Lecture 10 12 16 CHAPTER 11 Lipids Olives













- Slides: 42

Lecture: 10 -12 -16 CHAPTER 11 Lipids

Olives Tress in the Mediterranean Region

Chapter 11 Outline

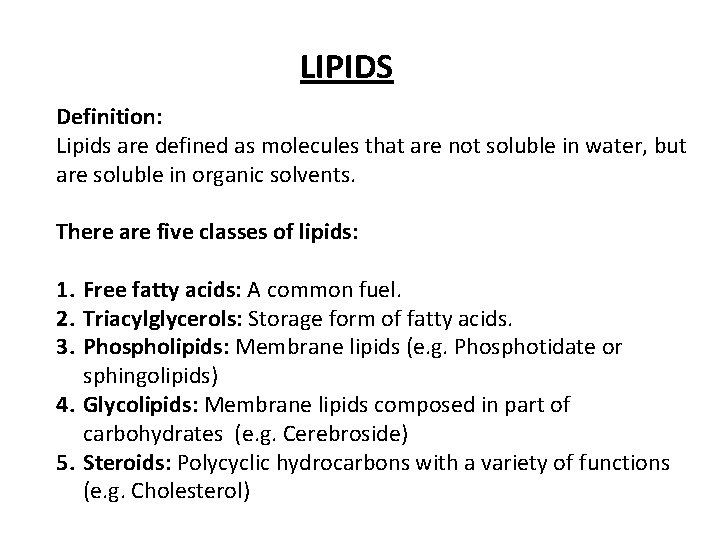
LIPIDS Definition: Lipids are defined as molecules that are not soluble in water, but are soluble in organic solvents. There are five classes of lipids: 1. Free fatty acids: A common fuel. 2. Triacylglycerols: Storage form of fatty acids. 3. Phospholipids: Membrane lipids (e. g. Phosphotidate or sphingolipids) 4. Glycolipids: Membrane lipids composed in part of carbohydrates (e. g. Cerebroside) 5. Steroids: Polycyclic hydrocarbons with a variety of functions (e. g. Cholesterol)

Free fatty acids: Definition: Fatty acids are chains of hydrogen-bearing carbon atoms that have a carboxylic acid at one end a methyl group at the other end. Fatty acids may be saturated or unsaturated.

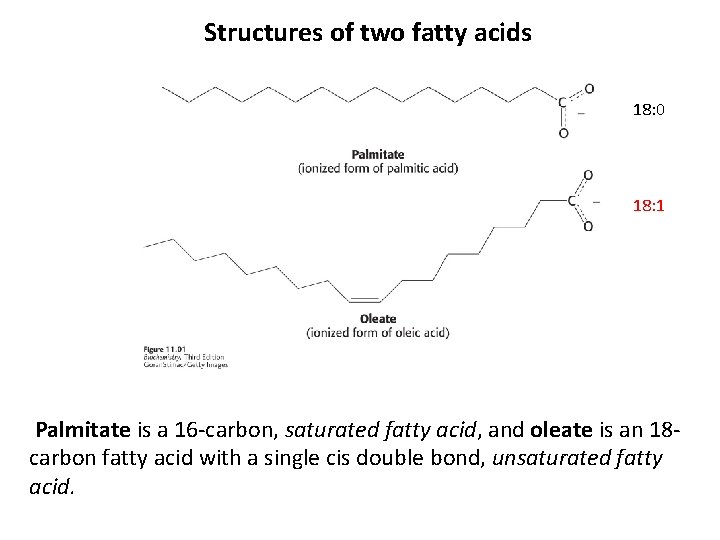
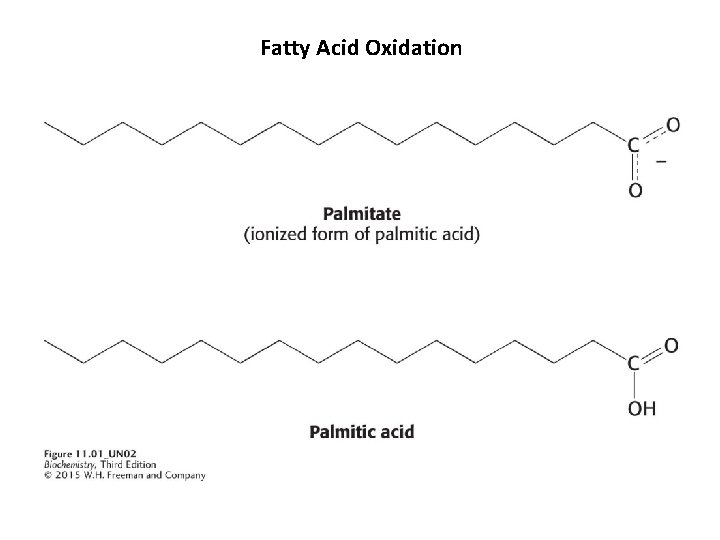
Structures of two fatty acids 18: 0 18: 1 Palmitate is a 16 -carbon, saturated fatty acid, and oleate is an 18 carbon fatty acid with a single cis double bond, unsaturated fatty acid.

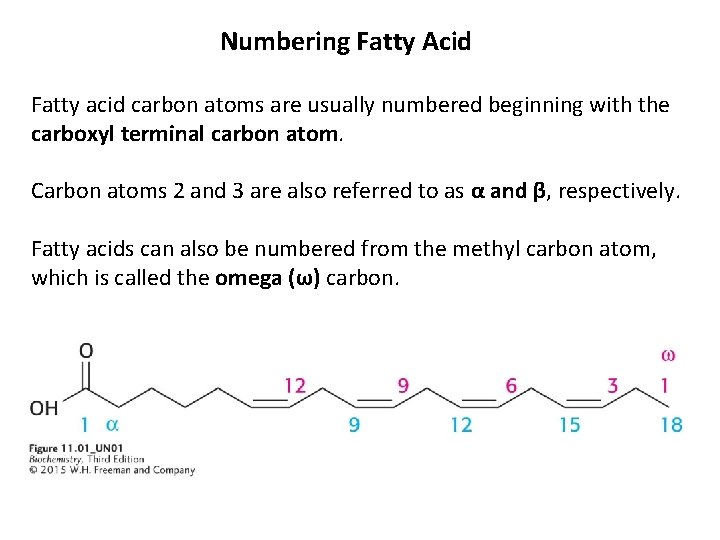
Numbering Fatty Acid Fatty acid carbon atoms are usually numbered beginning with the carboxyl terminal carbon atom. Carbon atoms 2 and 3 are also referred to as α and β, respectively. Fatty acids can also be numbered from the methyl carbon atom, which is called the omega (ω) carbon.

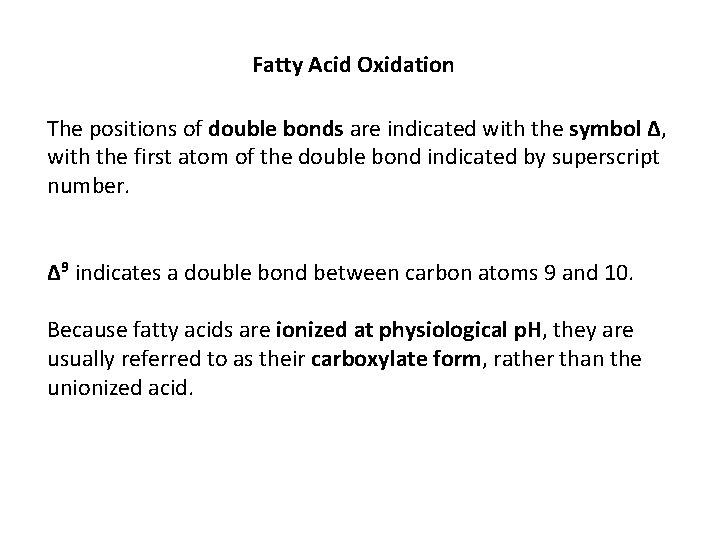
Fatty Acid Oxidation The positions of double bonds are indicated with the symbol Δ, with the first atom of the double bond indicated by superscript number. Δ 9 indicates a double bond between carbon atoms 9 and 10. Because fatty acids are ionized at physiological p. H, they are usually referred to as their carboxylate form, rather than the unionized acid.

Fatty Acid Oxidation

Fatty acids in biological systems usually contain an even number of carbon atoms, with the 16 and 18 carbon atom chains the most common. When double bonds are present, they are commonly in the cis configuration. In polyunsaturated fatty acids, the double bonds are separated by at least one methylene group.

The properties of fatty acids are dependent on chain length and degree of unsaturation. *Short chain length and the presence of cis double bonds enhances the fluidity of fatty acids.

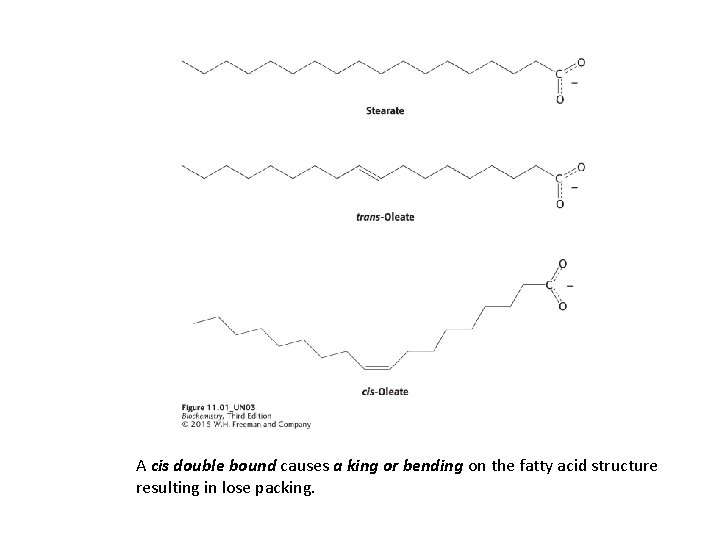
A cis double bound causes a king or bending on the fatty acid structure resulting in lose packing.

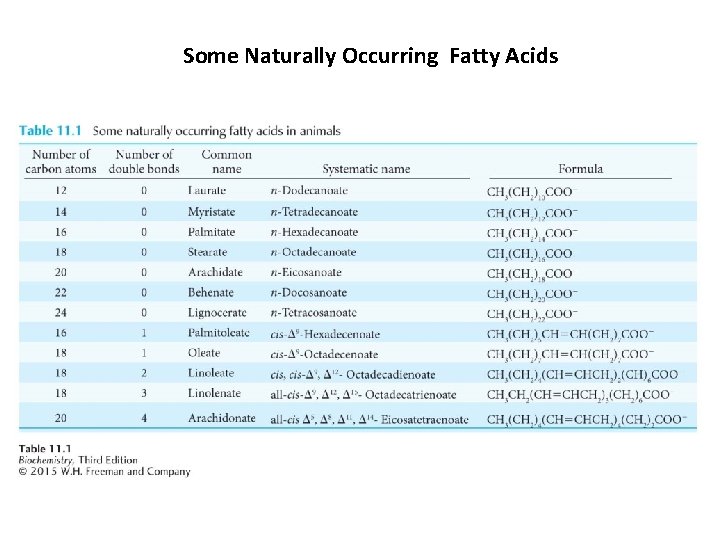
Some Naturally Occurring Fatty Acids


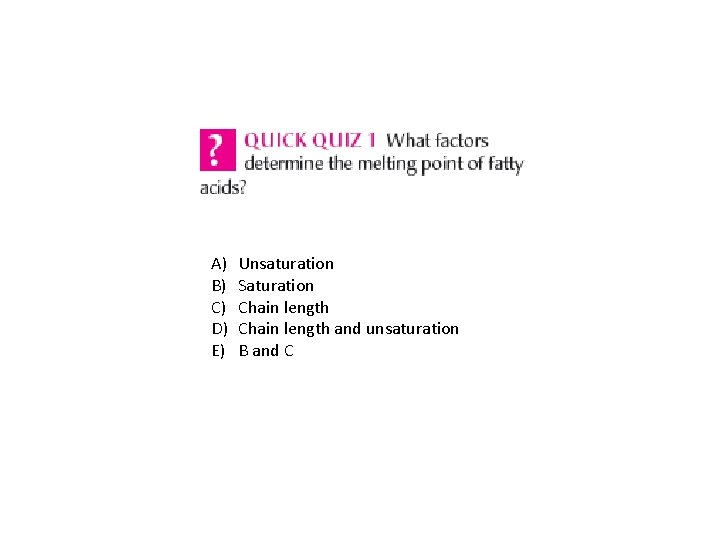
A) B) C) D) E) Unsaturation Saturation Chain length and unsaturation B and C

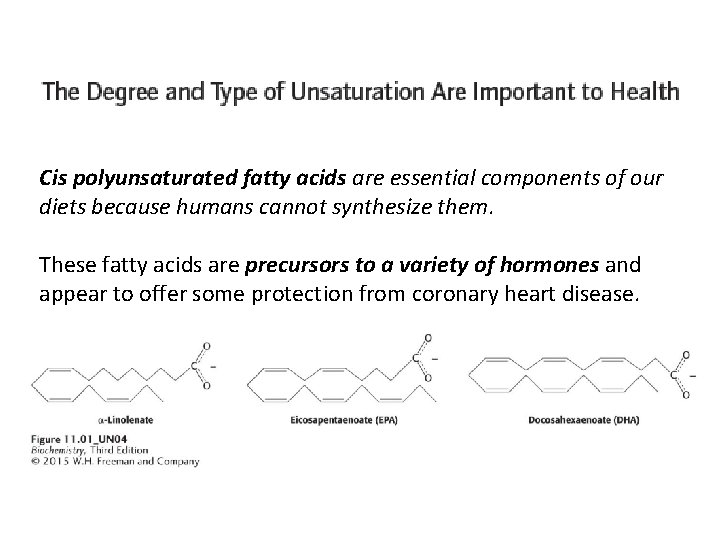
Cis polyunsaturated fatty acids are essential components of our diets because humans cannot synthesize them. These fatty acids are precursors to a variety of hormones and appear to offer some protection from coronary heart disease.

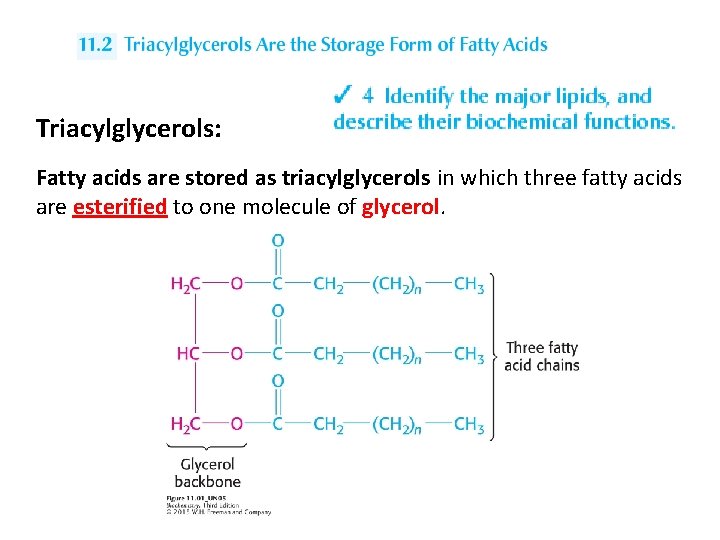
Triacylglycerols: Fatty acids are stored as triacylglycerols in which three fatty acids are esterified to one molecule of glycerol.

Soaps are the sodium or potassium salts of long chain fatty acids generated by treating triacylglycerols with a strong base.

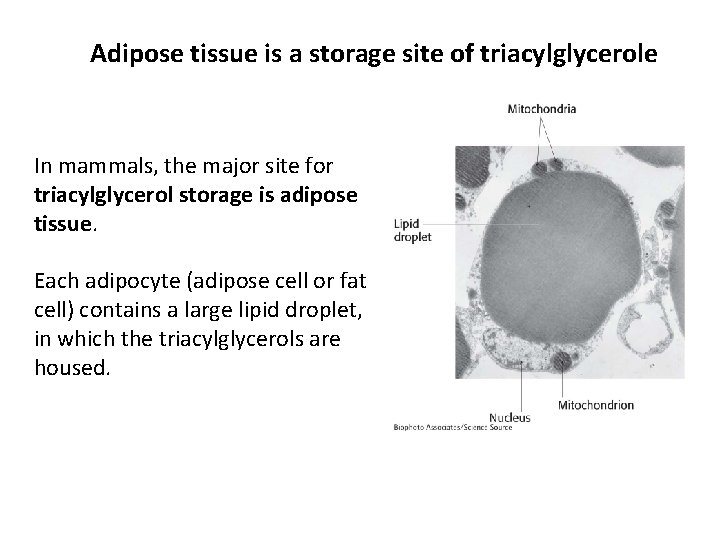
Adipose tissue is a storage site of triacylglycerole In mammals, the major site for triacylglycerol storage is adipose tissue. Each adipocyte (adipose cell or fat cell) contains a large lipid droplet, in which the triacylglycerols are housed.

Triacylglycerols are energy rich. Because they are hydrophobic and reduced, a gram of anhydrous fat stores more than six times the energy of a gram of hydrated glycogen. Migratory birds use fat stores to power long flights without the opportunity to feed. Ruby-throated hummingbird

The common types of membrane lipids are: 1. Phospholipids 2. Glycolipids 3. Cholesterol

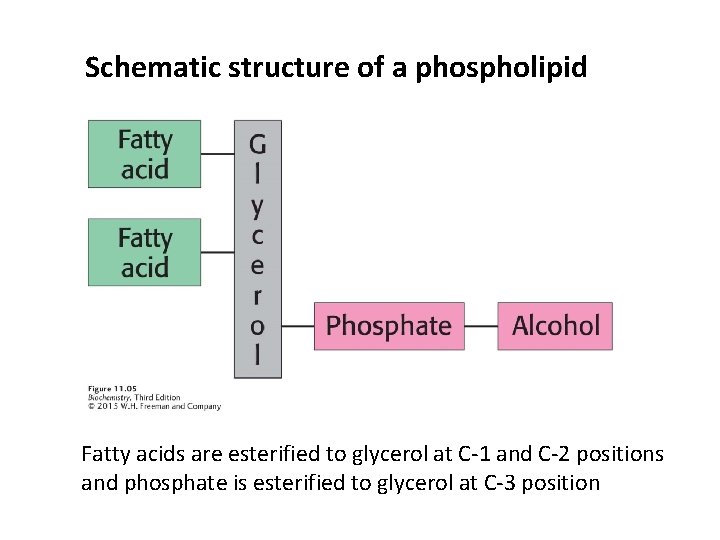
1) PHOSPHOLIPID Phospholipids are composed of four components: Fatty acids (2 or more), a platform, a phosphate, and an alcohol. Two common platforms are glycerol and sphingosine. Phospholipids with a glycerol platform are called phosphoglycerides or phosphoglycerols. The major phospholipids are derived from phosphatidate.

Schematic structure of a phospholipid Fatty acids are esterified to glycerol at C-1 and C-2 positions and phosphate is esterified to glycerol at C-3 position

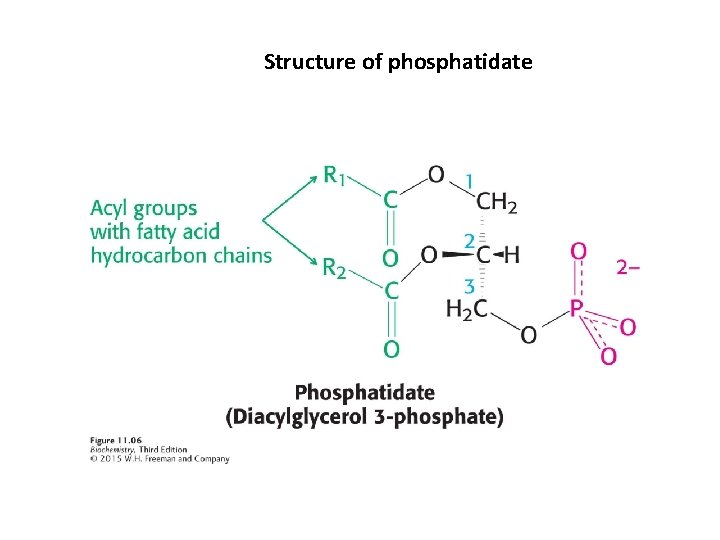
Structure of phosphatidate

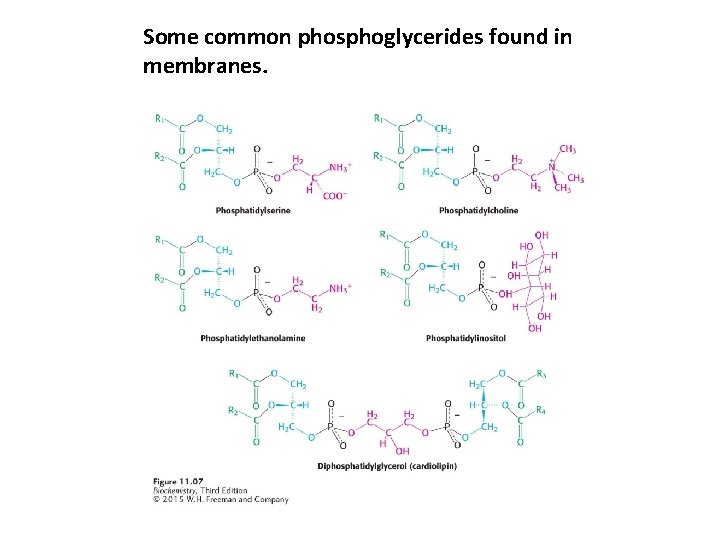
Some common phosphoglycerides found in membranes.

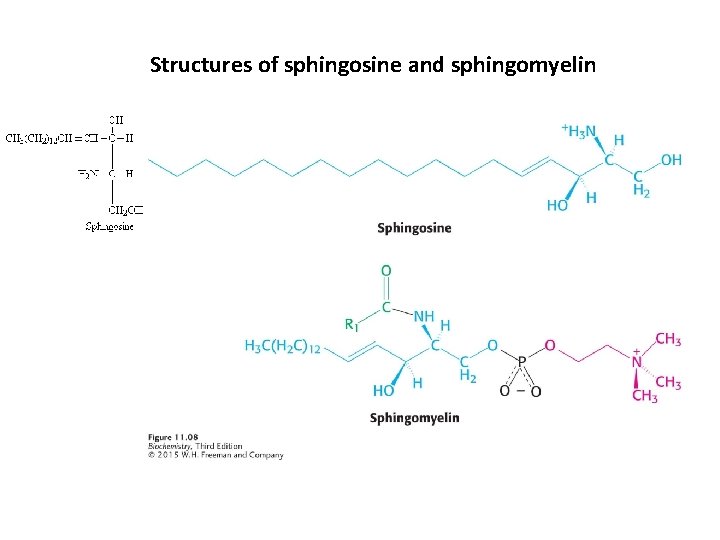
Phospholipids built on a sphingosine platform are called sphingolipids. Sphingomylein is a common membrane sphingolipid and is especially common in the myelin sheath of nerve cells.

Structures of sphingosine and sphingomyelin

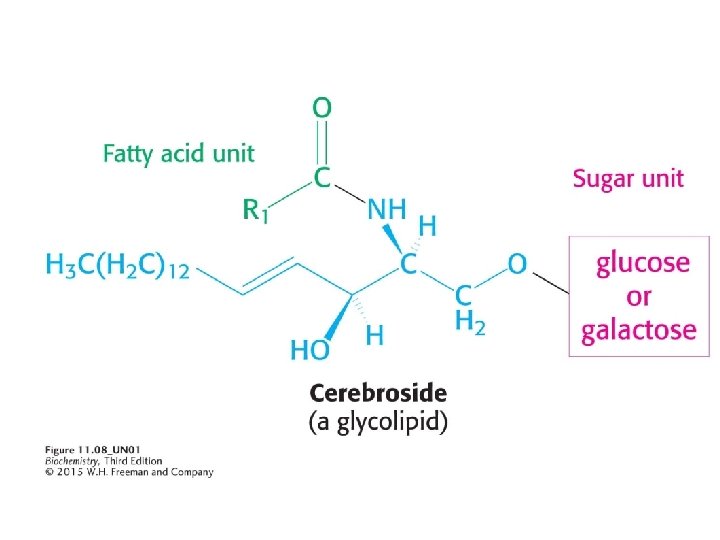
2) GLYCOLIPIS (Sweet Lipids!) Glycolipids are carbohydrate-containing lipids. The carbohydrate components of glycolipids are on the extracellular surface of the cell membrane, where they play a role in cell-cell recognition. Cerebrosides are the simplest glycolipids.


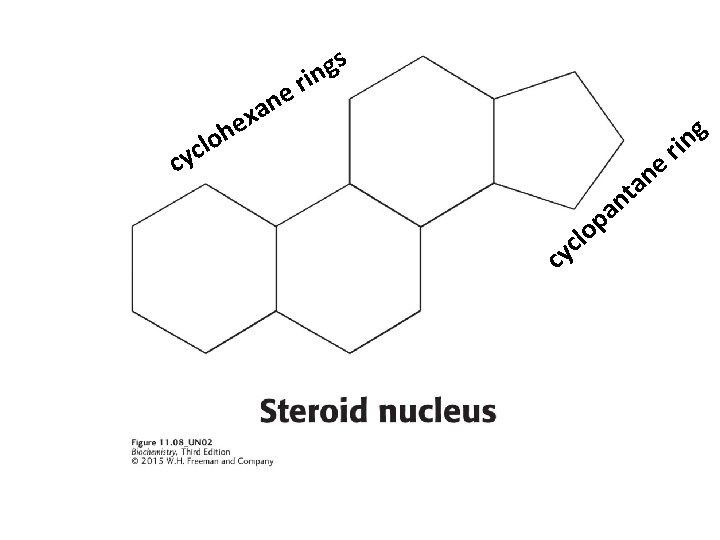
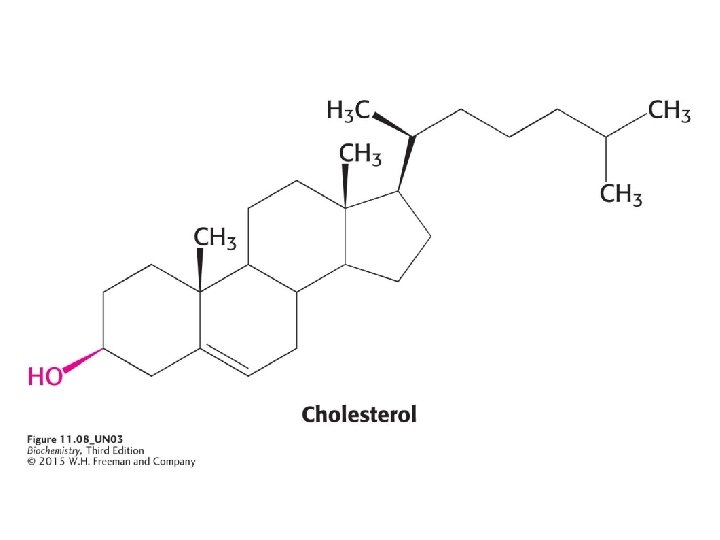
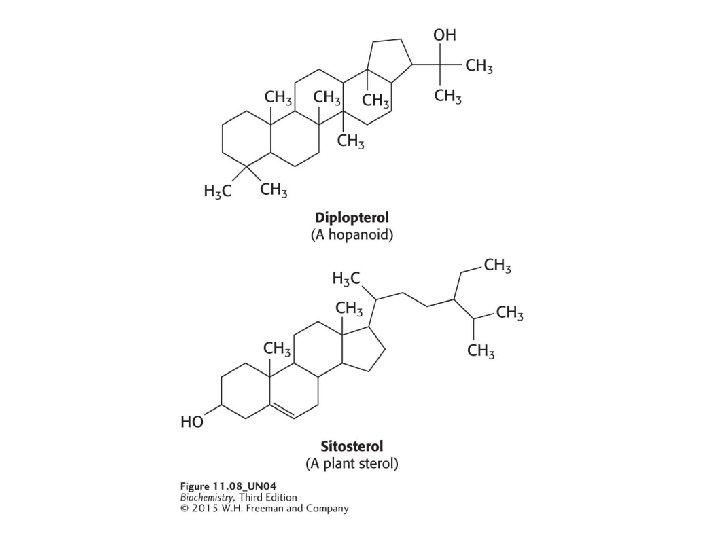
3) CHOLESTEROL Steroids are built on a tetracyclic platform, consisting of three cyclohexane rings and a cyclopentane ring fused together. Cholesterol is the most common steroid and plays a role in maintaining membrane fluidity. Cholesterol is also a precursor to steroid hormones. *Only mammalians have cholesterol among eukaryotes Hopaniods and various sterols play the role of cholesterol in prokaryotes and plants.

s g in r e n a x he g n o l c cy i r e n a t cy n a p o cl



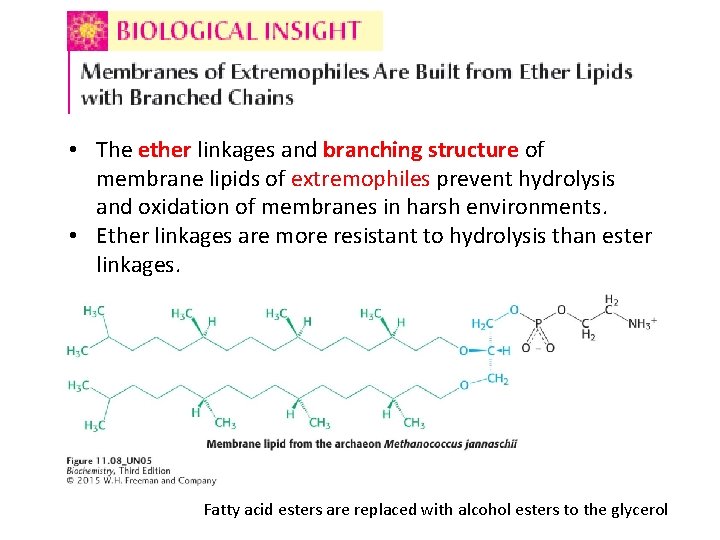
• The ether linkages and branching structure of membrane lipids of extremophiles prevent hydrolysis and oxidation of membranes in harsh environments. • Ether linkages are more resistant to hydrolysis than ester linkages. Fatty acid esters are replaced with alcohol esters to the glycerol

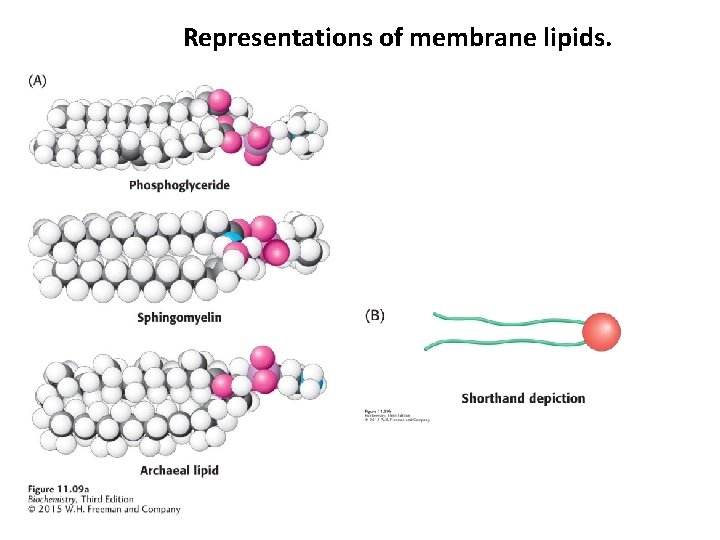
Membrane lipids are amphipathic molecules, containing hydrophobic and hydrophilic properties. The fatty acid components provide the hydrophobic properties, while the alcohol and phosphate components, called the polar head group, provide the hydrophilic properties.

Representations of membrane lipids.


A) B) C) D) E) Glycolipid, cholesterol, triacylglycerol Phospholipid, fatty acids, cholesterol Glycolipid, triacylglycerol, phospholipid Phospholipid, cholesterol, glycolipid None of the above

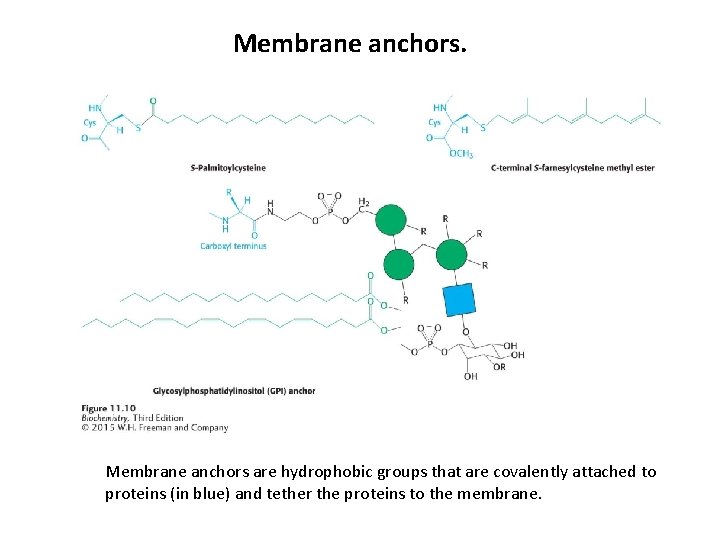
Proteins are sometimes covalently bound to lipids to localize the protein to the cell membrane.

Membrane anchors are hydrophobic groups that are covalently attached to proteins (in blue) and tether the proteins to the membrane.

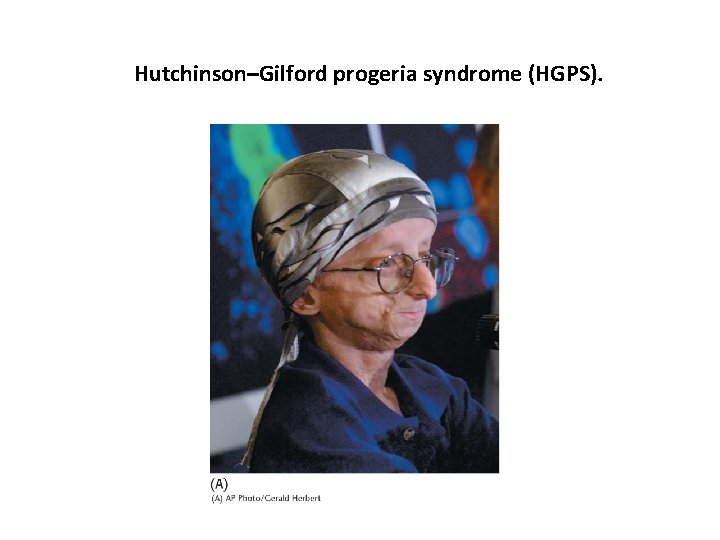
Inappropriate farnesylation is a cause of Hutchinson-Gilford progeria syndrome, a disease of premature aging. Failure to remove a farnesyl group from the nuclear protein lamin results in a deformed nuclear membrane and impaired nuclear function.

Hutchinson–Gilford progeria syndrome (HGPS).