ROLES OF LIPIDS IN FOOD INDUSTRY They serve













- Slides: 21

ROLES OF LIPIDS IN FOOD INDUSTRY § They serve as carriers or sources of fat-soluble vitamins § They are sources of essential fatty acids(linoleic, linolenic and arachidonic acids) §Lipids play a distinctive role in the natural flavour of a wide variety of foods-lipids generally improve the palatability of foods §Lipids determine the body and texture characteristic of a large number of food productsthe richiness and texture of ice creams are due to the lipid fraction §Phospholipids serve as emulsifying agents

• Lipids play an important role as lubricants in bread and the cereal products • Fats/oils serve as concentrated form of energy • Unsaturated fatty acid component of lipids are the causes of the undesirable off-flavours and offcolours when they become rancid

§ § NON-ENZYMATIC BROWNING Non Enzymatic browning involves thermal decomposition of poly-hydroxyl carbonyl compounds in the presence or absence of amino compounds It is divided into two: caramelisation and Maillard reactions depending on whether the decomposition is in the absence or presence of amino compounds respectively. Caramelisation occurs when polyhydroxyl carbonyl compounds are heated to 1000 c and above in the absence of amino compounds. Caramelisation reaction involves enolisation, isomerisation, dehydration, fragmentation and polymerisation.

• The first stage of caramelisation is a keto-enol tautomerism. This means there is reversible isomerization of an aldose sugar to the corresponding ketose. The enediol is readily dehydrated in an acidic environment to form hydroxymethylfurfural(HMF). • HMF(colourless) polymerises to form a brown complex called caramel • Caramelisation requires more energy to get started than the Maillard reaction. • Factors that affect caramelisation include: temperature, the nature of sugar(carbonyl radical), alkaline p. H, influence by acidic medium

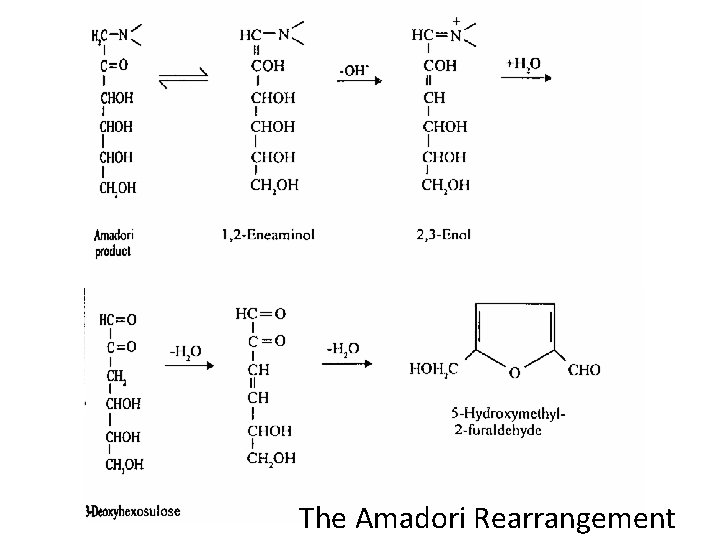
• Maillard reaction is the sequence of events that begins with reaction of the amino group of amino acids with a glycosidic hydroxyl group of sugars; the sequence terminates with the formation of brown nitrogenous polymers or melanoidins. • It produces flavor, colour, antioxidant products, toxic products and destroys nutrients(lysine).



The Amadori Rearrangement

• Maillard reaction is the browning reaction resulting from the carbonyl-amino interactions. • This reaction occurs more rapidly than caramelisation. • The Maillard pathway involves the initial condensation reaction of a carbonyl compound with an amine to form Schiff’s base which is then stabilised by undergoing an Amadori rearrangement. • The sequence terminates with the formation of brown nitrogenous polymers or melanoidins.

• Factors that affect Maillard reaction include low water activity, lower temperature(<caramelisation temperature), nature of sugar, alkaline p. H • Maillard reaction is the commonest type of browning encountered in food processing. • The application of heat in food processing produces non-enzymatic browning. Examples are baking, roasting e. t. c • Desirable non-enzymatic browning occurs in baked foods, breakfast cereals, fried plantain, bean balls, roasted meat • Undesirable non-enzymatic browning occurs in powdered milk and egg productions

• • • PLANT GUMS AND THEIR USES IN FOODS Plant gums are hydrocolloidal substances that give viscous solutions when treated with cold or hot water Most gums are polysaccharides and examples include: seed gums, plant exudate gums, sea weed gums Gums are usually classified according to their sources. They are incorporated into foods when they have been listed as GRAS by Federal Code. However, limits are set on the amounts that can be added to various food products.

• Most commonly added plant gums in food industry are: Seed gums: Guar and locust bean gums are examples of seed gums. • Guar gum consists of long chains of 1 -4 linked β-Dmannopyranose unit to every other mannopyranose unit being attached to an α-Dgalactopyranose unit through a 1 -6 linkage. • Locust bean gum structure is similar but with the branched units spaced more widely. • They both have high molecular weights and can yield high viscosity

• Their rheology and hydrocolloidal stability are least affected by freezing, heat processing, addition of acids/salts. • Plant exudates: These gums are collected manually from the wounds of tropical trees and shrubs. • Examples are gums from Acacia, ghatti, karaya. • Compositional and structural differences among the exudate gums result in wide differences in their solubilities and viscosities. • Sea weed extracts: Red and brown algae are examples of sea weed extracts. Agar used in microbiology laboratories are got from seaweed extracts.

• Examples of foods that are much improved by gum additions are: • Ice cream, gelled desserts, salad dressing, baked goods e. t. c • Gums function as moisture retaining agents in baked goods, thickeners in gravies and sauces, emulsion stabilizer in salad dressing e. t. c

Properties of gum depend on: 1) Size and shape – Linear structures: • More viscous (occupy more space for same weight as branched) • Lower gel stability get syneresis on storage (i. e. water squeezes out of the gel) – Branched structures • Less viscous • Higher gel stability more interactions 15

Properties of gum also depend on: 2) Ionization and p. H – Non-ionized gums = little effect of p. H and salts – Negatively charged gums • Low p. H = deionization = aggregation precipitation – Can modify by placing a strong acidic group on gum so it remains ionized at low p. H (important in fruit juices) • High p. H = highly ionized = soluble viscous • Ions (e. g. Ca 2+) = salt bridges = gels 3) Interactions with other components – Proteins – Sugars 16

• • • PECTIC SUBSTANCES Pectic substances are got from middle lamella of cell walls of plants in conjunction with cellulose They are group designated for colloidal carbohydrate derivatives which occur in plants They function to move H 2 O and cement materials for the cellulose network. They are composed of 4 classes/forms/substances Pectin is got when pectic substances (citrus peel etc. ) are heated in water(60 -950 C) and in the presence of acidic p. H of 2. 5 The pectins are precipitated with ethanol and removed by centrifugation. • Pectins from different sources may differ in chemical and functional details. • Pectins are water soluble and make viscous solutions or gels, similar in composition to the gums.

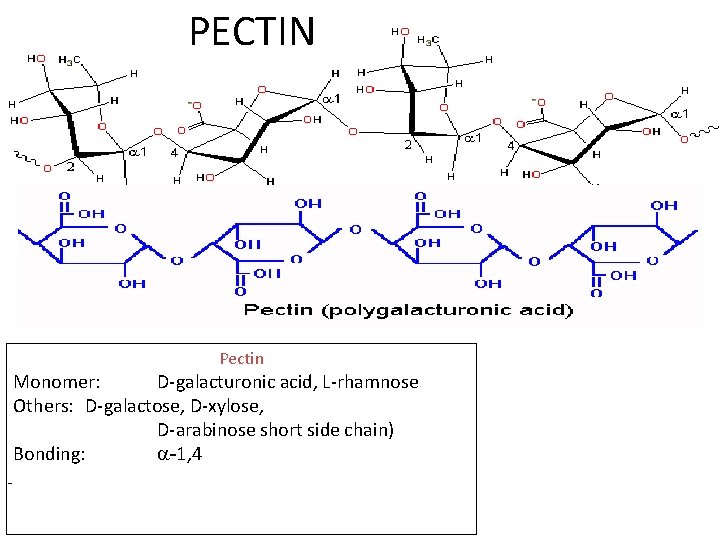
PECTIN Pectin Monomer: D-galacturonic acid, L-rhamnose Others: D-galactose, D-xylose, D-arabinose short side chain) Bonding: -1, 4 -

Pectins • Unbranched polymers of 200 - 1, 000 Galactose units, linked b 1 -4 Glucosidic bonds • Degree of esterification controls setting rate • • • >50% High Ester Pectins (HM) <50% Low Ester Pectins (LM) 70 - 85% (DE) = Rapid Set 44 - 65% (DE) = Slow Set Calcium required to gel LM Pectins USES: • Amidated LM Pectins used to gel natural fruit preserves • High ester (HM) Pectins stabilize sour milk drinks - react with casein • Low ester (LM) Pectins used for milk gels

• Pectin consists of polygalacturonic acids linked together by 1, 4 linkage similar to amylose in starch. • It is a polymer of heteroglycan. • The carboxyl radicals can either be methylated, esterified or neutralised (addition of alkaline) or replaced by metals. • It is found in climateric fruits. As fruits mature, pectinases usually break down the pectins between cells and make the fruit soft and edible. . It forms a gel in the presence of sugar and acid under suitable condition because it contains substantial degree of esterification(50 -80%) • The number of methoxy groups present is important in determination of the properties of any particular pectin. • The peels of many fruits serve as convenient sources of pectins • Most commercial pectins come from "apple pomace" or waste from manufacture of apple products (10 -15% pectins), citrus wastes (20 -30% pectins) and from sugar beet processing.

• As is true for gums, humans are incapable of digesting pectins. • It is used in making fruit preserve(jam, gelly, marmalade) • Many candies (especially gum drops) involve pectins as a gelling agent. • Also used in ice cream(frozen food because it’s gel is stable either hot or cold), cosmetics, adhesives, and in hardening steel. • Used in many foods as a thickener and stabilizer.
 Application of lipids in food industry
Application of lipids in food industry Food handlers can contaminate food when they answer
Food handlers can contaminate food when they answer When should hand antiseptics be used?
When should hand antiseptics be used? Unit 2 food food food
Unit 2 food food food Eltonian pyramid
Eltonian pyramid Competitive analysis grid example
Competitive analysis grid example Rankings: what are they and do they matter?
Rankings: what are they and do they matter? If they have time at the weekend they will come to see us
If they have time at the weekend they will come to see us They seek him here they seek him there
They seek him here they seek him there It is not you they are rejecting but me
It is not you they are rejecting but me Jordan 14
Jordan 14 Grammar rules frustrate me they're not logical they are so
Grammar rules frustrate me they're not logical they are so For they not know what they do
For they not know what they do Although they knew god they did not glorify him
Although they knew god they did not glorify him Food and fiber industry
Food and fiber industry Food and beverage industry in sri lanka
Food and beverage industry in sri lanka Key success factors fast food industry
Key success factors fast food industry Distillation application
Distillation application Freezing techniques in food industry
Freezing techniques in food industry Historical entrepreneurs in the food industry
Historical entrepreneurs in the food industry Cross counter flow heat exchanger
Cross counter flow heat exchanger Single pass and multi pass heat exchanger
Single pass and multi pass heat exchanger