Chapter 10 Notes Lipids Natures Flavor Enhancers Too













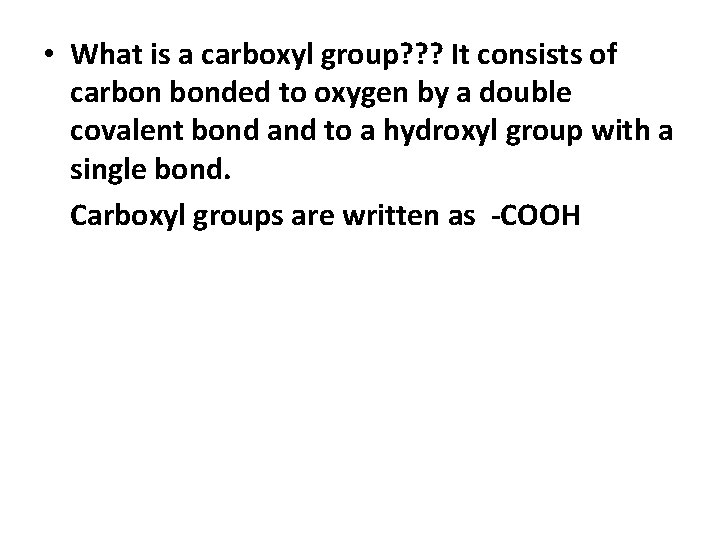







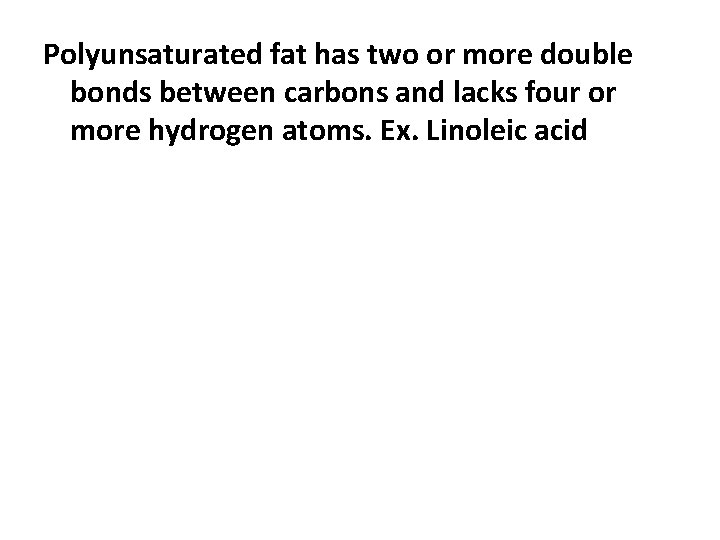



















- Slides: 44

Chapter 10 Notes Lipids: Nature’s Flavor Enhancers

Too much fat can be harmful however, fat plays an important role both in food preparation and general health.

You will: • Learn functions of fat in food preparation • Why fat is a vital part of a diet • Describe molecular structure of glycerides, phospholipids, and sterols. • Define monounsaturated, and polyunsaturated fatty acids. • List categories of lipids based on physical state and dietary sources. • Relate physical characteristics of lipids to their performance in foods.

I. Chemical Structure of Lipids are a category of organic compounds that are insoluble in water and have a greasy feel. • Fats, • Oils • Shortening • Grease • Phospholipids • Sterols • Cholesterol are all terms used for lipids and their related compounds.

Lipids are like carbohydrates because they contain • 1. carbon • 2. hydrogen • 3. oxygen.

Lipids differ from carbohydrates because • lipids are not polymers • they do not provide structure to food products • they cannot be dissolved in water.

The three types of lipids found in foods and the human body are • 1. triglycerides • 2. phospholipids • 3. sterols.

What Are Lipids? Triglycerides: • Most lipids found in foods and in the body are triglycerides. • They include nearly all of the fats and oils people typically eat. • Triglycerides can have three different fatty acids attached to the glycerol base.

Phospholipids: • These lipids have a structure that enables them to dissolve in both fat and water. • The food industry uses them as emulsifiers that mix fats with water in products like mayonnaise. • They are also found naturally in some foods like eggs and peanuts.

Sterols: • Sterols include important compounds. Bile acids and certain hormones, for example perform vital functions. • Both plant and animal foods contain sterols but only animal foods contain the most well known, cholesterol.

Triglycerides • The main function of triglycerides, or fat, is to fuel the body and keep it warm. • Triglycerides help keep body temperature relatively constant. • When triglycerides are stored in the body, they collect in adipose tissue: pockets of fatstoring cells.

• The adipose tissue under each kidney forms a cushion to help protect it from impact. • Natural body oils keep skin supple and hair glossy. • Fat carries certain vitamins.

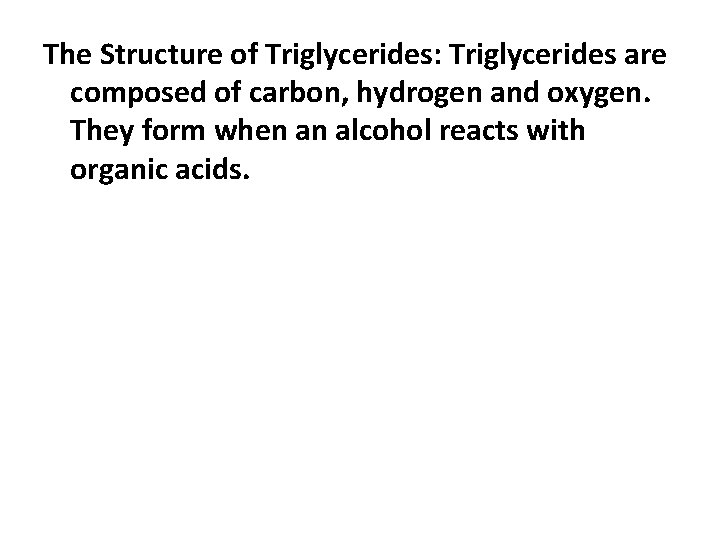
The Structure of Triglycerides: Triglycerides are composed of carbon, hydrogen and oxygen. They form when an alcohol reacts with organic acids.

The organic acids in triglycerides are called fatty acids. • Fatty acids: Organic compounds that have a carbon chain with attached hydrogen atoms and a carboxyl group at one end.
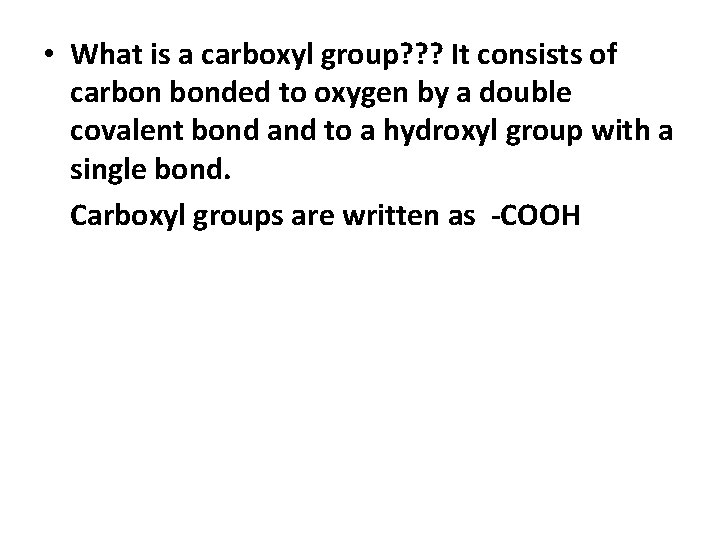
• What is a carboxyl group? ? ? It consists of carbon bonded to oxygen by a double covalent bond and to a hydroxyl group with a single bond. Carboxyl groups are written as -COOH

Essential Fatty Acids: The human body can make all but two fatty acids. • linoleic • linolenic Both are needed for normal growth and development. They are found in vegetables, grains, nuts, seeds and soybeans.

Saturated and Unsaturated Fat: These terms describe how carbon atoms are bonded in fatty acids. The bonds can be • single • double

Single bond: • Covalent bond in which each atom donates only one electron to form the bond. • Two atoms share one pair of electrons between them.

Double bond: • Covalent bond in which each atom donates two electrons to form the bond. • Two atoms share two pairs of electrons between them.

Saturated Fat: • the fatty acids contain all the hydrogen atoms their molecular structure can hold. • As long as no hydrogen atoms are missing, each bond between a carbon atom and its four partnering atoms will be single.

Unsaturated Fat: • missing hydrogen atoms. • Where hydrogen atoms are missing due to a chemical change, single bonds can’t form. • To make up the difference for a missingle bond, a double bond forms. • two carbon atoms that are each missing a hydrogen atom will bond to each other with a double bond.

Monounsaturated fat lacks two hydrogen's, which creates one double bond between carbons. Ex. Oleic acid
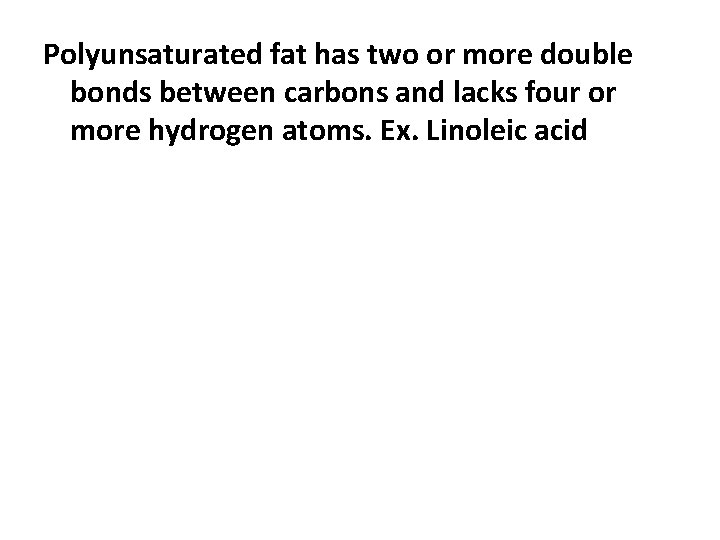
Polyunsaturated fat has two or more double bonds between carbons and lacks four or more hydrogen atoms. Ex. Linoleic acid

• • • Properties of Triglycerides: Energy Value Solubility Phase Differences Melting Range Solidification point

• Energy Value: Energy is released by oxidation, the reaction of a substance with oxygen. Triglyceride molecules are high in carbon and hydrogen, making them supply over twice the energy of glucose – nine kcalories per gram compared to four.

• Solubility: Slightly soluble in water, they resist hydrogen bonding that dissolves sugars in water. That is why you have to shake a bottle or oil and vinegar dressing.

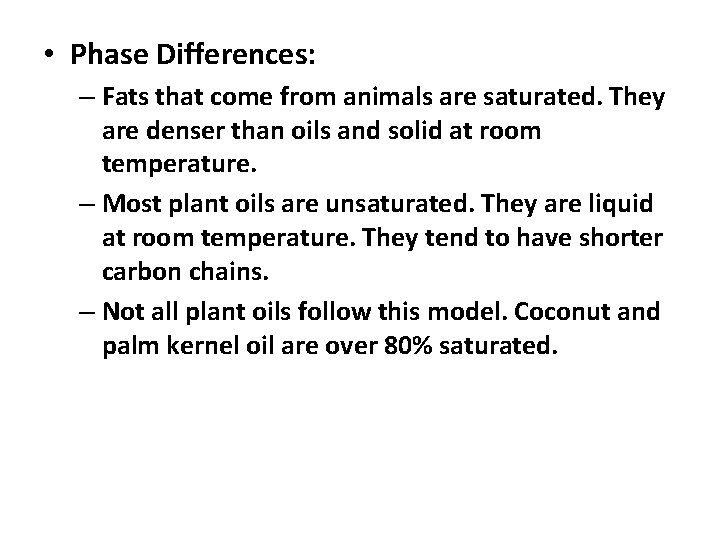
• Phase Differences: – Fats that come from animals are saturated. They are denser than oils and solid at room temperature. – Most plant oils are unsaturated. They are liquid at room temperature. They tend to have shorter carbon chains. – Not all plant oils follow this model. Coconut and palm kernel oil are over 80% saturated.

• Melting Range: Fat melts gradually. In a pat of butter, some fats are in liquid phase and some are solid crystals. Fats with a greater proportion of unsaturated fatty acids melt at lower temperatures.

• 5. Solidification Point: Definition is the temperature at which a melted fat regains its original firmness. Fat’s solidification point is lower than its melting range. Fats with more similar fatty acids solidify with a grainier texture.

Functions of Fats in Food • Tenderizing • Aeration • Emulsions • Flavor

• Tenderize: In baked products, fat coats the flour particles, creating a flaky, delicate, lighter texture. Without fat, cakes would crumble, pizza dough and pie crust would be tough.

• Aeration: Fats add air or gas to batters and doughs. The fat forms a bubble around air molecules, which are incorporated in the batter.

• Emulsions: Fats can either be part of an emulsion or influence an emulsion. Ex. Oils are used as an emulsifier in mayonnaise.
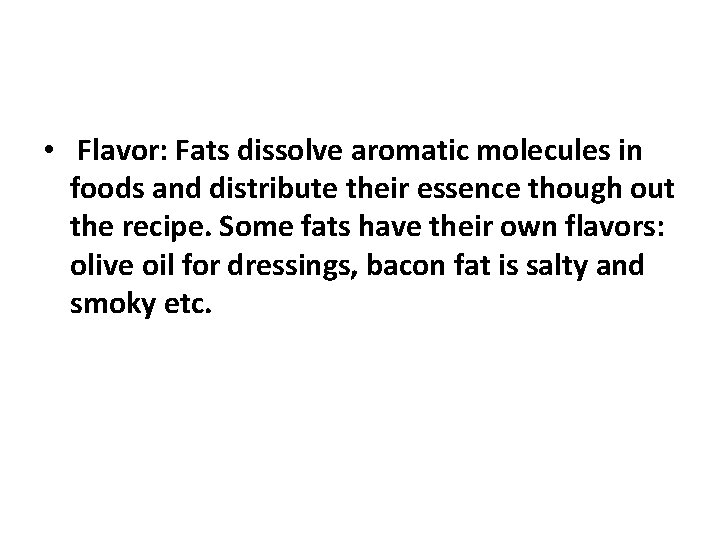
• Flavor: Fats dissolve aromatic molecules in foods and distribute their essence though out the recipe. Some fats have their own flavors: olive oil for dressings, bacon fat is salty and smoky etc.

Oxidation: This occurs when the surface of foods react with oxygen. • Oxidation involves the loss of a hydrogen atom from a single-bonded carbon next to a double bond. • Unsaturated fats, with more double bonds, are more prone to oxidization. • Heat speeds the rate of oxidation. • Cooling the environment, even freezing, slows but doesn’t stop the process. • Water inhibits oxidation. • Rancid: Describes the unpleasant flavors that develop as fats oxidize.

How do you control oxidation? • Potato chips are packaged in pure nitrogen. • Bacon is vacuum sealed. • Oils may be bottled in dark glass. • Dried foods that lack water may be treated with substances that prevent oxidation. They are called antioxidants. +

Commercial Uses of Fats Two groups: Animal fats and plant oils. • Animal fats: Butter, lard, beef fat. – Butter fat is a natural emulsion with 80% fat and 18% water. – Lard, or rendered hog fat, is popular in baking pastries, cakes, frostings. – Beef fat is usually combined with vegetable fats for use in foods.

• Plant lipids: derived from oil rich seeds. – Corn, olive, canola, and oil blends are popular.

Hydrogenated Oils: Solid vegetable shortening • Hydrogenation is: a chemical process in which hydrogen is added to unsaturated fat molecules, breaking some double bonds and replacing them with single bonds.

Why do this? • Good texture to the fat • It is more stable • It resists rancidity better than liquid oil • Doesn’t develop stale flavor and odor as quickly. • Helps make margarine!

Cooking with Fats • Fats form so many bonds between carbon chains that it takes temperatures of at least 260 degrees C to separate the molecule. • Effects on Fats: Frying can be tough on fats. Here is what could happen when frying: • Repeated exposure to intense heat causes decomposition. • Foods release some of their own fat into the oil, causing the oil to change color and change flavor.

• Triglycerides in the Diet: The fats you eat become fats in your body. A typical American diet gets 45% of its calories from fat. Low Fat Options: Low-fat foods in grocery stores Watch calorie intake

Fat Replacers: substances that mimic fats in foods but not in the body. • Fat based replacers are manufactured from very short and long carbon-chain acids • Protein based replacers use milk or protein particles to stabilize and texturize dairy products, baked goods, sauces and soups. • Carbohydrate fat replacers include cellulose, gums and modified food starch. • Newer fat replacer consists of fatty acids attached to a sucrose molecule. This is the first artificial fat that is suitable for frying. It cannot be digested and it is calorie-free.

• Cholesterol: Not a triglyceride but a sterol, a fatty alcohol made from glucose or saturated fatty acids. • Cholesterol is vital in producing vitamin D and some hormones. It strengthens cell membranes • The liver makes cholesterol • In your diet, cholesterol is found in animal products. It is usless and possibly harmful. • Cholesterol in the blood is thought to contribute to plaque that can lodge in artery walls.