Lecture Presentation Chapter 13 Properties of Solutions 2015






- Slides: 23

Lecture Presentation Chapter 13 Properties of Solutions . © 2015 Pearson Education, Inc. James F. Kirby Quinnipiac University Hamden, CT Solutions

13. 1 The Solution Process Solution – a homogeneous mixture of 2 or more pure substances. Solute – substance being dissolved. Solvent – liquid the substance is dissolved in. The ability of a substance to dissolve in another substance depends on 1. Natural tendency of the substance to increase randomness, and 2. The intermolecular forces between the two substances. Copyright © Cengage Learning. All rights reserved 2

13. 2 Types of Solutions The solution process and crystallization are opposing processes. Saturated solution: Rate of solution = rate of crystallization. The amount of solute is the threshold amount that is required to cause crystallization. Unsaturated solution: The amount of solute is below the threshold amount required to cause crystallization. Supersaturated solution: The amount of solute exceeds the threshold amount to cause crystallization. The solvent can hold more solute than is normally possible.

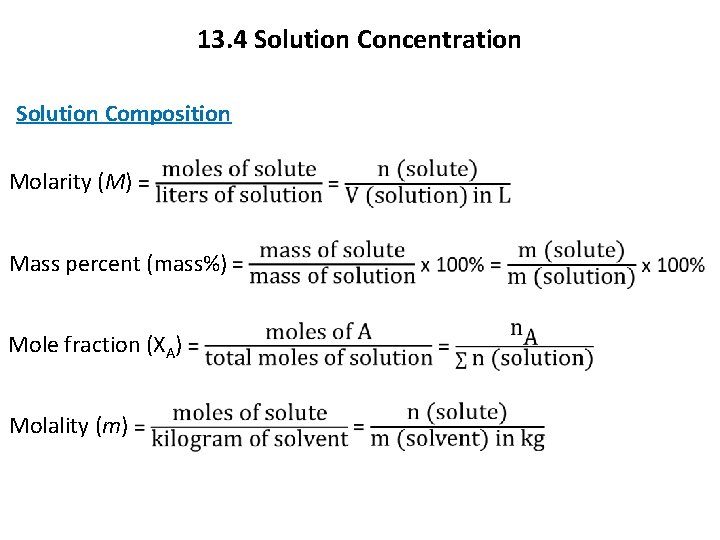
13. 4 Solution Concentration Solution Composition Molarity (M) Mass percent (mass%) Mole fraction (XA) Molality (m)

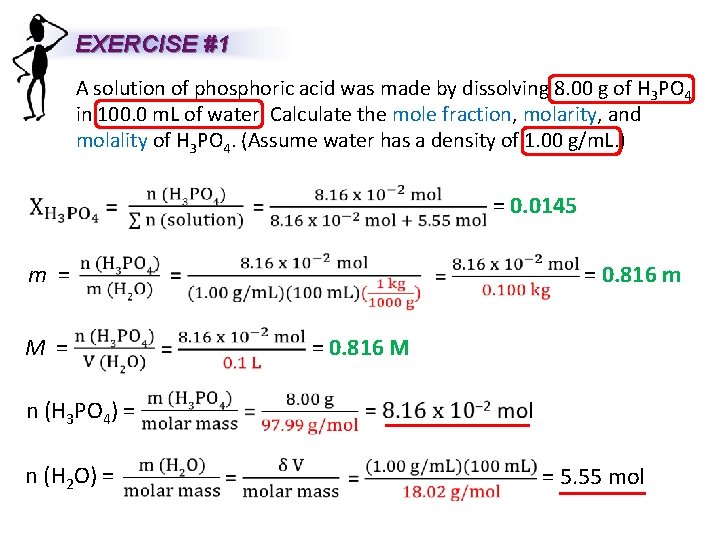
EXERCISE #1 A solution of phosphoric acid was made by dissolving 8. 00 g of H 3 PO 4 in 100. 0 m. L of water. Calculate the mole fraction, molarity, and molality of H 3 PO 4. (Assume water has a density of 1. 00 g/m. L. ) = 0. 0145 = 0. 816 m m = M = = 0. 816 M n (H 3 PO 4) = n (H 2 O) = = 5. 55 mol

13. 2 Solubility is the maximum amount of solute that can dissolve in a given amount of solvent at a given temperature. Saturated solutions have that amount of solute dissolved. Unsaturated solutions have any amount of solute less than the maximum amount dissolved in solution. Supersaturated solutions have amount of solute more than the maximum amount dissolved in solution.

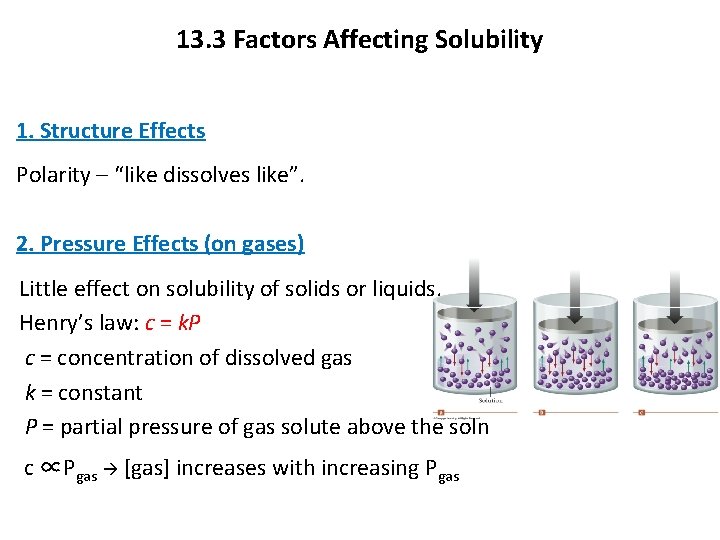
13. 3 Factors Affecting Solubility 1. Structure Effects Polarity – “like dissolves like”. 2. Pressure Effects (on gases) Little effect on solubility of solids or liquids. Henry’s law: c = k. P c = concentration of dissolved gas k = constant P = partial pressure of gas solute above the soln c ∝Pgas [gas] increases with increasing Pgas

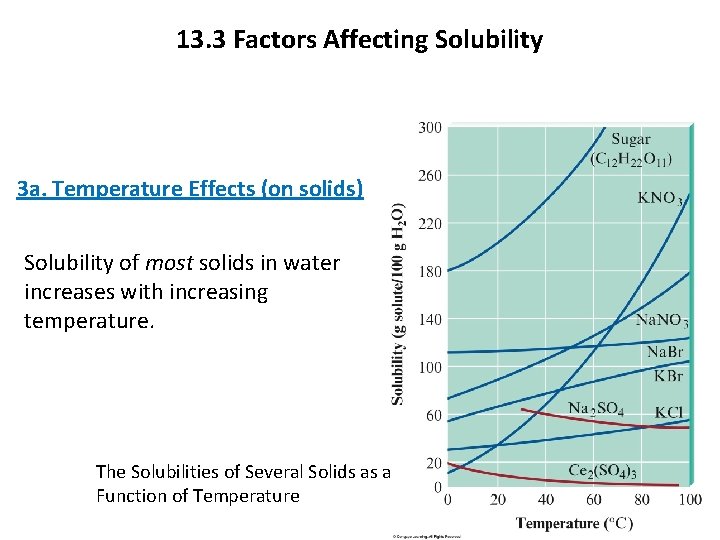
13. 3 Factors Affecting Solubility 3 a. Temperature Effects (on solids) Solubility of most solids in water increases with increasing temperature. The Solubilities of Several Solids as a Function of Temperature Copyright © Cengage Learning. All rights reserved 8

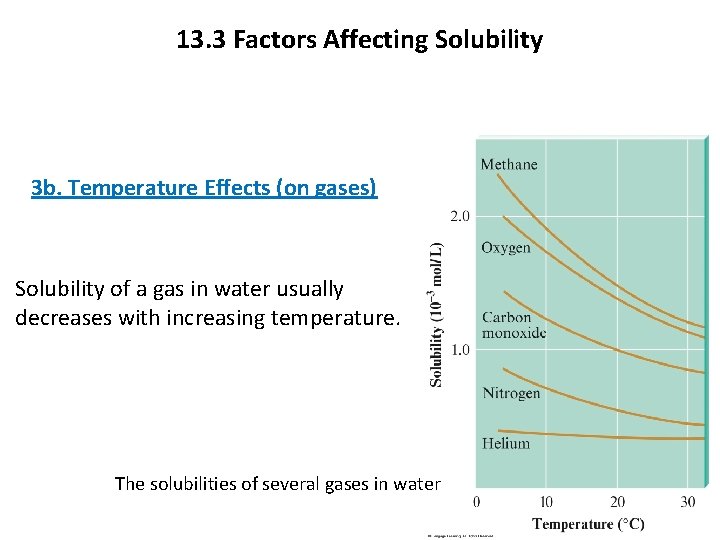
13. 3 Factors Affecting Solubility 3 b. Temperature Effects (on gases) Solubility of a gas in water usually decreases with increasing temperature. The solubilities of several gases in water 9

13. 5 Colligative Properties of Non-Electrolyte Solutions Depend only on the number of solute particles in solution and not on the nature of the solute particles. § § Vapor-pressure Boiling-point elevation Freezing-point depression Osmotic pressure Copyright © Cengage Learning. All rights reserved 10

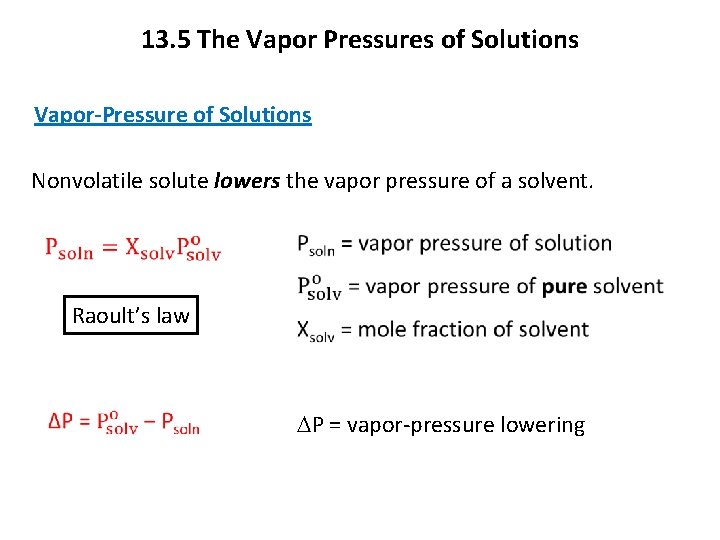
13. 5 The Vapor Pressures of Solutions Vapor-Pressure of Solutions Nonvolatile solute lowers the vapor pressure of a solvent. Raoult’s law DP = vapor-pressure lowering

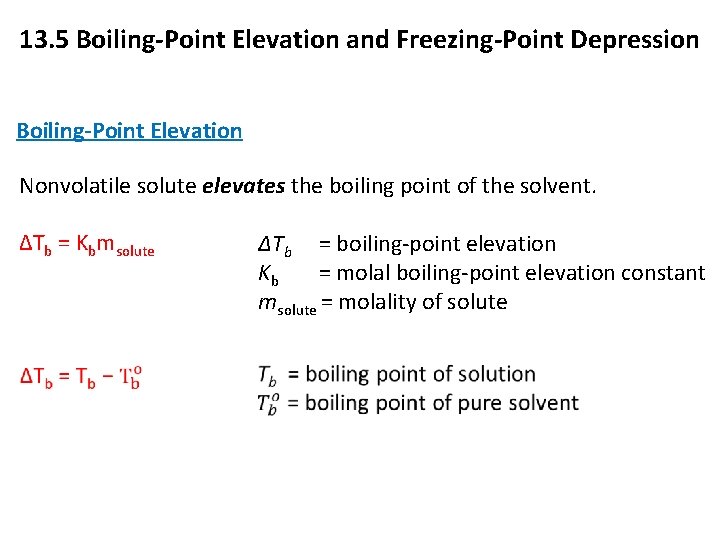
13. 5 Boiling-Point Elevation and Freezing-Point Depression Boiling-Point Elevation Nonvolatile solute elevates the boiling point of the solvent. ΔTb = Kbmsolute ΔTb = boiling-point elevation Kb = molal boiling-point elevation constant msolute = molality of solute 12

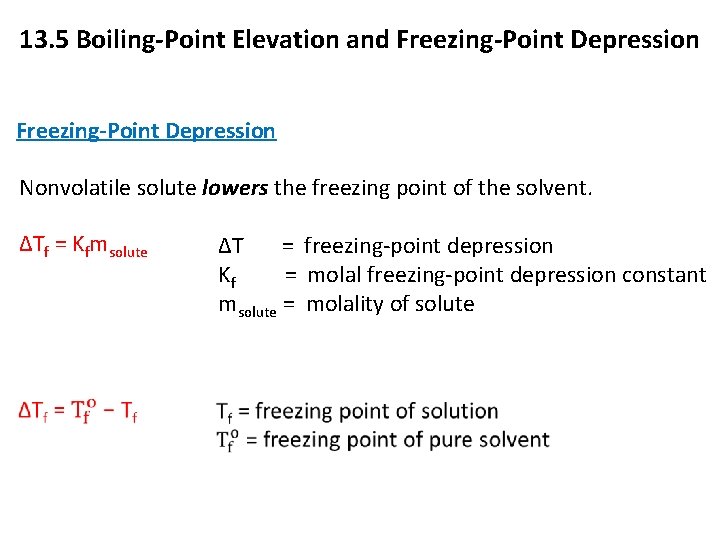
13. 5 Boiling-Point Elevation and Freezing-Point Depression Nonvolatile solute lowers the freezing point of the solvent. ΔTf = Kfmsolute Copyright © Cengage Learning. All rights reserved ΔT = freezing-point depression Kf = molal freezing-point depression constant msolute = molality of solute 13

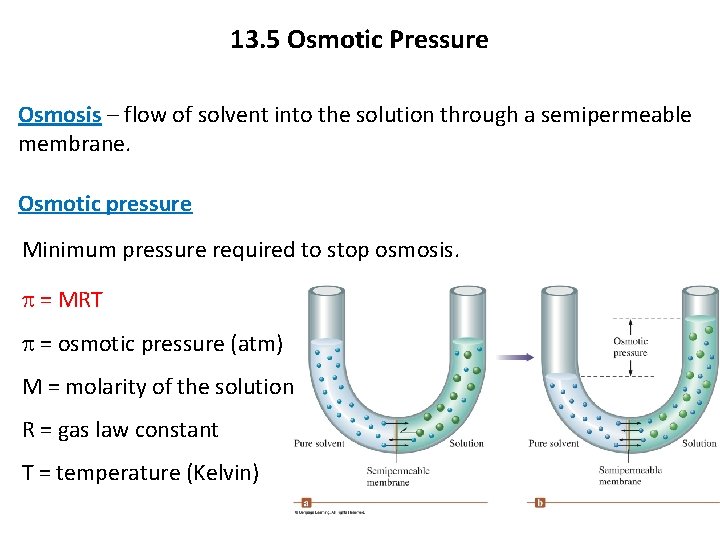
13. 5 Osmotic Pressure Osmosis – flow of solvent into the solution through a semipermeable membrane. Osmotic pressure Minimum pressure required to stop osmosis. p = MRT p = osmotic pressure (atm) M = molarity of the solution R = gas law constant T = temperature (Kelvin)

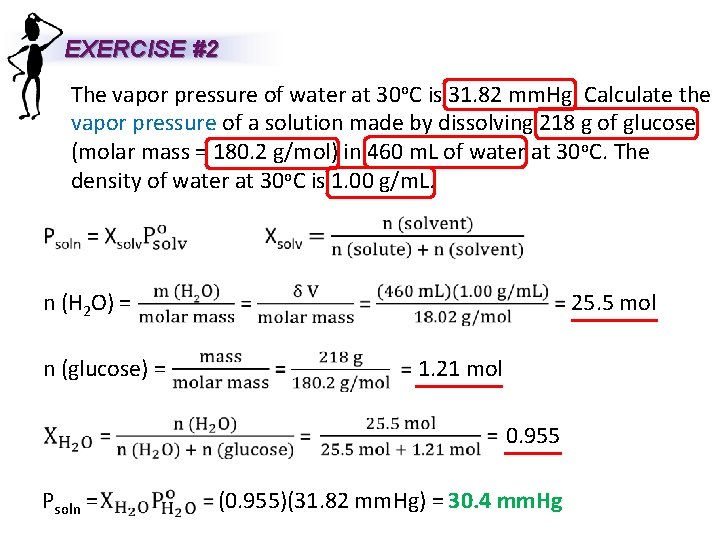
EXERCISE #2 The vapor pressure of water at 30 o. C is 31. 82 mm. Hg. Calculate the vapor pressure of a solution made by dissolving 218 g of glucose (molar mass = 180. 2 g/mol) in 460 m. L of water at 30 o. C. The density of water at 30 o. C is 1. 00 g/m. L. n (H 2 O) = n (glucose) = 25. 5 mol 1. 21 mol 0. 955 Psoln = (0. 955)(31. 82 mm. Hg) = 30. 4 mm. Hg

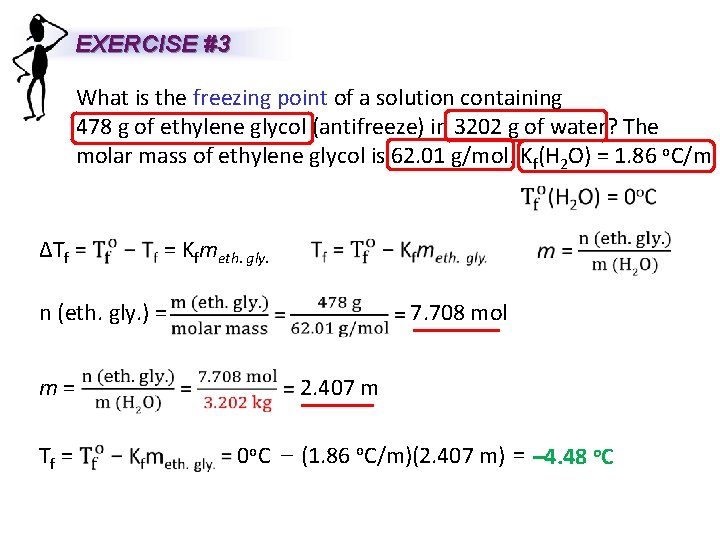
EXERCISE #3 What is the freezing point of a solution containing 478 g of ethylene glycol (antifreeze) in 3202 g of water? The molar mass of ethylene glycol is 62. 01 g/mol. Kf(H 2 O) = 1. 86 o. C/m ΔTf = Kfmeth. gly. n (eth. gly. ) = m= Tf = 7. 708 mol 2. 407 m 0 o. C – (1. 86 o. C/m)(2. 407 m) = – 4. 48 o. C

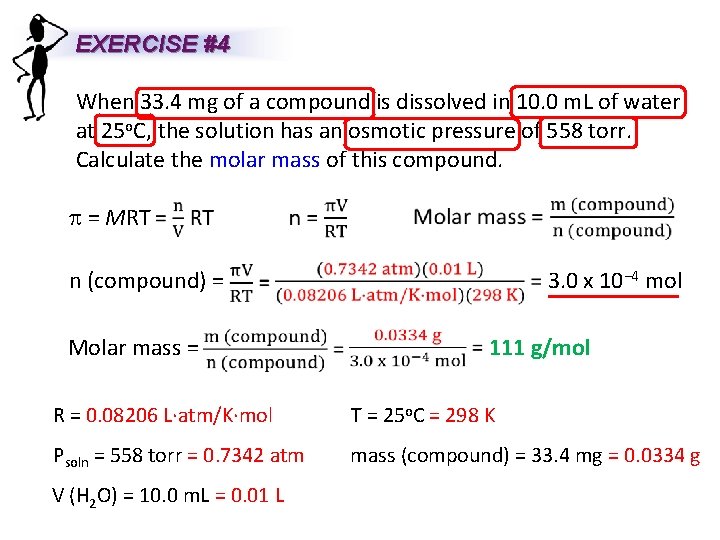
EXERCISE #4 When 33. 4 mg of a compound is dissolved in 10. 0 m. L of water at 25 o. C, the solution has an osmotic pressure of 558 torr. Calculate the molar mass of this compound. p = MRT n (compound) = Molar mass = 3. 0 x 10− 4 mol 111 g/mol R = 0. 08206 L∙atm/K∙mol T = 25 o. C = 298 K Psoln = 558 torr = 0. 7342 atm mass (compound) = 33. 4 mg = 0. 0334 g V (H 2 O) = 10. 0 m. L = 0. 01 L

13. 5 Colligative Properties of Non-Electrolyte Solutions Review Colligative Properties of Nonelectrolyte Solutions Vapor-pressure Boiling-Point Elevation DTb = Kb m Freezing-Point Depression DTf = Kf m Osmotic Pressure (p) p = MRT

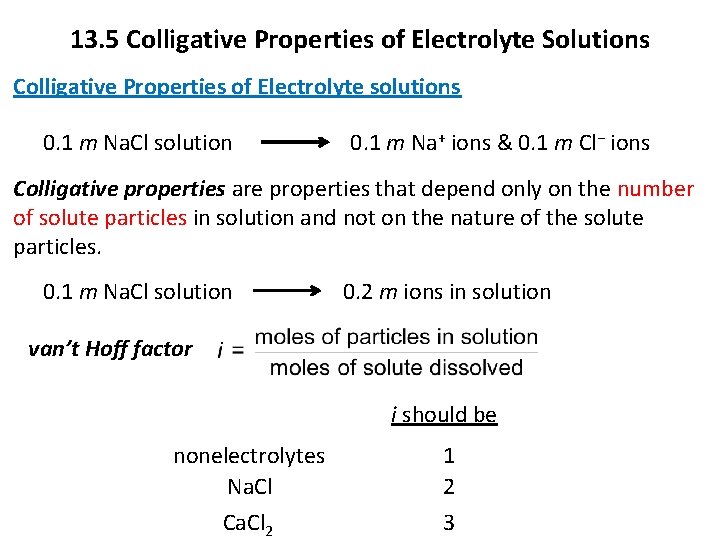
13. 5 Colligative Properties of Electrolyte Solutions Colligative Properties of Electrolyte solutions 0. 1 m Na. Cl solution 0. 1 m Na+ ions & 0. 1 m Cl− ions Colligative properties are properties that depend only on the number of solute particles in solution and not on the nature of the solute particles. 0. 1 m Na. Cl solution 0. 2 m ions in solution van’t Hoff factor i should be nonelectrolytes Na. Cl Ca. Cl 2 1 2 3

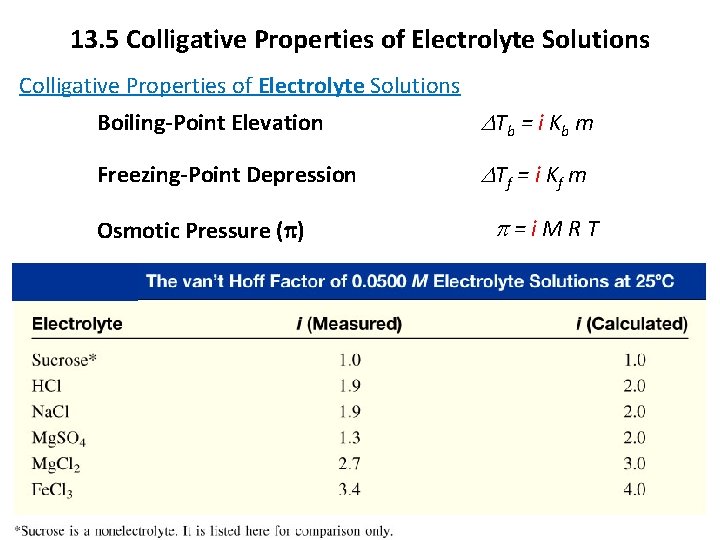
13. 5 Colligative Properties of Electrolyte Solutions Boiling-Point Elevation D Tb = i K b m Freezing-Point Depression D Tf = i K f m Osmotic Pressure (p) p=i. MRT

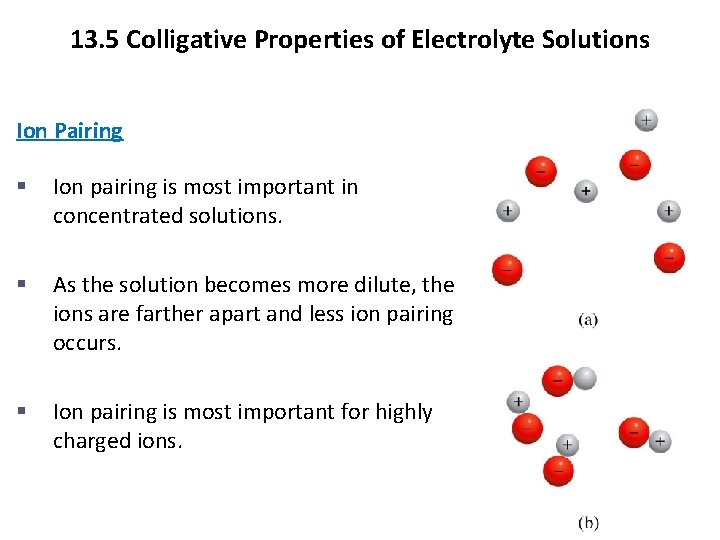
13. 5 Colligative Properties of Electrolyte Solutions Ion Pairing § Ion pairing is most important in concentrated solutions. § As the solution becomes more dilute, the ions are farther apart and less ion pairing occurs. § Ion pairing is most important for highly charged ions. Copyright © Cengage Learning. All rights reserved 21

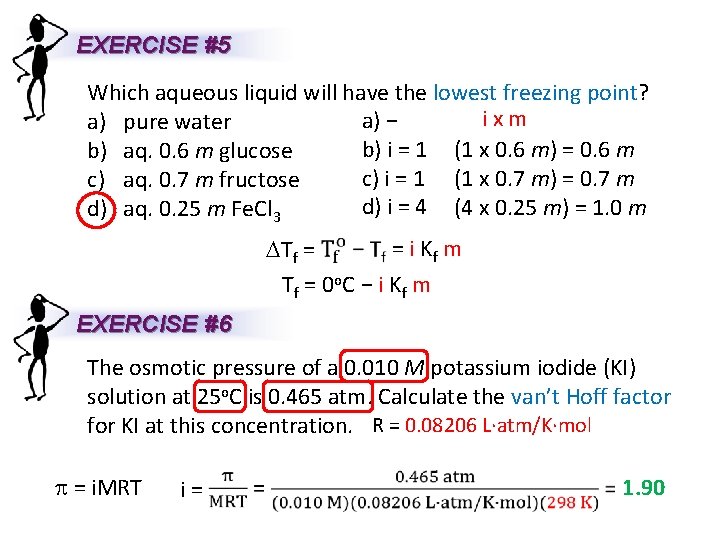
EXERCISE #5 Which aqueous liquid will have the lowest freezing point? ixm a) − a) pure water b) i = 1 (1 x 0. 6 m) = 0. 6 m b) aq. 0. 6 m glucose c) i = 1 (1 x 0. 7 m) = 0. 7 m c) aq. 0. 7 m fructose d) i = 4 (4 x 0. 25 m) = 1. 0 m d) aq. 0. 25 m Fe. Cl 3 = i Kf m DTf = T f = 0 o. C − i K f m EXERCISE #6 The osmotic pressure of a 0. 010 M potassium iodide (KI) solution at 25 o. C is 0. 465 atm. Calculate the van’t Hoff factor for KI at this concentration. R = 0. 08206 L∙atm/K∙mol p = i. MRT i= 1. 90

END OF CHAPTER 13