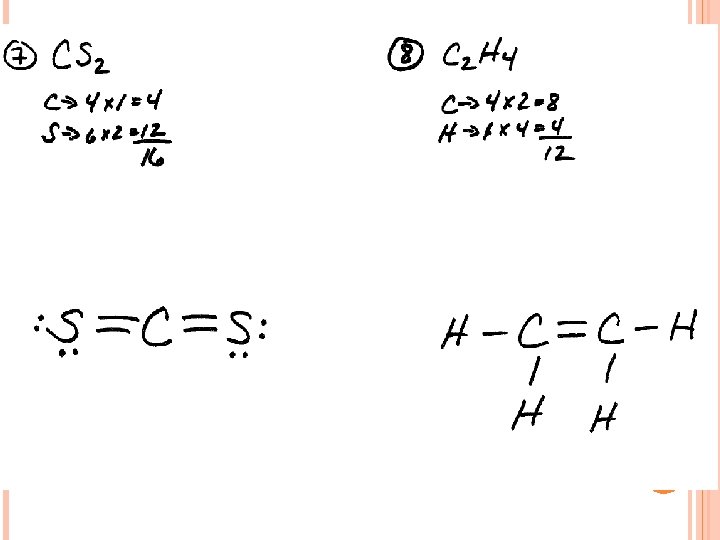
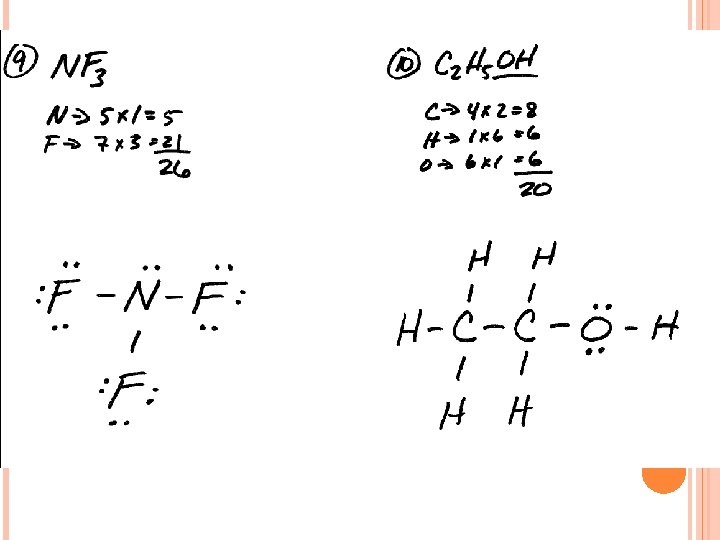
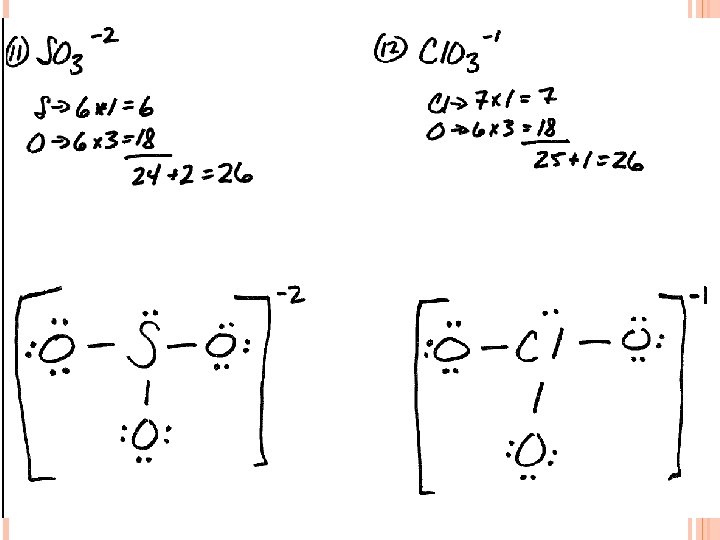
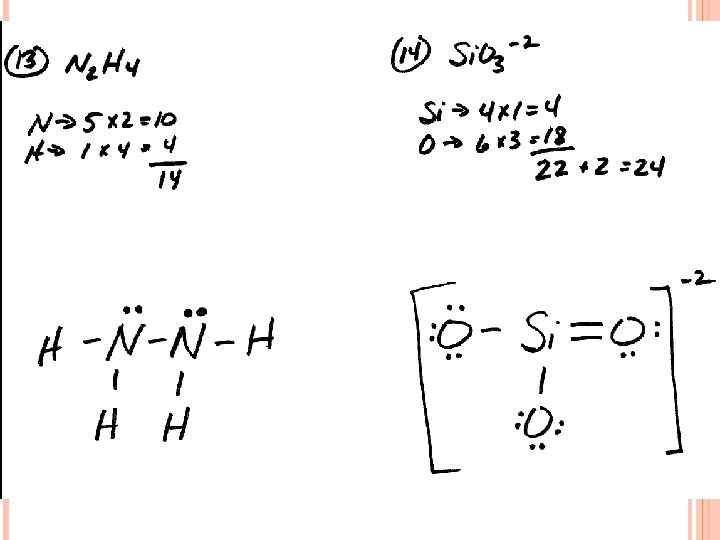
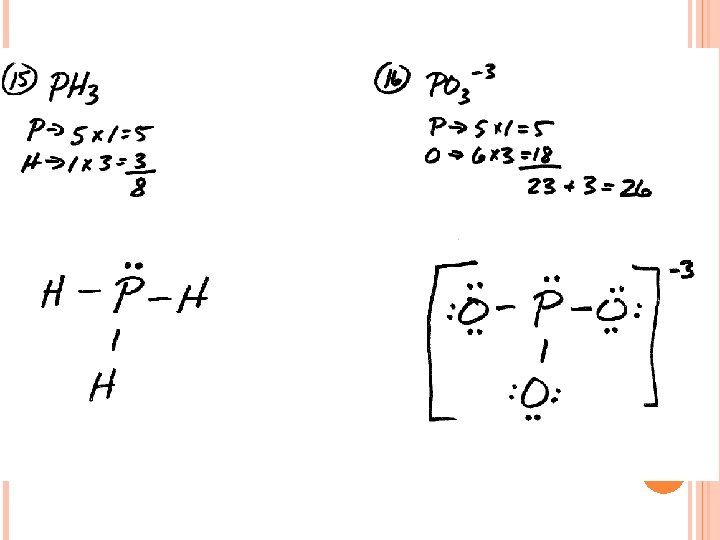
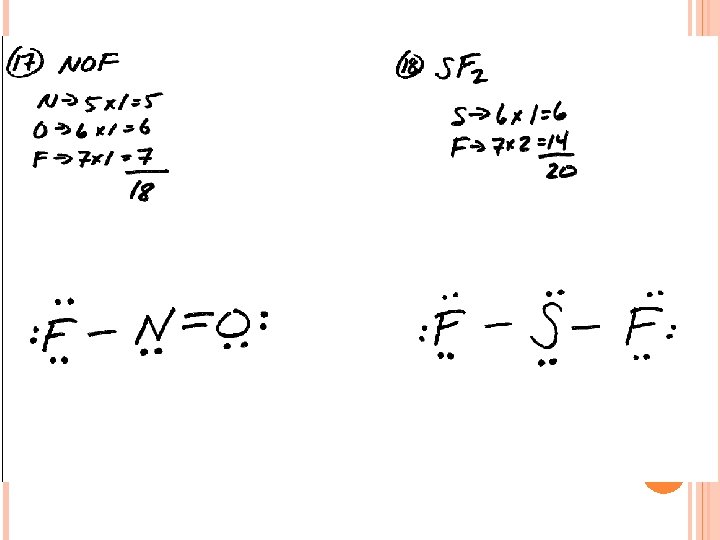
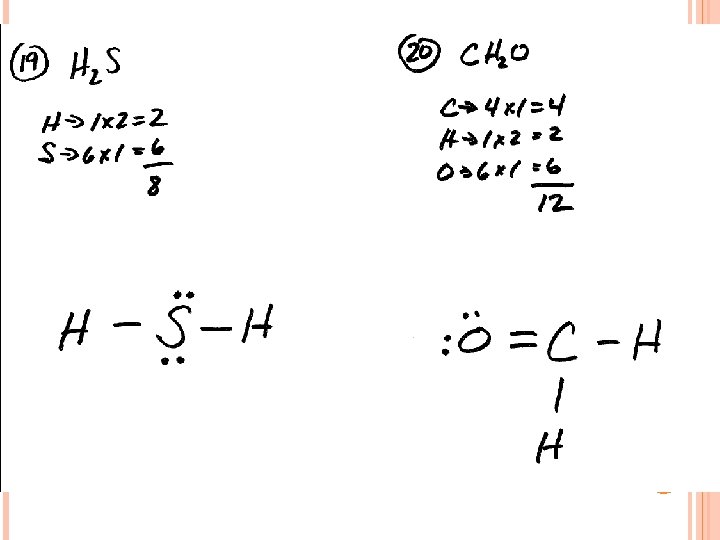













- Slides: 42

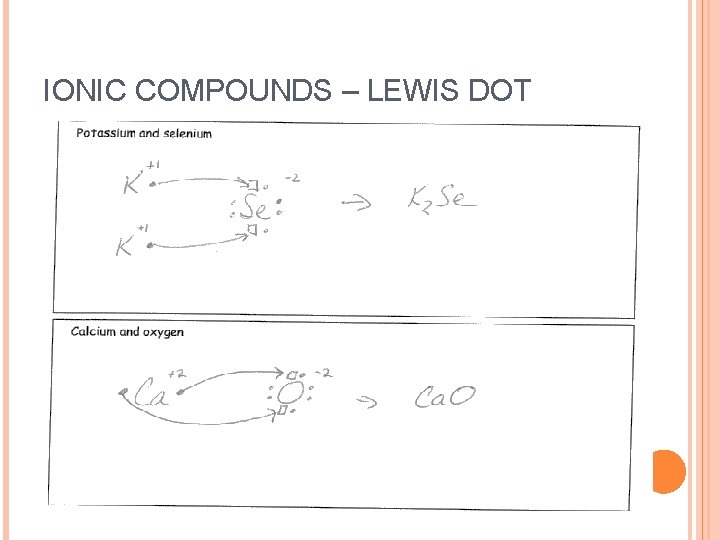
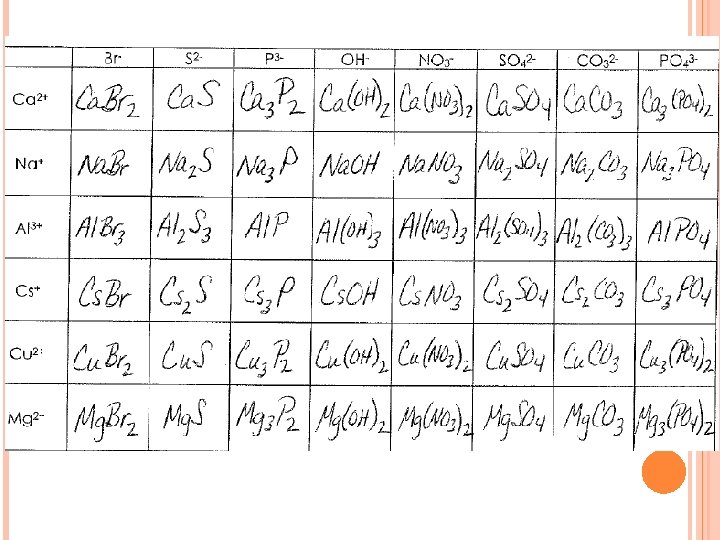
IONIC COMPOUNDS – LEWIS DOT

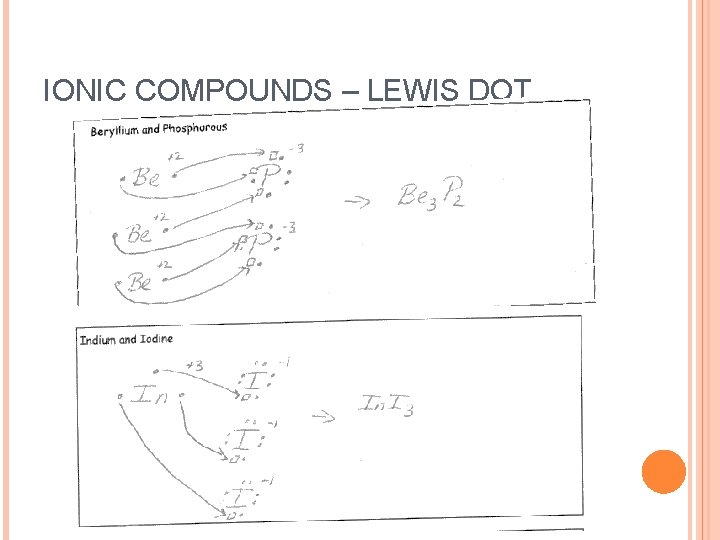
IONIC COMPOUNDS – LEWIS DOT

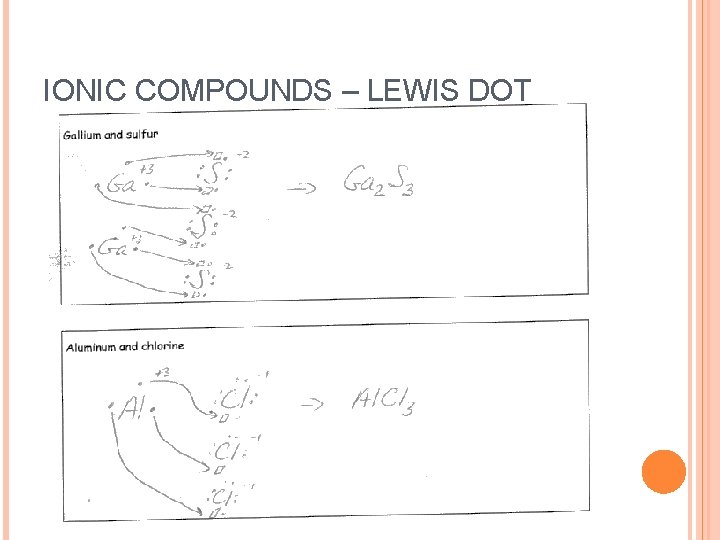
IONIC COMPOUNDS – LEWIS DOT

CRISS CROSS #1 1. KBr 2. Li 2 O 3. Ca. Cl 2 4. Al 2 S 3 5. Fe. S 6. Na 3 N 7. Mg. Se 8. Al. Br 3 9. Fe 2 P 3 10. Ag 3 P

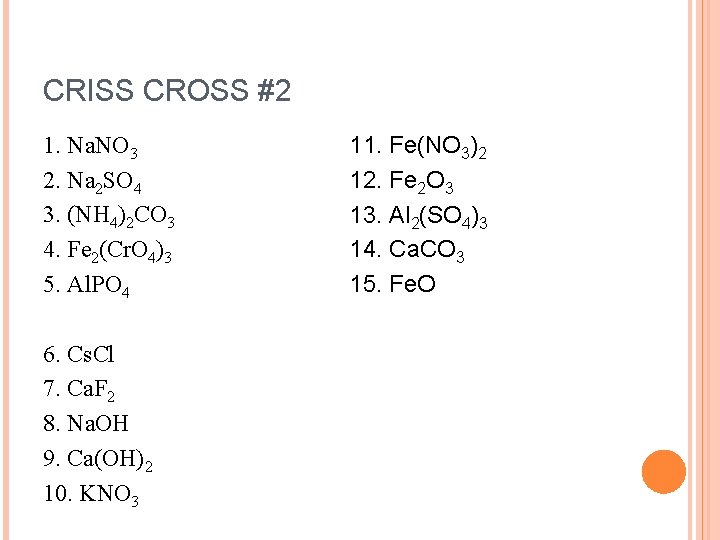
CRISS CROSS #2 1. Na. NO 3 2. Na 2 SO 4 3. (NH 4)2 CO 3 4. Fe 2(Cr. O 4)3 5. Al. PO 4 6. Cs. Cl 7. Ca. F 2 8. Na. OH 9. Ca(OH)2 10. KNO 3 11. Fe(NO 3)2 12. Fe 2 O 3 13. Al 2(SO 4)3 14. Ca. CO 3 15. Fe. O



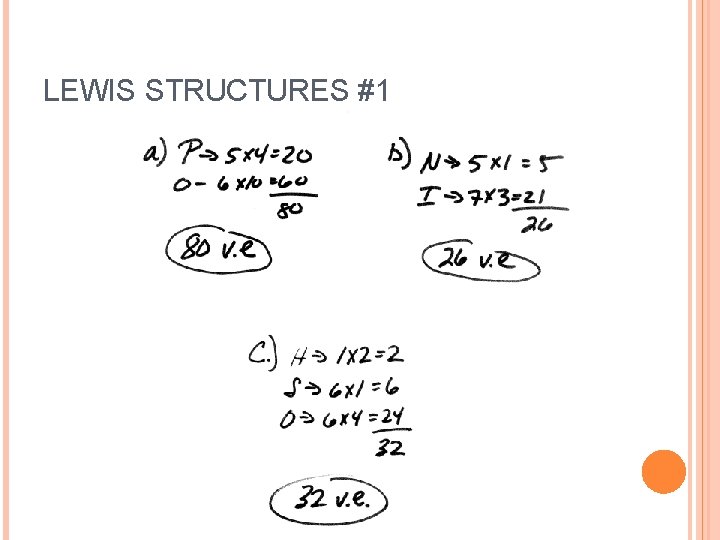
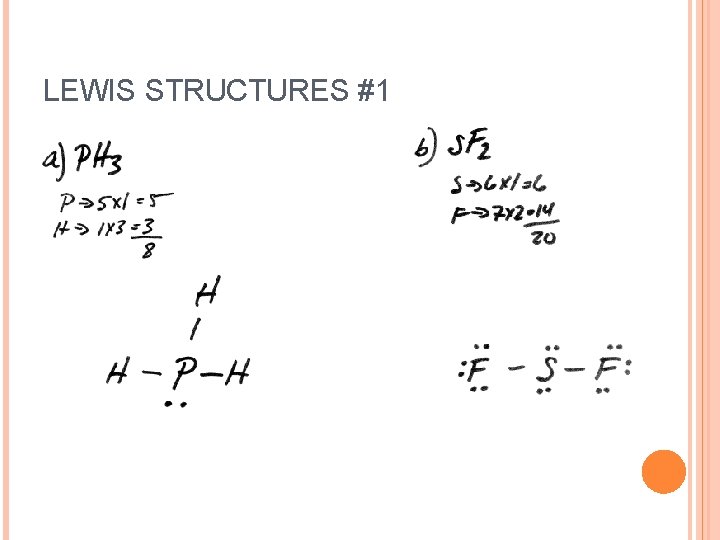
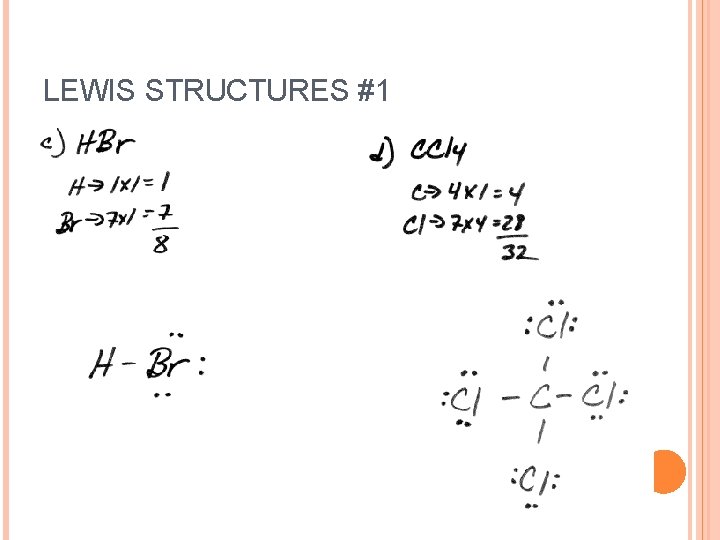
LEWIS STRUCTURES #1

LEWIS STRUCTURES #1

LEWIS STRUCTURES #1

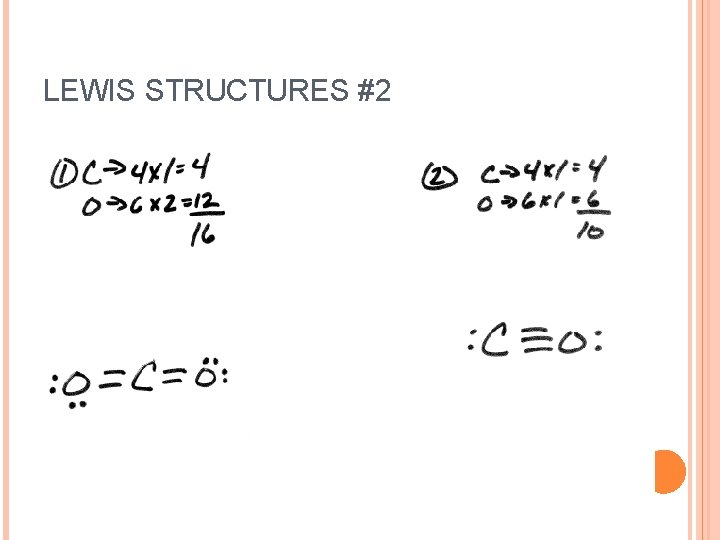
LEWIS STRUCTURES #2

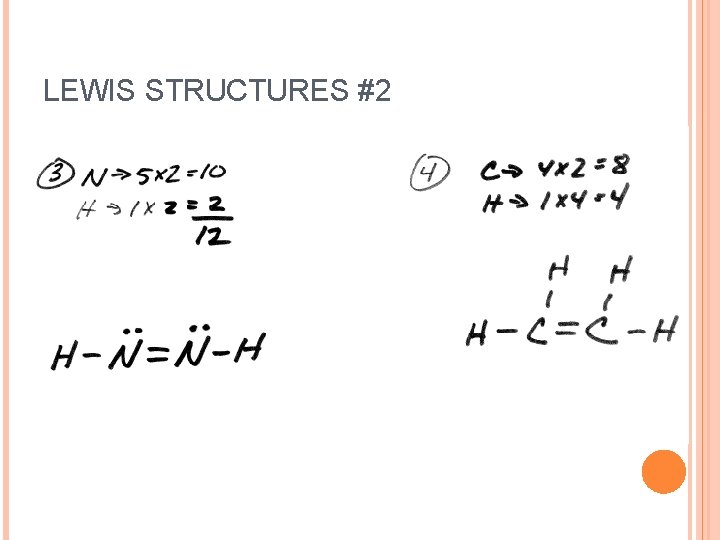
LEWIS STRUCTURES #2

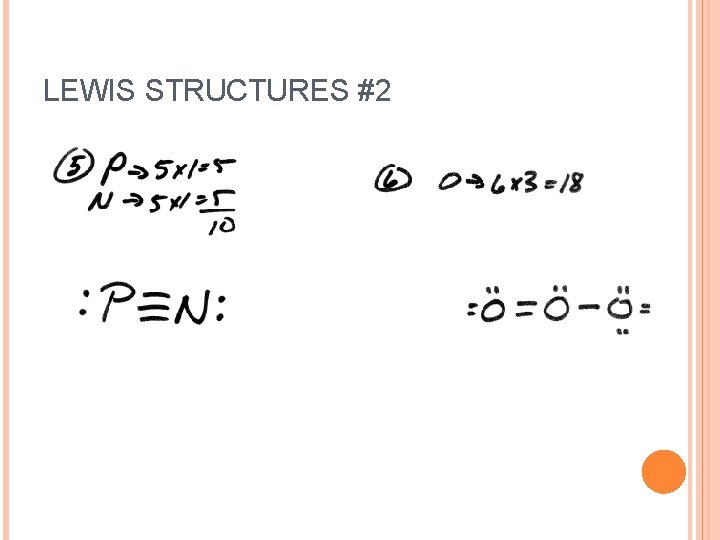
LEWIS STRUCTURES #2

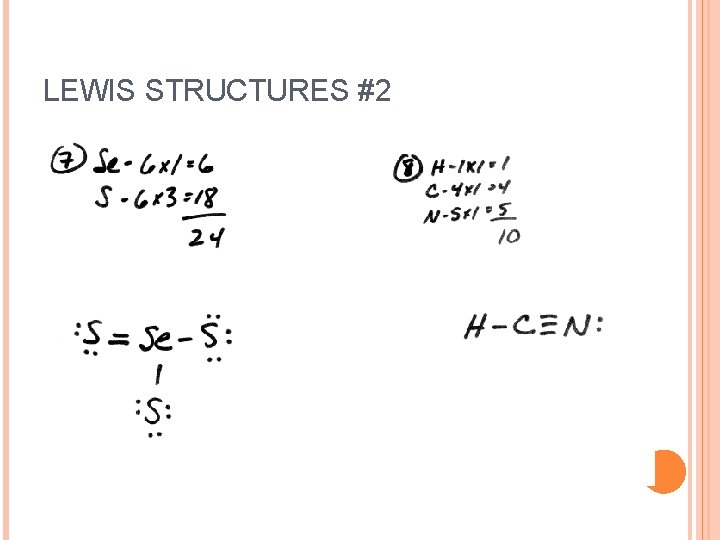
LEWIS STRUCTURES #2

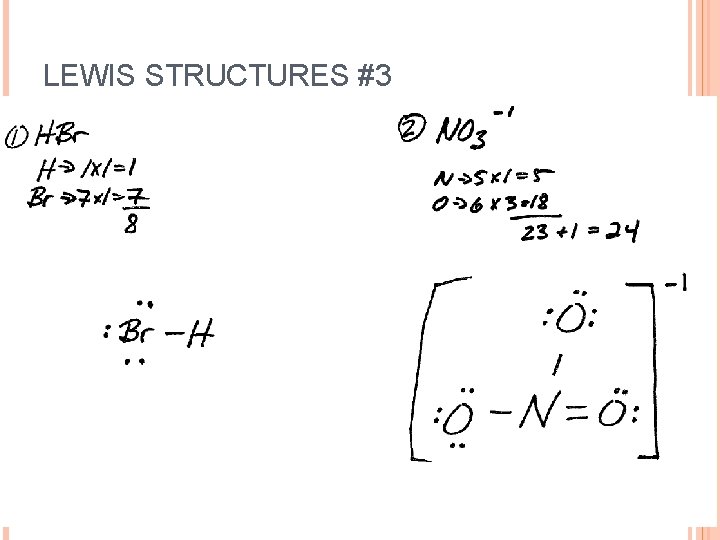
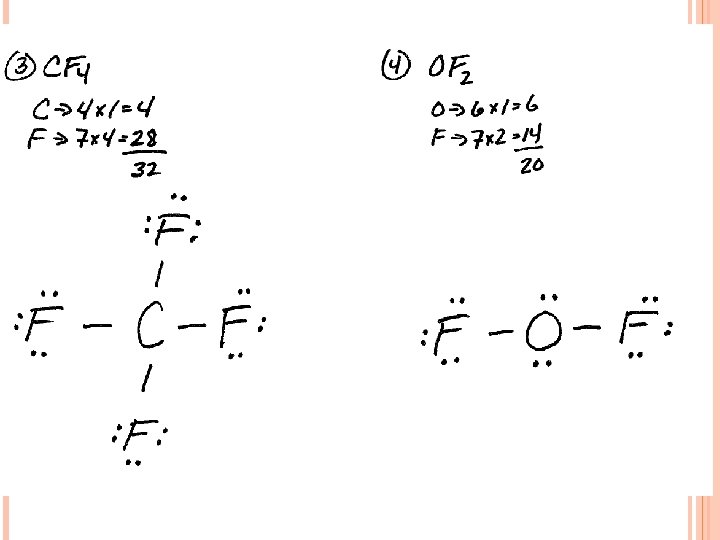
LEWIS STRUCTURES #3

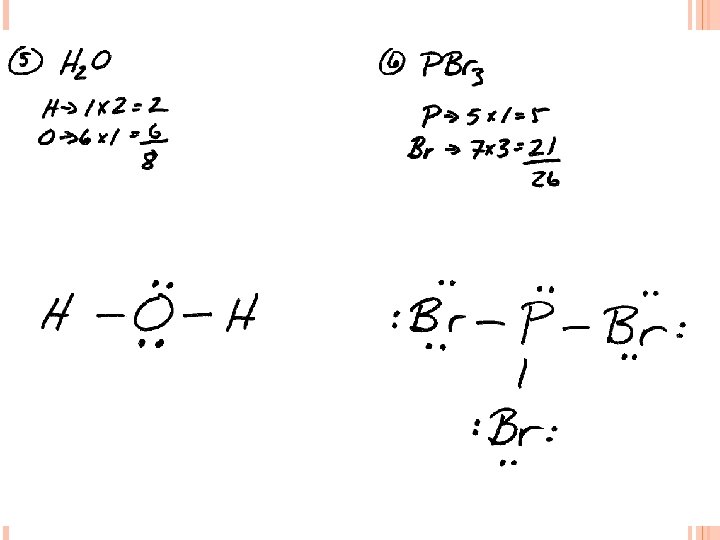
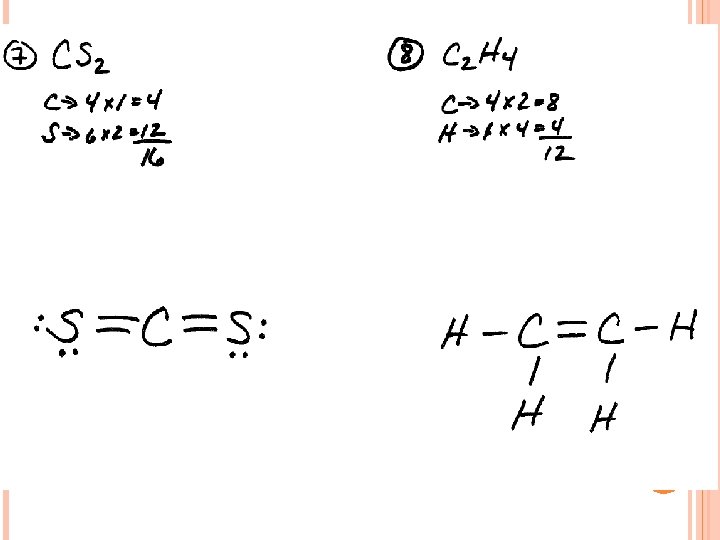
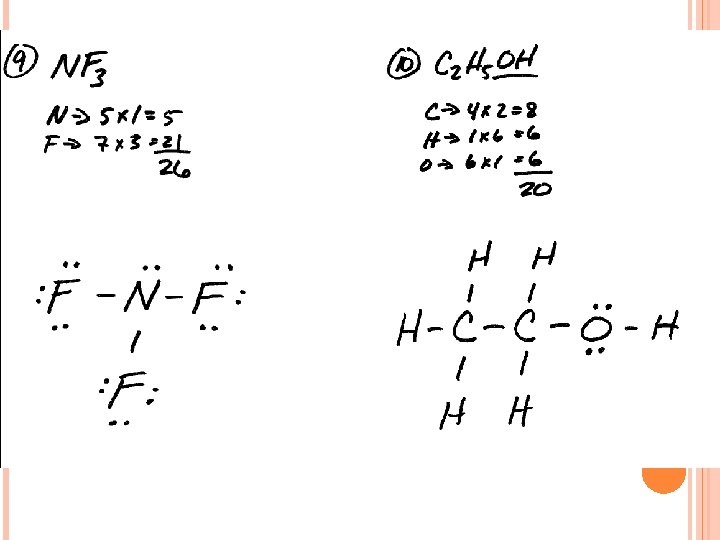
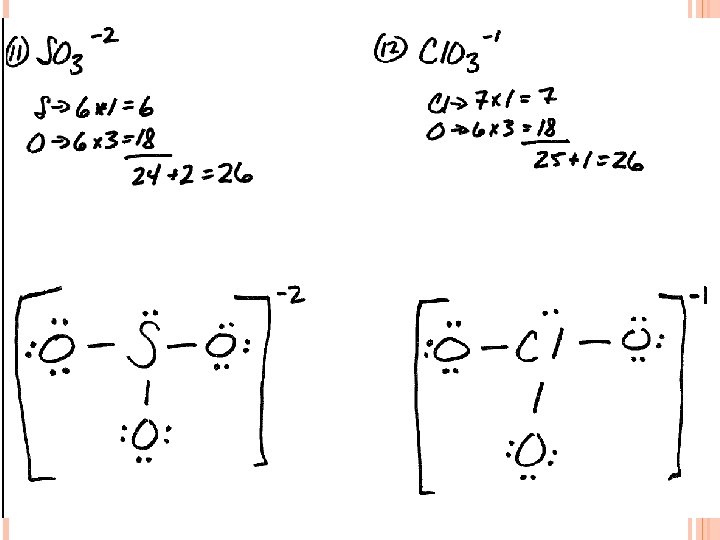
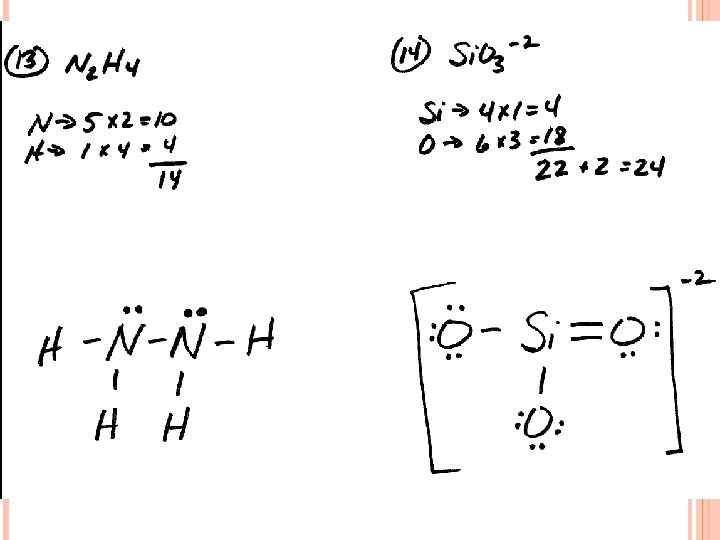
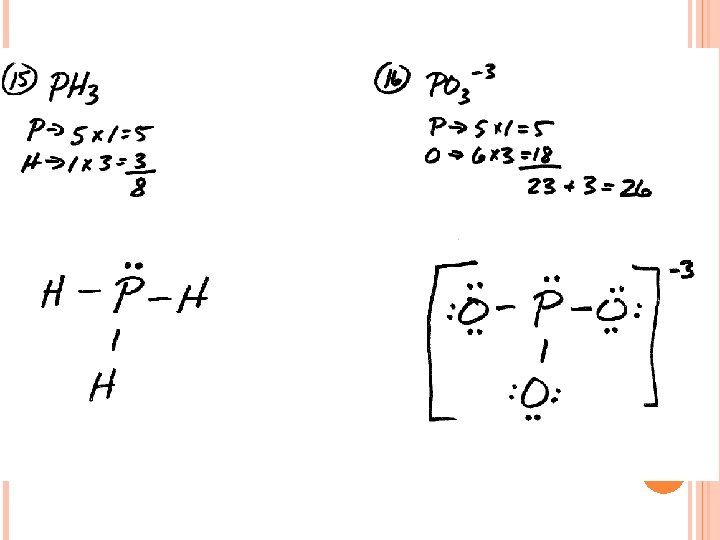
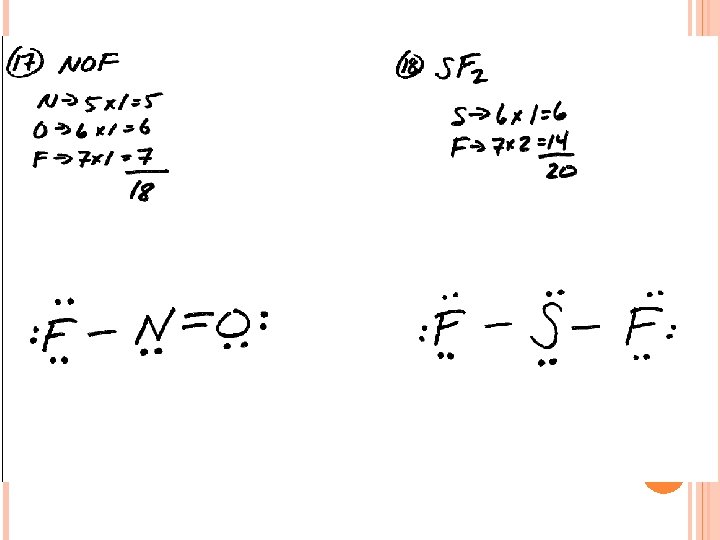
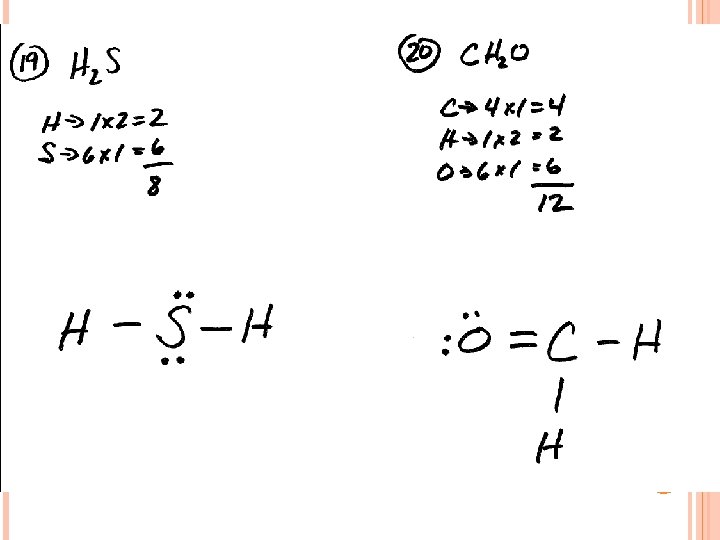

IONIC COMPOUNDS - #1 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Ba(C 2 H 3 O 2)2 Al. I 3 Ca(NO 3)2 Na. HCO 3 Ba. SO 4 Rb 2 O Sr. S K 2 CO 3 Mg. SO 3 Be. Br 2 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Magnesium phosphate Aluminum sulfide Calcium phosphate Magnesium hydroxide Potassium chlorate Ammonium dichromate Potassium hydrogen sulfate Calcium sulfite Calcium acetate Aluminum bromide

Ionic Compounds: Formulas and Names #2 1. Pb(C 2 H 3 O 2)2 2. Fe(OH)3 3. Cr 2(SO 4)3 4. Ni. O 5. Fe. I 2 6. Zn. O 7. Ag. I 8. Ni 2(CO 3)3 9. Sn. Br 2 10. Fe. Br 3

Ionic Compounds: Formulas and Names #2 (ANSWERS) 11. lead (II) dichromate 12. zinc chromate 13. chromium (III) fluoride 14. mercury (II) chloride 15. silver sulfide 16. manganese (II) bromide 17. copper (I) sulfate 18. copper (II) hydroxide 19. nickel (II) acetate 20. silver bromide

Ionic Compounds: Formulas and Names #3 1) sodium carbonate 2) copper (I) hydroxide 3) magnesium bromide 4) potassium iodide 5) iron (II) chloride 6) iron (III) chloride 7) zinc hydroxide 8) beryllium sulfate 9) chromium (II) fluoride 10) aluminum sulfide 11) lead (II) oxide 12) lithium phosphate 13) tin (IV) iodide 14) cobalt (II) nitride 15) cobalt (III) phosphide 16) gallium nitrite 17) silver sulfite 18) cobalt (III) hydroxide 19) aluminum cyanide 20) beryllium acetate

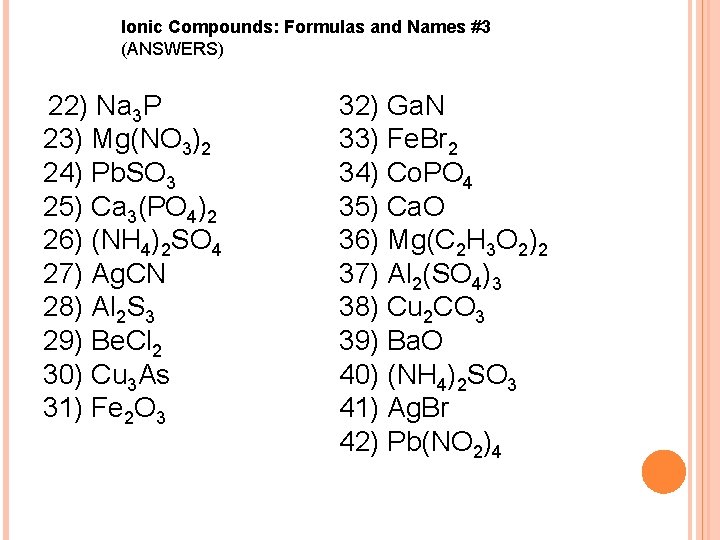
Ionic Compounds: Formulas and Names #3 (ANSWERS) 22) Na 3 P 23) Mg(NO 3)2 24) Pb. SO 3 25) Ca 3(PO 4)2 26) (NH 4)2 SO 4 27) Ag. CN 28) Al 2 S 3 29) Be. Cl 2 30) Cu 3 As 31) Fe 2 O 3 32) Ga. N 33) Fe. Br 2 34) Co. PO 4 35) Ca. O 36) Mg(C 2 H 3 O 2)2 37) Al 2(SO 4)3 38) Cu 2 CO 3 39) Ba. O 40) (NH 4)2 SO 3 41) Ag. Br 42) Pb(NO 2)4

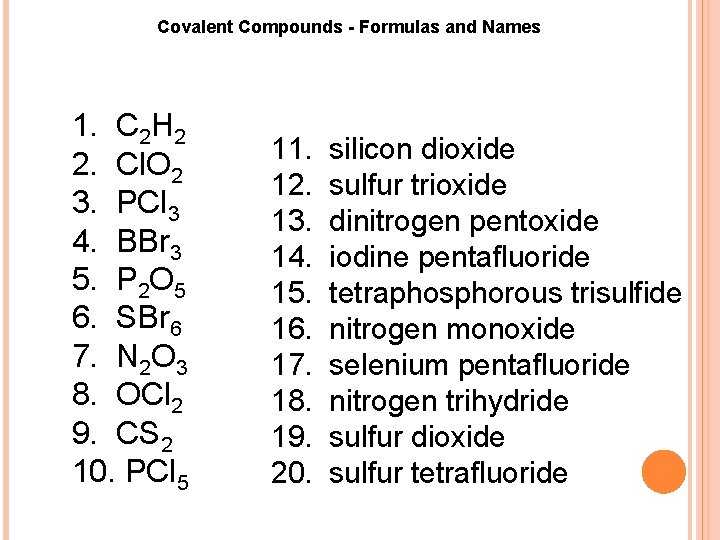
Covalent Compounds Formulas and Names 1. C 2 H 2 2. Cl. O 2 3. PCl 3 4. BBr 3 5. P 2 O 5 6. SBr 6 7. N 2 O 3 8. OCl 2 9. CS 2 10. PCl 5 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. silicon dioxide sulfur trioxide dinitrogen pentoxide iodine pentafluoride tetraphosphorous trisulfide nitrogen monoxide selenium pentafluoride nitrogen trihydride sulfur dioxide sulfur tetrafluoride

Covalent Compounds Formulas and Names 21. calcium carbonate - ionic 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. (NH 4)3 PO 4 - ionic nitrogen trioxide - covalent CO - covalent zinc sulfate - ionic N 2 O - covalent sodium hydrogen carbonate - ionic Mg(OH)2 - ionic phosphorous pentabromide - covalent Al 2(SO 3)3 - ionic carbon tetrachloride - covalent KNO 3 - ionic

Mixed Ionic/Covalent Naming Review #1 1. Cr. CO 3 2. Zn. S 3. Si. Cl 4 4. Ba. SO 4 5. Cl. O 6. Na 2 S 7. Na 2 SO 3 8. Be(Mn. O 4)2 9. K 3 PO 3 10. Mg. SO 4 11. (NH 4)2 SO 3 12. CCl 4 13. P 2 O 5 14. Pb(NO 2)2 15. N 2 O 2 16. Ca. Cr. O 4 17. Sr(OH)2 18. Al 2(Cr. O 4)3 19. Na. I 20. BI 4

Mixed Ionic/Covalent Naming Review 21. iron (III) bromide 22. cobalt (II) sulfide 23. tin (IV) chloride 24. lithium nitride 25. magnesium oxide 26. barium sulfite 27. potassium dichromate 28. nitrogen triiodide 29. ammonium nitrate 30. lead (II) phosphate 31. phosphorous dichloride 32. aluminum sulfite 33. dinitrogen tetroxide 34. cesium phosphate 35. calcium perchlorate 36. selenium monobromide 37. magnesium acetate 38. potassium permanganat 39. chlorine monofluoride 40. copper (I) phosphide

Mixed Ionic/Covalent Review #2 1) sodium bromide 11) Si. O 2 2) calcium acetate 12) Ni 2 S 3 3) diphosphorus pentoxide 13) Mn 3(PO 4)2 4) titanium(IV) sulfate 14) Ag. C 2 H 3 O 2 5) iron(III) phosphate 15) B 2 Br 4 6) potassium nitride 16) PF 5 7) sulfur dioxide 17) K 2 CO 3 8) copper(I) hydroxide 18) (NH 4)2 O 9) zinc nitrite 19) Sn. Se 2 10) Cobalt (III) sulfide 20) C 2 H 4

(Still) More Naming Practice 1) boron tribromide 11) P 4 Se 3 2) calcium sulfate 12) KC 2 H 3 O 2 3) dicarbon hexabromide 13) Fe 3 P 2 4) chromium (VI) carbonate 14) Si 2 Br 6 5) silver phosphide 15) Ti(NO 3)4 6) iodine dioxide 16) Se 2 I 2 7) tin (IV) oxide 17) Cu 3 PO 4 8) lead (II) sulfide 18) Ga 2 O 3 9) methane carbon tetrahydride 19) S 4 N 2 10) dinitrogen trioxide 20) Ba 3 P 2

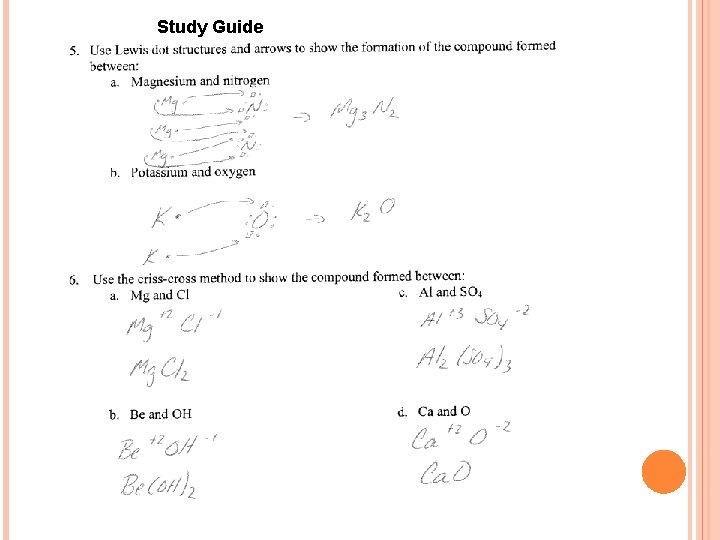
Study Guide

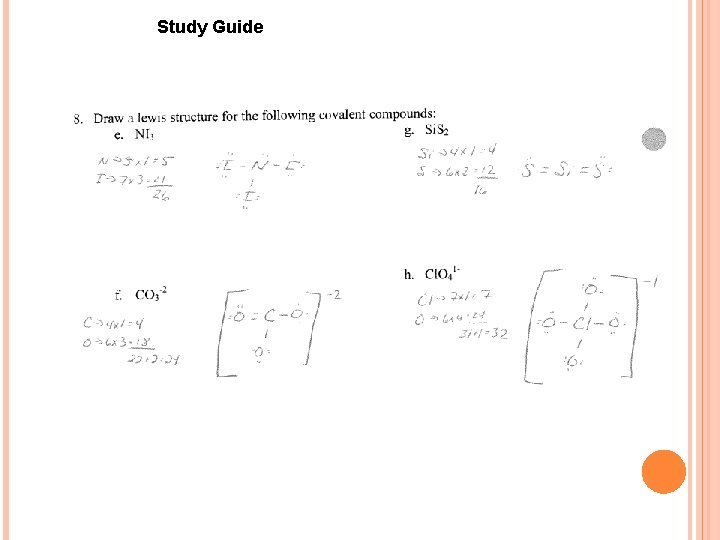
Study Guide

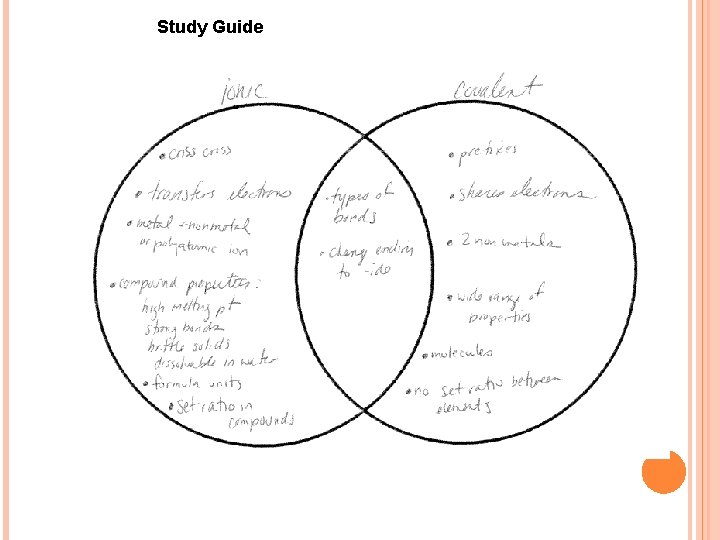
Study Guide

Study Guide

Study Guide

Ch 7 Practice Test

Ch 7 Practice Test